Abstract
Sclerostin, the product of the SOST gene, is a secreted inhibitor of Wnt signaling that is produced by osteocytes to regulate bone formation. While it is often considered an osteocyte-specific protein, SOST expression has been reported in numerous other cell types, including hypertrophic chondrocytes and cementocytes. Of interest, SOST/sclerostin expression is altered in certain pathogenic conditions, including osteoarthritis and rheumatic joint disease, and it is unclear whether sclerostin plays a protective role or whether sclerostin may mediate disease pathogenesis. Therefore, as anti-sclerostin antibodies are being developed for the treatment of osteoporosis, it is important to understand the functions of sclerostin beyond the regulation of bone formation.
Keywords: Sclerostin, Wnt, osteoarthritis, ankylosing spondylitis, rheumatoid arthritis
1. Introduction
Sclerostin, the product of the SOST gene, is a negative regulator of Wnt signaling and bone formation that was identified in the study of the bone sclerosing conditions sclerosteosis (1, 2) and Van Buchem Disease (3, 4). Clinical studies show that targeting sclerostin via monoclonal antibodies is a powerful strategy to promote new bone formation (5–8). While sclerostin has been widely viewed as an osteocyte-specific protein, recent studies have shown that several additional cell types express SOST and are capable of producing sclerostin protein (9–12). Moreover, altered SOST expression and serum sclerostin have been noted in numerous diseases including osteoarthritis (13) and ankylosing spondylitis (14), but a role for sclerostin in the pathogenesis of these disorders is unclear. Therefore, as sclerostin antibodies move beyond clinical trials, it is important to understand the potential implications of targeting sclerostin for all bone cells and non-bone tissues. In this review we will detail the expression of SOST/sclerostin beyond osteocytes and discuss the current understanding of sclerostin in certain pathogenic conditions, including osteoarthritis and rheumatic joint disease.
2. Sclerostin expression beyond the osteocyte
Sclerostin has widely been viewed as an osteocyte-specific protein, despite early studies noting SOST RNA in multiple human and mouse tissues, including cartilage, kidney, heart, and liver (1, 2, 15). More recently, using Sost promoter LacZ reporter mice, Collette, et al., further expanded this list, documenting Sost LacZ reporter expression in the epididymis and vas deferens of the testis, the pyloric sphincter, the carotid arteries, and parts of the cerebellum (16).
As suggested by occasional hand defects, such as syndactyly, in sclerosteosis patients, SOST is expressed in the developing embryo where it plays a role in limb patterning. Collette, et al., documented Sost LacZ reporter in the distal limb bud ectoderm beginning at E9.5. With progression of limb development, Sost LacZ reporter remains restricted to the ectoderm, and by E14.5, is restricted to the digits. Sost LacZ reporter expression is also evident on cells lining the edges of neural folds, as well as symmetrically at the base of the spinal cord at E11.5 marking motor neurons migrating into the limbs. However, by E16.5, Sost LacZ reporter expression is restricted primarily to the skeleton (16). Overexpression of SOST disrupted the anterior-posterior and proximal-distal signaling centers in the developing limb leading to a loss of posterior structures in the zeugopod and autopod (17). Similar to sclerosteosis patients, Sost knockout (KO) in mice resulted in hand defects in four percent of neonates. Knocking out both Sost and its paralog Sostdc1, which is 55% similar in sequence and also inhibits Wnt signaling, increased the number of hand defects to approximately fifty percent suggesting partially redundant and complementary roles for these proteins in limb development (16).
Sclerostin protein was first detected in adult human osteocytes by Winkler, et al (9). Consistent with RNA data showing cartilage expression of SOST(2), Winkler, et al., demonstrated that hypertrophic chondrocytes were also positive for sclerostin protein (9). Van Bezooijen, et al., confirmed osteocyte protein expression but the authors were unable to detect sclerostin protein in non-mineralized articular cartilage (18); however, non-osteocyte sclerostin protein expression was confirmed in a later study showing sclerostin protein in mineralized hypertrophic chondrocytes in the human adolescent growth plate. Van Bezooijen, et al., also demonstrated sclerostin protein in cementocytes of the cellular cementum in the dental root from five healthy adults. Cellular cementum cementocytes stained positive for sclerostin, however, dental pulp and odontoblasts were negative for sclerostin (19). Sclerostin expression has also been reported in a newly developed cementocyte cell line, IDG-CM6, which shows many similarities to osteocytes (11). These data are consistent with the hypothesis that sclerostin protein is expressed by terminally differentiated cells within mineralized matrices (19).
