Abstract
Background
High-resolution impedance manometry (HRIM) allows evaluation of esophageal bolus retention, flow, and pressurization. We aimed to perform a collaborative analysis of HRIM metrics to evaluate patients with non-obstructive dysphagia.
Methods
14 asymptomatic controls (58% female; ages 20 – 50) and 41 patients (63% female; ages 24 – 82), 18 evaluated for dysphagia, 23 for reflux (‘non-dysphagia patients’), with esophageal motility diagnoses of normal motility or ineffective esophageal motility were evaluated with HRIM and a global dysphagia symptom score (Brief Esophageal Dysphagia Questionnaire). HRIM were analyzed to assess Chicago Classification metrics, automated pressure-flow metrics, the esophageal impedance integral (EII) ratio, and the bolus flow time (BFT).
Key Results
Significant symptom-metric correlations were detected only with basal EGJ pressure, EII ratio, and BFT. The EII ratio, BFT, and impedance ratio differed between controls and dysphagia patients, while the EII ratio in the upright position was the only measure that differentiated dysphagia from non-dysphagia patients.
Conclusions & Inferences
The EII ratio and BFT appear to offer an improved diagnostic evaluation in patients with non-obstructive dysphagia without a major esophageal motility disorder. Bolus retention as measured with the EII ratio appears to carry the strongest association with dysphagia, and thus may aid in the characterization of symptomatic patients with otherwise normal manometry.
Keywords: dysphagia, high-resolution manometry, impedance, esophageal motility
Introduction
Esophageal manometry is the primary method to evaluate esophageal motility and sphincter function making it the test of choice to evaluate patients with non-obstructive dysphagia, i.e. dysphagia without an identified mechanical obstruction on upper endoscopy. High-resolution manometry (HRM) with esophageal pressure topography (EPT) has allowed improved characterization of esophageal contractile activity and sphincter function over conventional manometry.(1) Esophageal motility evaluation with HRM/EPT also provides objective metrics of esophageal function that can be applied to a standardized, hierarchical classification scheme of esophageal motility diagnoses.(2, 3) However, despite the improved characterization, the association of esophageal symptoms with HRM metrics remains limited.(4) Frequent, and sometime difficult, clinical scenarios occur when symptomatic patients have esophageal motility patterns that are also observed in asymptomatic controls such as ineffective esophageal motility (IEM) or normal motility, prompting a diagnosis of a functional gastrointestinal syndrome.(3)
Incorporation of multichannel, intraluminal impedance sensors onto the HRM catheter, high-resolution impedance manometry (HRIM), allows for the enhanced assessment of intra-bolus pressure (IBP), bolus flow, and bolus retention. Hence, HRIM may enhance the HRM evaluation of esophageal symptoms and ultimately, the approach to diagnosis and management of esophageal disease. Although early use of impedance-technology was limited by dichotomous evaluation of bolus flow as complete or incomplete,(5) novel HRIM analysis paradigms may advance the clinical utility of HRIM. Newer approaches have combined the intraluminal impedance and pressure measurements facilitating the objective derivation of novel ‘pressure-flow’ variables. It has been proposed that pressure flow variables may identify subtle abnormalities of flow resistance in patients with non-obstructive dysphagia and post-fundoplication dysphagia.(6-8) Furthermore, key pressure-plot landmarks can be defined and used to map spatio-temporal domains within which pressure and impedance data can be simultaneously accessed. This has led to the derivation of additional novel HRIM metrics to predict flow across the EJG or bolus residual due to esophageal stasis based on studies utilizing simultaneous videofluoroscopy.(9, 10).
We hypothesized that variables that quantify bolus distension pressure, esophagogastric junction (EGJ) flow, and bolus residual would aid in the evaluation of patients with non-obstructive dysphagia. As these advanced HRIM metrics have not been fully applied to patient populations with non-obstructive dysphagia assessed using solid state HRIM, we undertook a study to compare and contrast these measures and to assess their relationship to symptoms of dysphagia and esophageal chest pain. To further demonstrate the potential role for HRIM metrics in clinical practice, we aimed to compare these measures across patients with and without dysphagia, as well as with asymptomatic controls.
Methods
Subjects
We retrospectively evaluated consecutive patients that completed upper endoscopy, HRIM, and symptom questionnaires during evaluation at the Esophageal Center of Northwestern between January 2012 and May 2015. ‘Dysphagia patients’ were evaluated with a primary HRIM-indication of dysphagia and included when a Brief Esophageal Dysphagia Questionnaire (BEDQ) score was > 6. To provide a patient group for comparison, patients who were evaluated for reflux-related symptoms were included when a BEDQ score was ≤ 6: ‘non-dysphagia patients’. Only patients meeting Chicago Classification esophageal motility diagnoses of normal motility and IEM were included.(3) Patients with manometric findings of EGJ outflow obstruction or major disorders of peristalsis were not included. Patients were excluded for endoscopic findings of mechanical obstruction (including eosinophilic esophagitis via distal and proximal esophageal biopsies among dysphagia patients), esophagitis (more severe than grade A by the Los Angeles classification), hiatal hernia ≥ 3-cm, or Barrett's metaplasia.
Additionally, 14 asymptomatic, healthy volunteers (‘controls’; 58% female, ages 20-50) without history of gastrointestinal surgery were included for comparison. These subjects have been previously described, though one subject was excluded due to impedance sensor malfunction.(9) Informed consent was obtained from each subject. The study protocol was approved by the Northwestern University Institutional Review Board.
Symptom assessment
Symptoms of chest pain and dysphagia were evaluated using the BEDQ (a recently validated questionnaire that was previously termed the impaction dysphagia questionnaire), which was completed at the time of manometric investigation.(11) The questionnaire consists of eight 6-point Likert scale questions (scored 0-5) that assess frequency of symptoms and severity of symptoms and 2 open-ended questions regarding frequency of food impactions and related emergency room visits.(12) Scores range from 0 (asymptomatic) – 50; a BEDQ score ≤ 6 was considered normal. Symptom scores were also generated separately for the six dysphagia/food impaction items (Dysphagia score; 0 – 30) and four chest pain items (Chest pain score; 0 – 20). Patients also completed the GERDQ.(13)
High-resolution impedance manometry
Manometry studies were completed using a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals and 18 impedance segments at 2-cm intervals (Medtronic Inc, Shoreview, MN). After a minimum 6-hour fast, the HRIM assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with approximately three intragastric pressure sensors. The HRIM protocol included a 5-minute baseline recording, 10 5-ml swallows in a supine position, and five 5-ml swallows in the upright position using 0.45% saline for test swallows at 20-30 second intervals.
Data analysis
Standard Esophageal Pressure Topography Measures
Manometry studies were analyzed using ManoView version 3.0 analysis software to measure basal EGJ pressure (EGJP), integrated relaxation pressure (IRP), distal contractile integral (DCI), and distal latency. The EGJP was measured at end-expiration during the baseline recording. An esophageal motility diagnoses was derived using the Chicago Classification v3.0; a median IRP of > 15 mmHg was utilized as the upper-limit of normal.(3) The HRIM data for each subject were exported to MATLAB™ (The MathWorks Inc., Natick, MA, U.S.A.) to apply to a customized programs for further analysis of HRIM parameters.
Measures of Bolus Residual
Two measures of esophageal bolus residual were calculated: the esophageal impedance integral (EII) ratio and the nadir impedance to peak pressure impedance ratio (IR).
The EII ratio was measured by creating a measurement region-of-interest (ROI) ranging from the distal border of the upper esophageal sphincter to the proximal border of the EGJ and from the upper esophageal sphincter relaxation and to the completion of peristalsis or 12 seconds (Figure 1).(10) A best-fit diagonal straight line that demarcated the present or expected peristaltic wave front was defined to divide the swallow ROI into swallow (Z1) and post-swallow (Z2) impedance domains. The amount of bolus present within each domain (Z1 and Z2) was then quantified by measuring the esophageal impedance integral (EII), which was proportional to the volume of intra-esophageal liquid present within each domain (Z1 and Z2). The EII was measured by first determining the times of bolus presence by assessing the mean baseline impedance and the nadir impedance at all times within the ROI; domains of bolus presence were defined when the impedance value decreased to 50% from baseline. The EII was then calculated by measuring the impedance-pixel density (impedance value × time × axial length). Finally, the EII ratio was calculated as the EII-Z2 divided by EII-Z1; i.e. the ratio of residual bolus volume (Z2) relative to the intra-esophageal bolus volume following the swallow, but before the peristaltic wave. A greater EII ratio indicated a greater degree of bolus retention.(10)
Figure 1. The esophageal impedance integral (EII) ratio.
A) The pressure topography plot of a normal, single swallow from a dysphagia patient is displayed. The region-of-interest for the EII ratio is designated with the red-dashed box. The black-dashed box is displayed in Figure 2 for generation of the bolus flow time. B) Areas of bolus presence are enclosed within the white lines. The EII ratio was calculated as the measured bolus volume (impedance pixel-density within areas of bolus presence) in Z2, the post-swallow-EII, divided by the bolus volume in Z1, the swallow-EII (C). The displayed swallow yielded a normal EII-ratio of 0.2. Figure used with permission from the Esophageal Center at Northwestern.
The IR was measured by creating an analysis region-of-interest ranging from the distal border of the upper esophageal sphincter to the proximal border of the EGJ, beginning at the upper esophageal sphincter relaxation and lasting to the completion of peristalsis (Figure 2A).(14) The contractile peaks of the peristaltic wave and corresponding nadir impedance time points preceding the peaks (corresponding to maximum distension) were identified along the ROI. To determine the IR the nadir impedance value was divided by the impedance value mapped to the timing of peak contraction (peak pressure impedance). IR was calculated at each position along the ROI and the average IR was then calculated for the entire esophagus. A greater IR indicated a greater degree of bolus retention.(14)
Figure 2. Pressure Flow Analysis.
A. Pressure topography plot of a 5-ml saline swallow from a control subject showing the two regions of interest (ROI) used; ROI 1 from upper esophageal spincter (UES) to esophago-gastric junction (EGJ) and RO2 from transition zone (TZ) to EGJ.
B. Pressure isocontour plot of ROI 1 showing the time and position of nadir impedance, indicating timing of bolus distension, and peak pressure, indicating maximum contraction. Plots right show impedance values mapped to these locations and the impedance ratio (calculated as nadir impedance/peak pressure impedance). Average values along ROI 1 were determined for each swallow.
C. Pressure-impedance plot derived at sensor position number 14. Pressure (black line) and impedance (purple line) are shown for a 12s period from from swallow onset (0s). Note that impedance values have been reversed (lowest impedance at the top) for ease of presentation. This is a representative example showing how four key pressure-flow variables were calculated, these were; pressure at nadir impedance (1.PNI) representing the pressure at maximum luminal distension, intrabolus pressure (2.IBP) and intrabolus pressure slope (3.IBP slope), representing the median pressure and gradient of pressure change respectively during luminal closure (defined by the period from nadir impedance to the midpoint between nadir impedance and peak pressure), and the time from nadir impedance to peak pressure (4.TNIPP) representing the latency period from maximum distension to maximum contraction.
D. Plots of the four key pressure variables and the pressure-flow index composite score based on values which were calculated at all axial locations along ROI 2 (using the operations shown in C). Average values along ROI 2 were determined for each swallow. Note the marked increase in bolus pressurisation and shortening of distention-contraction latency below sensor position number 18. This correspondes to an increase in bolus flow resistance associated with the transiton from compartmentalised bolus transport along the esophagus to esophageal emptying across a (variably resistive) EGJ opening.