While articular cartilage is negative for sclerostin protein in healthy joint tissue (18), numerous studies have reported sclerostin protein expression in articular chondrocytes isolated from osteoarthritic joints (13, 20–22). Sclerostin expression has also been reported to be altered in rheumatic joint diseases (14, 23, 24);
3. Sclerostin in Osteoarthritis and Rheumatic Joint Disease
Articular cartilage is maintained through a delicate balance of anabolic and catabolic processes, and disruption of this balance is associated with joint disease (25). Proper control of the canonical Wnt signaling pathway is essential for maintaining cartilage homeostasis (25, 26). Numerous studies have reported upregulated canonical Wnt signaling in osteoarthritis (OA) and ankylosing spondylitis (14, 27), where it is thought that this pathway promotes chondrocyte hypertrophy and breakdown of adult cartilage (20). Consistent with this, activation of -catenin, the primary mediator of canonical Wnt signaling, in articular chondrocytes led to an OA-like phenotype in mice (28). In contrast, rheumatoid arthritis (RA), which is associated with bone erosion, is characterized by reduced Wnt signaling (23). Given the importance of Wnt signaling in maintaining cartilage homeostasis and its disruption in joint disease, understanding the role of sclerostin in these pathologies and how this is modulated by anti-sclerostin antibody therapies is of great interest.
Osteoarthritis (OA), a degenerative joint disease causing cartilage breakdown, osteophyte (bone spur) formation, and subchondral bone thickening, is the most common form of arthritis, affecting an estimated 27 percent of adults over the age of 65 (29). OA joints exhibit increased canonical Wnt signaling, leading to increased aggrecanase and matrix metalloproteinase expression and cartilage destruction. While normal articular chondrocytes are negative for sclerostin protein (18), articular chondrocytes isolated from OA-affected joints express sclerostin (13, 20–22). This has been reported in surgically-induced OA models in sheep (20) and mice (13, 20), the STR/Ort OA mouse model (30), as well as human OA (13, 20–22). Papathanasiou, et al., found that sclerostin expression in OA articular chondrocytes isolated from patients undergoing knee replacement surgery correlated with hypomethylation of the CpG region of the SOST promoter, which is associated with open chromatin and increased gene expression (22). Despite increased Wnt signaling being associated with cartilage destruction in OA, aged sclerostin KO mice, which would be expected to exhibit higher levels of Wnt signaling, exhibited no significant differences in articular cartilage compared to wild type mice, and sclerostin antibody treatment of Sprague Dawley rats did not affect rat articular cartilage (13). Florio, et al., recently showed that anti-sclerostin antibody treatment and Sost KO results in compensatory increased expression of the Wnt receptor antagonist Dkk1 (31); it is possible that the up-regulation of Dkk1 may account for the lack of effect of sclerostin inhibition on articular cartilage. A separate study utilizing Sost KO mice showed that destabilization of the medial meniscus to induce OA resulted in significantly higher OA scores in Sost KO compared to wild type mice, with significant increases in aggrecanase and type X collagen expression (32). In contrast, sclerostin antibody treatment of rats with surgical-induced OA did not have any significant effect on the OA disease score (13). This discrepancy between the Sost KO mice and sclerostin antibody treatment in destabilization of the medial meniscus-induced OA model is unclear. It is possible that tissue distribution of the sclerostin antibody in rat cartilage is not sufficient to see an effect. Alternatively, this may be a species specific effect.
In contrast to the increase in sclerostin expression in OA articular chondrocytes, the subchondral bone associated with sclerosis in OA shows reduced osteocyte sclerostin expression (14, 20, 30, 33). It is unknown whether this is due to a subchondral bone defect or whether this results from the increased mechanical strain (load) on the joint. Serum levels of sclerostin are significantly reduced in OA patients (34), suggesting that the reduced osteocyte sclerostin contributes to systemic changes consistent with data that osteoarthritis is protective against hip fracture (35). Because of the subchondral bone sclerosis, Radin, et al., have postulated that the increased stiffness contributes to the cartilage destruction (36). However, the studies performed by Roudier, et al., and Bouaziz, et al., showing no effect of the increased bone mass related to sclerostin knockout and sclerostin antibody treatment on articular cartilage suggest that an additional trigger is needed to establish OA pathogenesis (13, 32).