Pressure-Flow Measures of Distension Pressure, Flow Timing and Bolus Pressurization
Pressure-flow parameters were measured for the distal esophagus proximal of the EGJ. A measurement ROI was defined from the mid-point of the transition zone to the proximal border of the EGJ (Figure 2B).(14) Note, the transition zone midpoint was defined by the lowest pressure between proximal and distal esophageal pressure segments or the distal margin of the proximal esophageal segment in the case of large 20mmHg iso-baric contour breaks (>5cm). The peaks of the peristaltic contraction and corresponding nadir impedance time points preceding the peaks (indicative of maximal luminal cross-sectional area) were identified along the ROI. Guided by the timing of nadir impedance and peak pressure, four pressure-flow variables were then determined as described below:
The pressure at nadir Impedance (PNI) was used to define the discrete intrabolus distension pressure occurring at the point of maximal luminal distention.
The intrabolus pressure slope (IBP slope) was defined by calculating the average gradient of pressure change from the nadir impedance point to the midpoint in time between nadir impedance and peak pressure. This quantifies the rate of pressure change (or pressure ‘ramp’) during the isotonic/auxotonic phase of esophageal contraction preceding luminal occlusion.
The time from nadir impedance to peak pressure (TNIPP) was used to define the flow latency from maximum distension to maximum contraction.
Finally, the pressure-flow index (PFI) was calculated using the formula PFI = (intrabolus distension pressure × IBP slope)/(TNIPP – peak pressure). The intrabolus distension pressure was defined by calculating the median distension pressure from the nadir impedance point to the midpoint in time between nadir impedance and peak pressure. The peak pressure was the pressure recorded at the maximum wave amplitude.
Bolus Flow Time
Finally, a measure of the time of trans-EGJ bolus flow (and thus a surrogate of esophageal emptying), the bolus flow time (BFT) was assessed. To measure the BFT, three impedance and three manometry signals were positioned through the EGJ at 1-cm intervals (impedance and pressure signals were interpolated by the analysis software). The distal impedance and pressure signals were positioned within the hiatus as identified by crural contractions.(9, 15) An example swallow is displayed in Figure 3. Using the impedance signals, the duration of bolus presence was determined: The onset of bolus presence was defined as the point at which the impedance dropped to 90% of the nadir; the offset of bolus presence was defined as the return to 50% of the impedance baseline. Using the three manometry signals, periods of a trans-EGJ flow-permissive pressure gradient (i.e. when the esophageal pressure was greater than both the crural and intra-gastric pressure signals) were determined. The BFT was then derived as the sum of all periods meeting the criteria of both bolus presence and a flow-permissive pressure gradient time. Reduced BFT values were indicative of a reduction in esophageal emptying.(15) If the impedance drop was not greater than 50% at each axial location and/or a flow-permissive pressure gradient was not achieved, the BFT was considered to be zero.
Figure 3. Bolus flow time (BFT).
The top panel is the esophageal pressure topography of the distal esophagus; the overlaid horizontal lines represent the placement of the EGJ and gastric impedance and manometry signals. The middle panel represents the impedance signals which were used to determine the time of bolus presence. The bottom panel represents the pressure signals used to determine periods of a flow-permissive pressure gradient, i.e. when the esophageal pressure, red line, was greater than both the hiatal (crural diaphragm, CD) and intra-gastric pressure signals. The BFT was then derived as the time when both criteria (1. bolus presence and 2. trans-EGJ flow permissive pressure gradient) were met. Figure used with permission from the Esophageal Center at Northwestern.
Statistical analysis
The median value of each HRIM parameter of the first five supine swallows and the five upright swallows was utilized for each patient. Descriptive statistics for all continuous and ordinal measures were presented as median and IQR, unless otherwise stated. Correlations were assessed using Spearman's rho. Groups were compared using the Kruskal-Wallis or Mann-Whitney U test for continuous variables and X2 or Fischer's Exact tests for dichotomous and categorical variables. For multiple comparisons, post-hoc tests were applied with a Bonferroni correction. Analyses otherwise assumed a 5% level of statistical significance.
Results
Subjects
Forty-one patients (63% female; median age 49 years; range 24 – 82), 18 dysphagia patients and 23 non-dysphagia patients, were included. Patients were older than controls (p < 0.001). Subject characteristics by group are displayed in Table 1. Esophageal motility diagnoses among patients included 30 (73%) with normal motility and 11 (27%) with IEM and had a similar distribution of motility diagnoses between dysphagia and non-dysphagia patients (p = 1.0). Four asymptomatic controls had a motility diagnosis of IEM; the remainder had normal motility. Endoscopic findings among the patients include two patients with LA grade A-esophagitis (1 dysphagia, 1 non-dysphagia), and 11 small hiatal hernia (n = 5, 28% dysphagia, n = 5, 21% non-dysphagia; p = 0.724); endoscopy was normal in the remainder.
Table 1. Subject characteristics.
| Controls | Dysphagia | Non-dysphagia | |
|---|---|---|---|
|
| |||
| n | 14 | 18 | 23 |
|
| |||
| Age | 33 (26 – 44) | 51 (37 – 62)1 | 49 (41 – 56)1 |
|
| |||
| Gender (F/M) | 8/6 | 13/5 | 13/10 |
|
| |||
| Motility diagnosis; n (%) | |||
| Normal motility | 10 (71) | 13 (72) | 17 (74) |
| IEM | 4 (29) | 5 (28) | 6 (26) |
|
| |||
| BEDQ score | -- | 16 (11– 30)2 | 2 (0 – 4)2 |
|
| |||
| BEDQ, dysphagia sub-score | -- | 10 (6 – 16)2 | 0 (0 – 3)2 |
|
| |||
| BEDQ, chest pain sub-score | -- | 9 (4 – 12)2 | 0 (0 – 1)2 |
|
| |||
| GERDQ score | -- | 9 (4 – 12) | 10 (4 – 14) |
Values expressed as median (IQR) unless otherwise specified.
p-value < 0.017 compared with controls.
p-value < 0.05 among comparisons between dysphagia and non-dysphagia patients.
One patient (dysphagia, IEM) had several faulty proximal impedance sensors. Consequently, the EII ratio and IR were not calculated for this patient (leaving 17 dysphagia patients for EII ratio and IR results); all other metrics were calculated for this patient.
Symptom and metric correlations
Correlation values (Spearman's rho) between symptoms (BEDQ scores: overall, dysphagia sub-score, and chest pain sub-score) and HRIM metrics among all patients (dysphagia and non-dysphagia) are shown in Table 2. Significant correlations were detected indicating higher EGJP (supine only), higher EII ratio, and shorter BFT with more severe symptoms. In the upright position, the EII ratio had a slightly weaker correlation with the chest pain than the total BEDQ or dysphagia sub-scores. Correlation was otherwise less robust and statistically insignificant for the remaining metrics, with similar correlations observed across the component sub-scores.
Table 2. Correlation of symptoms with high resolution impedance manometry metrics.
| BEDQ | BEDQ-dysphagia | BEDQ-chest pain | |
|---|---|---|---|
| Supine | |||
| IRP | -0.052 | -0.005 | -0.051 |
| DCI | 0.035 | -0.056 | 0.038 |
| EGJP | 0.368 | 0.347 | 0.363 |
| BFT | -0.367 | -0.349 | -0.392 |
| EII ratio | 0.466 | 0.449 | 0.400 |
| PNI | -0.007 | -0.117 | 0.123 |
| IBP slope | 0.083 | 0.070 | 0.070 |
| TNIPP | -0.102 | -0.084 | -0.144 |
| IR | 0.242 | 0.227 | 0.266 |
| PFI | 0.069 | 0.040 | 0.044 |
| Upright | |||
| BFT | -0.379 | -0.392 | -0.325 |
| EII ratio | 0.432 | 0.432 | 0.309 |
| PNI | -0.022 | -0.022 | 0.105 |
| IBP slope | -0.048 | -0.061 | 0.023 |
| TNIPP | -0.021 | 0.005 | -0.098 |
| IR | 0.000 | -0.029 | 0.079 |
| PFI | 0.035 | -0.018 | 0.214 |
Spearman's Rho is reported for each correlation; those with p-value < 0.05 are in bold. BEDQ – Brief Esophageal Dysphagia Questionnaire; BFT – bolus flow time; DCI – distal contractile integral; EGJP – esophagogastric junction pressure; EII – esophageal impedance integral; IBP – intrabolus pressure; IR – impedance ratio; IRP – integrated relaxation pressure; PNI – pressure at nadir impedance; TNIPP – time from nadir impedance to peak pressure
As some of the metrics evaluate similar features of esophageal function, we also evaluated the correlation between metrics (Supplementary Table 1). Measures of bolus retention, the EII ratio and the IR, demonstrated significant correlation values of 0.436 in the supine and 0.509 in the upright positions. Although both the TNIPP and BFT have been related to the duration of bolus flow across the EGJ in studies utilizing simultaneous HRIM and videofluoroscopy,(9, 16) the correlation of BFT and TNIPP was not significant in the supine (rho = 0.269) or upright positions (rho = 0.083). Consistent with esophageal emptying being related to bolus retention, the BFT was also significantly inversely correlated with the EII ratio (supine: -0.469; upright -0.509) and IR (supine: -0.517; upright: -0.396). The TNIPP was significantly correlated with the IR in the supine (rho = -0.223) but not the upright (rho = 0.179) position, nor with the EII ratio (rho values 0.035 and 0.017).
HRIM parameters
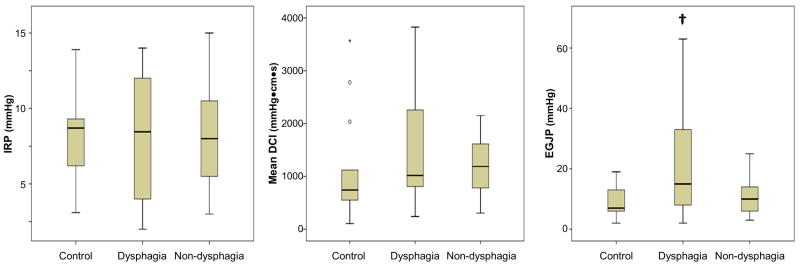
Comparison of traditional HRM metrics (Figure 4A) across subject groups demonstrated a significant difference in EGJP (p = 0.015), but not IRP or DCI. Post-hoc testing (which assumed statistical significance at a p-value of 0.017 based on a Bonferroni adjustment for multiple comparison) demonstrated that dysphagia patients had greater EGJP than controls and there were trends towards a greater EGJP in dysphagia than in non-dysphagia patients (p = 0.026) and greater EGJP in non-dysphagia patients than in controls (p = 0.048).
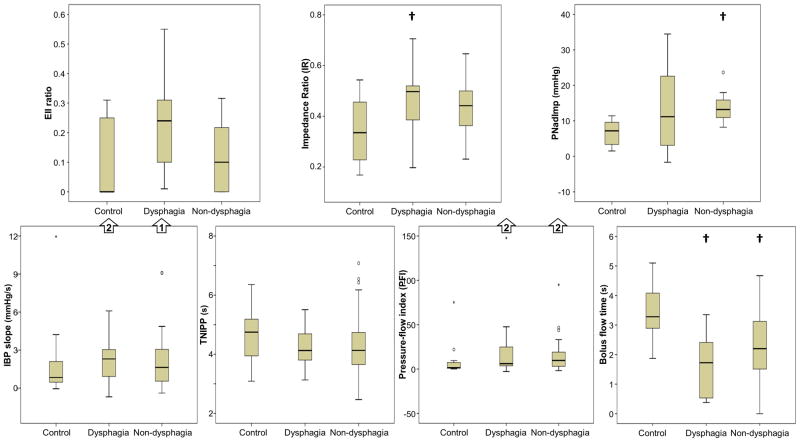
Figure 4. Comparison of high-resolution impedance manometry (HRIM) metrics.