Ankylosing spondylitis (AS), an inflammatory joint disease that is also associated with bony spur (syndesmophyte) formation, shows a similar reduction in osteocyte sclerostin expression in affected joints as well as reduced serum sclerostin. AS predominantly affects axial joints and interverterbral spaces, with bone deposition occurring at the inflamed entheses causing syndesmophyte formation, fused facet joints, back pain, and reduced mobility (14, 37). Analysis of zygapophyseal (ZA) joints obtained from AS patients, as well as healthy control tissue in the German SA Inception cohort study, showed that sclerostin positive osteocytes were significantly reduced in AS patient tissue (15% versus 53% positive in controls). Of interest, OA also exhibited significantly reduced sclerostin positive osteocytes in Lumbar ZA joints in this study (42% sclerostin positive), however joints collected from rheumatoid arthritis patients did not differ in osteocyte sclerostin (14).
Consistent with reduced sclerostin positive osteocytes in the ZA joints, serum sclerostin levels were significantly lower in AS patients compared to the healthy controls, and of interest, low serum sclerostin in the AS patients was associated with new syndesmophyte formation and radiographic progression. Because sclerostin was low before the syndesomphytes appeared, the data suggest that low sclerostin increased the susceptibility for syndesmophyte formation (14). This is consistent with the results of a more recent study by Sakellariou, et al., showing that sclerostin expression is blunted in patients with AS and patients with low serum sclerostin are more likely to develop ankyloses (37). This data showing low osteocyte sclerostin expression only in joint diseases with bony spurs (AS and OA) suggest low sclerostin as a biomarker for predicting the structural progression of bony disease (14).
In a related report, Tsui, et al., characterized the ank/ank mouse model of AS; these mice developed symptoms of human AS, and the joints showed increased β-catenin signaling. Consistent with the studies by Appel, et al., and Sakellariou, et al., Tsui, et al., confirmed reduced free sclerostin in AS patient sera; however their study also revealed the presence of sclerostin autoantibodies in AS patients and ank/ank mice. As stated above, AS is an autoimmune disease and these data suggest that sclerostin autoantibodies and immune complexes may contribute to AS disease development (38).
In contrast to OA and AS, rheumatoid arthritis (RA) is a chronic inflammatory disease causing bone erosion and decreased bone mass. Sclerostin protein expression was detected in the synovial tissue from RA patients. Sclerostin expression did not appear to co-localize with immune cells, but instead with fibroblast-like synoviocytes (23). In a mouse model of TNF-α induced RA (human TNF-α transgenic mice), Wehmeyer, et al., found that these fibroblast-like synoviocytes constituted a major source of sclerostin. Of interest, the sclerostin appeared to be protective, as Sost KO or sclerostin antibody treated mice exhibited accelerated RA-like disease with increased paw swelling, reduced grip strength, elevated synovial pannus, and greater bone erosion. This was specific to TNF-α mediated RA, since blocking sclerostin had no effect on the G6PI (partial TNF-α mediated disease) or K/BXN (TNF-α independent) RA models. The authors showed through in vitro studies that TNF-α induced sclerostin expression in RA fibroblast-like synoviocytes (23). TNF-α induced SOST/sclerostin expression has previously been described in osteoblasts (39). Additionally, sclerostin blocked TNF-α induced p38/ERK activity, suggesting a protective role of sclerostin in chronic inflammation. Of interest to RA bone disease, sclerostin blocked TNF-α induced RANKL expression by fibroblast-like synoviocytes, suggesting a possible mechanism by which the Sost KO RA mice exhibited worsened bone erosion (23). However, these data are in contrast to those of Chen, et al., who showed that anti-sclerostin antibodies prevented bone erosion as well as cartilage degradation in TNF-α induced RA (human TNF-α transgenic mouse) without modifying inflammation (40). It is unclear why sclerostin inhibition worsened versus improved bone erosion in these two studies.
A separate study by Marenzana, et al., showed that prophylactic and therapeutic administration of anti-sclerostin antibody prevented systemic bone loss in mice with collagen-induced arthritis, a model of RA (41). Consistent with Chen, et al. (40), Marenzana, et al., reported no effect on systemic inflammation; however, Marenzana, et al., found no protection or improvement in focal bone erosion (41). This discrepancy may be due to differences in inflammatory cytokines induced in the two mouse models. Additional experiments are required to determine the specific role of sclerostin in RA. If the results of Wehmeyer, et al., are correct, treatment of RA patients with TNF-α-mediated disease with anti-sclerostin antibody therapy would be contraindicated. However, based on the findings of Chen, et al., and Marenzana, et al., certain RA patients may benefit from anti-sclerostin therapy to prevent systemic bone loss.