Standard supine HRM metrics (A), supine HRIM metrics (B), and upright HRIM metrics (C) were compared across subject groups. † = p-value < 0.017 compared with controls. ‡ = p-value < 0.017 compared with dysphagia patients. Outliers omitted from the charts for display purposes are indicated with arrows. IRP – integrated relaxation pressure. DCI – distal contractile integral. EGJP – esophagogastric junction pressure. EII – esophageal impedance integral. PNI – pressure at nadir impedance. IBP – intrabolus pressure. TNIPP – time from nadir impedance to peak pressure.
Comparisons of supine HRIM metrics across subject groups demonstrated a significant difference with EII ratio (p = 0.025), BFT (p < 0.001), PNI (p = 0.004), and IR (p = 0.013) as illustrated in Figure 4B. Pairwise comparisons demonstrated trends towards greater supine EII ratios in dysphagia patients than in controls (p = 0.021) and in non-dysphagia patients (p = 0.019). A reduced supine BFT was evident in both dysphagia and non-dysphagia patients than in controls as was a trend toward lower supine BFT in dysphagia and in non-dysphagia patients (p = 0.078). PNI was greater in non-dysphagia patients than in controls, but was similar between dysphagia patients and controls and dysphagia patients and non-dysphagia patients. Supine IR was greater in dysphagia patients than controls with a numeric trend towards greater values in non-dysphagia patients than in controls (p = 0.033).
Comparisons of upright HRIM metrics across subject groups demonstrated a significant difference with EII ratio (p = 0.001), BFT (p < 0.001), and PNI (p = 0.004) as illustrated in Figure 4C. Pairwise comparisons demonstrated a greater upright EII ratio in dysphagia than controls and a greater upright EII ratio in dysphagia patients than non-dysphagia patients; controls and non-dysphagia patients were similar. Upright BFT was reduced in both dysphagia and non-dysphagia patients compared to controls with a numeric trend towards lower upright BFT in dysphagia than non-dysphagia patients (p = 0.019). Upright PNI was greater in non-dysphagia patients than in controls with a numeric trend towards greater PNI in dysphagia patients than in controls (p = 0.054). However, upright PNI was similar between dysphagia and non-dysphagia patients (p= 0.773).
As manometric variables are often better dichotomized as normal/abnormal, we also analyzed patients as abnormal if less than the 10th and/or greater than the 90th percentile of the control values, as appropriate for each metric. For the traditional HRM metrics, we applied historically utilized thresholds for IRP, DCI, and EGJP based on analysis of a larger control group.(2, 17, 18) Using these threshold values (Table 3), abnormal measures among the controls were observed in 0/14 for IRP, 3/14 (21%) for DCI, and 9/14 (64%) for EGJP; based on our inclusion criteria, all patients had a normal IRP. Using this approach, only the EII ratio in the upright position demonstrated a greater proportion of abnormal measures in dysphagia than non-dysphagia patients, although there was a statistical trend with the supine EII ratio.
Table 3. Abnormal high-resolution impedance manometry (HRIM) parameters.
| Normal definition1 | Dysphagia n (%) abnormal | Non-dysphagia n (%) abnormal | p-value | |
|---|---|---|---|---|
| Supine measures | ||||
| DCI | 450 – 5000 mmHg•s•cm | 2 (11) | 2 (9) | 0.438 |
| EGJP | 10 – 45 mmHg | 6 (33) | 11 (48) | 0.524 |
| BFT | > 1.9 s | 10 (56) | 9 (39) | 0.355 |
| EII ratio | <0.31 | 5 (29) | 1 (4) | 0.067 |
| PNI | < 11 mmHg | 9 (50) | 16 (70) | 0.334 |
| IBP slope | <8 mmHg/s | 2 (11) | 3 (13) | 1.0 |
| TNIPP | <3.4 s | 2 (11) | 3 (13) | 1.0 |
| IR | <0.64 | 2 (11) | 1 (4) | 0.565 |
| PFI | < 49 | 3 (17) | 3 (13) | 1.0 |
| Upright measures | ||||
| BFT | >1.1 s | 9 (50) | 6 (26) | 0.191 |
| EII ratio | <0.25 | 10 (59) | 4 (17) | 0.009 |
| PNI | < 7.7 mmHg | 10 (56) | 15 (65) | 0.748 |
| IBP slope | < 4.2 mmHg/s | 2 (11) | 3 (13) | 1.0 |
| TNIPP | < 3.0 | 1 (6) | 1 (4) | 1.0 |
| IR | <0.47 | 8 (47) | 5 (21) | 0.171 |
| PFI | < 27.9 | 2 (11) | 4 (17) | 0.679 |
Normal definitions for HRIM metrics were based upon the 10th or 90th percentile of the normal controls, except for distal contractile integral (DCI), and basal EGJ pressure (EGJP). BFT – bolus flow time; EII – esophageal impedance integral; IBP – intrabolus pressure; IR – impedance ratio; IRP – integrated relaxation pressure; PNI – pressure at nadir impedance; TNIPP – time from nadir impedance to peak pressure.
Discussion
The primary finding of our study is that HRIM parameters provide an improved diagnostic evaluation in non-obstructive dysphagia patients without a major esophageal motility disorder. Among the various HRIM-parameters derived for liquid swallows, in this study the EII ratio and BFT demonstrated the strongest correlation with a global dysphagia score. In the upright position, the parameters EII ratio and BFT were able to discriminate between non-obstructive dysphagia patients and controls. The EII ratio was also able to differentiate dysphagia patients from non-dysphagia patients. Additionally, the EII ratio appeared to be superior to the IR, the other parameter devised to quantify the extent of bolus residual. Other pressure-flow variables devised to quantify features of bolus distension and pressurization during compartmentalized bolus transport and esophageal emptying were also altered in patients compared to controls. However, these parameters did not differentiate dysphagia from non-dysphagia patients in this study. Thus, EII ratio and BFT may be helpful measures to illuminate generalized abnormalities in esophageal function in symptomatic dysphagia patients that would not otherwise be appreciated with endoscopy or HRM.
As the primary function of the esophagus is to clear intra-esophageal bolus, abnormalities in bolus flow, retention, and/or pressurization may account for symptom development in patients with otherwise normal endoscopic and manometric findings. Though simultaneous manometry and radiography allows for objective evaluation of these components, its clinical use is limited by its cumbersome nature and requisite radiation exposure. Early use of simultaneous manometry with multi-channel intraluminal impedance demonstrated utility in detecting bolus transit abnormalities in patients with otherwise normal manometry.(19, 20) Pressure-flow analysis methods were devised to objectively measure sub-components of bolus flow timing, bolus retention, luminal distension and pressurization that are not determined by the current HRM paradigms that focus on characterization of contractile strength and timing of peristalsis. Most recently, EII ratio and the BFT were devised in order to quantify bolus retention and trans-EGJ flow.(9, 10, 16)
Pressure-flow analysis was originally applied to ‘low resolution’ pressure-impedance recordings. Among three initial studies, pressure-flow variables (IBP, IBP slope, TNIPP) and the index composite score (PFI) were associated with post-fundoplication dysphagia and were able to differentiate asymptomatic controls from non-obstructive dysphagia patients.(7, 8, 21) Whilst apparent with both liquid and viscous boluses, the viscous consistency appeared to better discriminate these subtle abnormalities. While the novel pressure flow variables were unable to distinguish patients with ineffective esophageal motility from asymptomatic controls, due to the absence of propulsion, the IR appeared to be a useful adjunctive measure of the degree and extent of bolus retention as it was able differentiate IEM from controls.(8, 21)
More recently, pressure-flow analysis were applied to HRIM recordings to evaluate their association with the perception of bolus retention. These studies demonstrated associations of pressure-flow measures with solid bolus (but not liquid or viscous) perception in asymptomatic controls,(14, 22) and an association with viscous (but not liquid) perception among a broad pediatric dysphagia cohort, including the spectrum of esophageal motor disorders.(23) However, in symptomatic reflux patients, no relation was seen between pressure-flow measures during liquid and viscous swallows and dysphagia symptoms assessed by a validated dysphagia score (Dakkak score).(24) Hence, among the few available HRIM studies, differences in individual pressure-flow metrics have not been consistently observed in relation to symptom perception. When relationships are demonstrated, they are mostly apparent with results obtained from viscous and solid bolus consistencies.
In the current study, we attempted to further clarify and understand the potential clinical utility of novel pressure-impedance parameters by examining HRIM recordings obtained from a cohort of patients with non-obstructive dysphagia and without a major esophageal motility disorder on HRM. Importantly this study is the first of its kind to include a cohort of ‘non-dysphagia patient’ controls and well as asymptomatic controls. We did not discriminate patients and controls with IEM as the number of IEM cases was small and the proportions were similar among the three different groups. Pressure-flow parameters indicative of luminal distension and pressurization were altered in patients overall compared to controls, however they did not differentiate the patients with dysphagia from non-dysphagia patients with predominantly reflux symptoms. Hence, the pressure-flow signature, previously attributed to non-obstructive dysphagia patients, may be a more ubiquitous finding amongst patients presenting with upper GI symptoms and otherwise normal motility. While this suggests limited utility of these measures when quantified in relation to liquid swallows, utility in relation to assessment of viscous and solid swallows was not assessed in the current study.
In contrast to individual pressure-flow variables, which appear to best discriminate patients when used in relation to viscous and solid boluses and may have their greatest utility in patients with an increased esophageal resistance to flow,(7, 14, 22, 23) EII ratio and BFT measured in relation to liquid swallows better discriminated the three groups evaluated in this study. This represents the first systematic evaluation of the EII ratio and BFT in patients with non-obstructive dysphagia without a major esophageal motility disorder. A previous study evaluating patients with achalasia demonstrated a greater symptom association than the BFT than the IRP.(15) Esophageal pressure-flow characteristics may be more heterogeneous among non-obstructive dysphagia patients without achalasia given variation in peristaltic function, such as those included in this study. Thus, demonstrating manometry-symptom correlation may be expected to be more difficult. However, incorporation of the EII ratio and BFT demonstrated improved symptom-correlations and patient-group discrimination than the standard HRM measures (IRP, mean DCI, EJGP). Though we did find that the end-expiratory EGJP demonstrated a weak, but significant correlation with the BEDQ score, the significant overlap between controls, dysphagia patients, and non-dysphagia patients demonstrates the limited clinical utility of EGJP.
Although our aim was to differentiate patient groups related to a dysphagia symptom score, esophageal symptom development and perception is complex. Thus, specific abnormalities of esophageal function may not be completely specific to generate dysphagia, as opposed to reflux-symptoms, such as heartburn. For example, a previous study reported that most healthy volunteers actually reported heartburn, not chest pain, in response to intra-esophageal balloon distension.(25) Thus, although we choose our non-dysphagia, (patient-control) group from patients evaluated for reflux symptoms, the abnormal bolus retention, flow, and pressurization parameters we observed in some of our non-dysphagia cohort may not reflect falsely positive findings. Additionally, though we strive to improve our diagnostic evaluation to elucidate deficits in esophageal function, not all symptomatic patients may have functional abnormalities in bolus transit to account for symptoms, but may instead be solely related to perceptive, cognitive, or psychological factors.
Based on our study, it appears that bolus retention as measured with the EII ratio, which is related to abnormal trans-EGJ bolus flow (i.e. functional esophageal emptying as measured with the BFT) may be a stronger driver of dysphagia-symptoms than flow resistance (as inferred by IBP or IRP). However, as previously mentioned, a study that evaluated swallow-by-swallow perception in controls observed an association between higher bolus pressurization measured during solid swallows and solid-bolus perception. In that study solid bolus retention (based on IR) did not correlate with perception.(14) Thus, although our study reports that the EII ratio and BFT appear to better differentiate symptomatic patients in relation to liquid swallows further studies including different bolus consistencies are needed. We also recognize that BEDQ is a composite, global symptom score, and may not necessarily be comparable with swallow-by-swallow bolus perception.
In addition to the small sample size, this study is limited by its potential generalizability to clinical practice. The methods require both HRIM, which can be expensive to obtain and maintain, and customized research software programs not yet available for wide-spread use. Thus, future implementation of these metrics into commercially available software packages would help to expand the use of HRIM to general clinical practice and increase the evidence base that is required to underpin these methods.
In summary, HRIM metrics of EII ratio and BFT represent novel techniques to aid the evaluation of patients with non-obstructive dysphagia and distinguish disordered esophageal motility as an explanation for symptoms. Specific pressure flow variables appear less globally useful, but may play a role of distinguishing the pathophysiology underlying abnormal transit and/or EGJ flow. Future prospective and outcome studies are needed to demonstrate the benefits of these HRIM techniques to clinical practice, and may offer further insight into esophageal symptom generation.
Supplementary Material
Supplementary Table 1. Correlations of high-resolution impedance manometry metrics.
Key Points.
This study aimed to evaluate the association of non-obstructive dysphagia symptoms with pressure-flow parameters generated using high-resolution impedance manometry (HRIM) and to assess their ability to discriminate dysphagia patients from asymptomatic controls and patients without dysphagia.
The esophageal impedance integral (EII) ratio and bolus flow time demonstrated the strongest symptom correlations and differed between dysphagia patients and asymptomatic controls. The EII ratio also differed between dysphagia and non-dysphagia patient-controls.
Application of advanced HRIM parameters aids identification of abnormalities in esophageal function that are not detected with the standard manometric evaluation in non-obstructive dysphagia.
Acknowledgments
We would like to thank Zoe Listernick, Michael Tye, Katherine Ritter for their assistance with data acquisition.
Funding: This work was supported by T32 DK101363 (JEP) and R01 DK079902 (JEP) from the Public Health service.
Abbreviations
- BEDQ
Brief Esophageal Dysphagia Questionnaire
- BFT
bolus flow time
- CD
crural diaphragm
- DCI
distal contractile integral
- EGJP
esophagogastric junction pressure
- EII
esophageal impedance integral
- EPT
esophageal pressure topography
- HR(I)M
high-resolution (impedance) manometry
- IBP
intrabolus pressure
- IEM
ineffective esophageal motility
- IR
impedance ratio
- IRP
integrated relaxation pressure
- PNI
pressure at nadir impedance
- ROI
region of interest
- TNIPP
time from nadir impedance to peak pressure
- TZ
transition zone
- UES
upper esophageal sphincter
Footnotes
Author contributions: DAC contributed to study concept and design, data analysis, data interpretation, drafting of the manuscript, and approval of the final version. TO contributed to data analysis, data interpretation, drafting the manuscript, and approval of the final version. ZL contributed to data analysis, data interpretation, revising the manuscript and approval of the final version. NR, PJK, and JT contributed to manuscript revision, and approval of the final version. KS contributed to organization of data, recruitment of patients, and approval of the final version. JEP contributed to study concept and design, revising the manuscript critically, and approval of the final version.
Disclosures: John E. Pandolfino: Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking)
T Omari and N Rommel hold inventorship of Australian Patent 2011301768 which covers some of the analytical methods described.
Dustin A. Carlson, Zhiyue Lin, Kristen Starkey, Jan Tack: None
References
- 1.Clouse RE, Staiano A, Alrakawi A, Haroian L. Application of topographical methods to clinical esophageal manometry. Am J Gastroenterol. 2000;95(10):2720–30. doi: 10.1111/j.1572-0241.2000.03178.x. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfino JE, Ghosh SK, Rice J, Clarke JO, Kwiatek MA, Kahrilas PJ. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103(1):27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 3.Kahrilas PJ, Bredenoord AJ, Fox M, Gyawali CP, Roman S, Smout AJ, Pandolfino JE, International High Resolution Manometry Working G The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27(2):160–74. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao Y, Kahrilas PJ, Nicodeme F, Lin Z, Roman S, Pandolfino JE. Lack of correlation between HRM metrics and symptoms during the manometric protocol. Am J Gastroenterol. 2014;109(4):521–6. doi: 10.1038/ajg.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tutuian R, Castell DO. Clarification of the esophageal function defect in patients with manometric ineffective esophageal motility: studies using combined impedance-manometry. Clin Gastroenterol Hepatol. 2004;2(3):230–6. doi: 10.1016/s1542-3565(04)00010-2. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Mittal RK, Patel N, Ledgerwood M, Bhargava V. Esophageal distension during bolus transport: can it be detected by intraluminal impedance recordings? Neurogastroenterol Motil. 2014;26(8):1122–30. doi: 10.1111/nmo.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers JC, Nguyen NQ, Jamieson GG, Van't Hek JE, Ching K, Holloway RH, Dent J, Omari TI. Susceptibility to dysphagia after fundoplication revealed by novel automated impedance manometry analysis. Neurogastroenterol Motil. 2012;24(9):812–e393. doi: 10.1111/j.1365-2982.2012.01938.x. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen NQ, Holloway RH, Smout AJ, Omari TI. Automated impedance-manometry analysis detects esophageal motor dysfunction in patients who have non-obstructive dysphagia with normal manometry. Neurogastroenterol Motil. 2013;25(3):238–45. e164. doi: 10.1111/nmo.12040. [DOI] [PubMed] [Google Scholar]
- 9.Lin Z, Imam H, Nicodeme F, Carlson DA, Lin CY, Yim B, Kahrilas PJ, Pandolfino JE. Flow time through esophagogastric junction derived during high-resolution impedance-manometry studies: a novel parameter for assessing esophageal bolus transit. Am J Physiol Gastrointest Liver Physiol. 2014;307(2):G158–63. doi: 10.1152/ajpgi.00119.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Z, Nicodeme F, Lin CY, Mogni B, Friesen L, Kahrilas PJ, Pandolfino JE. Parameters for quantifying bolus retention with high-resolution impedance manometry. Neurogastroenterol Motil. 2014;26(7):929–36. doi: 10.1111/nmo.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taft TH, Riehl M, Sodikoff JB, Kahrilas PJ, Keefer L, Doerfler B, Pandolfino JE. Development and validation of the brief esophageal dysphagia questionnaire. Neurogastroenterol Motil. 2016 doi: 10.1111/nmo.12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roman S, Pandolfino JE, Chen J, Boris L, Luger D, Kahrilas PJ. Phenotypes and Clinical Context of Hypercontractility in High-Resolution Esophageal Pressure Topography (EPT) Am J Gastroenterol. 2011 doi: 10.1038/ajg.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jonasson C, Wernersson B, Hoff DA, Hatlebakk JG. Validation of the GerdQ questionnaire for the diagnosis of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2013;37(5):564–72. doi: 10.1111/apt.12204. [DOI] [PubMed] [Google Scholar]
- 14.Omari TI, Wauters L, Rommel N, Kritas S, Myers JC. Oesophageal pressure-flow metrics in relation to bolus volume, bolus consistency, and bolus perception. United European Gastroenterol J. 2013;1(4):249–58. doi: 10.1177/2050640613492157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Z, Carlson DA, Dykstra K, Sternbach J, Hungness E, Kahrilas PJ, Ciolino JD, Pandolfino JE. High-resolution impedance manometry measurement of bolus flow time in achalasia and its correlation with dysphagia. Neurogastroenterol Motil. 2015;27(9):1232–8. doi: 10.1111/nmo.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Omari TI, Szczesniak MM, Maclean J, Myers JC, Rommel N, Cock C, Cook IJ. Correlation of esophageal pressure-flow analysis findings with bolus transit patterns on videofluoroscopy. Dis Esophagus. 2016;29(2):166–73. doi: 10.1111/dote.12300. [DOI] [PubMed] [Google Scholar]
- 17.Pandolfino JE, Ghosh SK, Zhang Q, Jarosz A, Shah N, Kahrilas PJ. Quantifying EGJ morphology and relaxation with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290(5):G1033–40. doi: 10.1152/ajpgi.00444.2005. [DOI] [PubMed] [Google Scholar]
- 18.Xiao Y, Kahrilas PJ, Kwasny MJ, Roman S, Lin Z, Nicodeme F, Lu C, Pandolfino JE. High-Resolution Manometry Correlates of Ineffective Esophageal Motility. Am J Gastroenterol. 2012 doi: 10.1038/ajg.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conchillo JM, Nguyen NQ, Samsom M, Holloway RH, Smout AJ. Multichannel intraluminal impedance monitoring in the evaluation of patients with non-obstructive Dysphagia. Am J Gastroenterol. 2005;100(12):2624–32. doi: 10.1111/j.1572-0241.2005.00303.x. [DOI] [PubMed] [Google Scholar]
- 20.Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004;99(6):1011–9. doi: 10.1111/j.1572-0241.2004.30035.x. [DOI] [PubMed] [Google Scholar]
- 21.Chen CL, Yi CH, Liu TT, Hsu CS, Omari TI. Characterization of esophageal pressure-flow abnormalities in patients with non-obstructive dysphagia and normal manometry findings. J Gastroenterol Hepatol. 2013;28(6):946–53. doi: 10.1111/jgh.12176. [DOI] [PubMed] [Google Scholar]
- 22.Rommel N, Van Oudenhove L, Tack J, Omari TI. Automated impedance manometry analysis as a method to assess esophageal function. Neurogastroenterol Motil. 2014;26(5):636–45. doi: 10.1111/nmo.12308. [DOI] [PubMed] [Google Scholar]
- 23.Singendonk MM, Kritas S, Cock C, Ferris LF, McCall L, Rommel N, van Wijk MP, Benninga MA, et al. Pressure-flow characteristics of normal and disordered esophageal motor patterns. J Pediatr. 2015;166(3):690–6 e1. doi: 10.1016/j.jpeds.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Rohof WO, Myers JC, Estremera FA, Ferris LS, van de Pol J, Boeckxstaens GE, Omari TI. Inter- and intra-rater reproducibility of automated and integrated pressure-flow analysis of esophageal pressure-impedance recordings. Neurogastroenterol Motil. 2014;26(2):168–75. doi: 10.1111/nmo.12246. [DOI] [PubMed] [Google Scholar]
- 25.Takeda T, Nabae T, Kassab G, Liu J, Mittal RK. Oesophageal wall stretch: the stimulus for distension induced oesophageal sensation. Neurogastroenterol Motil. 2004;16(6):721–8. doi: 10.1111/j.1365-2982.2004.00620.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. Correlations of high-resolution impedance manometry metrics.