Juvenile idiopathic arthritis, an autoimmune condition with onset prior to 16 years of age, also causes reduced bone mass and patients have significantly increased levels of serum sclerostin, although it is unclear whether this is due to changes in chondrocyte or subchondral bone expression of sclerostin (24). Similar to RA, anti-sclerostin antibody therapy may benefit these patients by preventing systemic bone loss; however, it will be important to determine whether sclerostin plays an anti-inflammatory role in this disease setting.
4. Sclerostin and cancer
SOST/sclerostin has been noted in bone tumors and bone cancer cell lines (42). Early studies into regulation of the SOST promoter showed that the osteoblast transcription factor Runt-related transcription factor 2 (Runx2) promoted SOST expression in the Saos2 human osteosarcoma cell line through binding the proximal promoter (43). Consistent with this, a recent report by Inagaki, et al., showed patchy sclerostin protein expression in mineralized osteoid and bone forming tumors, including osteosarcomas. Positive staining for sclerostin was also found in hypertrophic chondrocytes in osteochondroma and chondroblasts in chondroblastoma. However, other bone tumors in this study, such as Multiple Myeloma (MM) and metastatic carcinoma were negative for sclerostin protein (42).
More than eighty percent of MM patients develop osteolytic lesions (44) due to increased osteoclast-mediated bone resorption and suppressed osteoblast function (45). A study by Terpos, et al., found that newly diagnosed MM patients had significantly increased levels of circulating sclerostin, and elevated serum levels of sclerostin correlated with advanced bone disease and reduced survival time (27 months vs. 98 months) in MM patients (46). Increased circulating sclerostin in MM compared to precursor disease states was confirmed by Eda, et al.; the authors of the latter study used a humanized xenograft mouse model and showed that the human MM-bearing mice exhibited increased mouse-derived sclerostin, suggesting that MM cells induce sclerostin expression by the bone microenvironment. Consistent with this, eight of twelve MM patients showed elevated SOST expression in bone-marrow isolated mesenchymal stem cells and osteoblasts, and MM cells stimulated SOST/sclerostin expression by immature osteoblasts in in vitro co-culture experiments; this effect could be prevented through neutralization of DKK1, suggesting a mechanism by which DKK1 may promote sclerostin expression (44). A separate study by Delgado-Calle, et al, reported that MM also induces SOST/sclerostin expression by osteocytes (47). Importantly, suppression of osteoblast differentiation by MM cells was prevented by sclerostin neutralizing antibodies, and neutralizing sclerostin in MM-bearing mice reversed osteolytic bone disease, suggesting sclerostin as a therapeutic target for MM bone disease (44).
In a separate study, Colucci, et al., reported SOST expression and sclerostin protein in MM cell lines (H929, RPMI-8226, U266 and Karpas 909) and CD138+ plasma cells isolated from MM patient bone marrow. Of interest, sclerostin levels were increased in MM patients with osteolytic lesions as compared to patients without bone disease (48). While Terpos, et al.(46), and Eda, et al.(44), reported elevated circulating sclerostin, Brunetti, et al., found no change in serum sclerostin, only in bone marrow sclerostin, suggesting only local microenvironment changes in sclerostin (49). Consistent with the data reported by Eda, et al.(44), H929 MM cells and CD138+ cells harvested from MM patients inhibited osteoblast mineralization and the expression of osteoblast-specific proteins in vitro, and normal osteoblast differentiation and function was restored by neutralization of sclerostin (48). Additionally, H929 cells increased BMSC RANKL expression while decreasing OPG expression, and this was prevented by neutralization of sclerostin, suggesting that MM-derived sclerostin is involved not only in the inhibition of osteoblast differentiation, but also in the up-regulation of osteoclast mediated resorption in osteolytic lesions (48).
Similar to the findings that Runx2 promoted SOST expression in Saos2 osteosarcoma cells (43), Mendoza-Villanueva, et al., reported that the bone metastatic breast cancer cell line MDA-MB-231 abnormally expresses Runx2, leading to sclerostin expression by these cells (50), and overexpression of Runx2 by non-metastatic MCF-7 breast cancer cells induced SOST expression in vitro. Sclerostin secretion by MDA-MB-231 cells strongly inhibited osteoblast differentiation and function in vitro implicating breast cancer-derived sclerostin in the suppression of bone formation and progression of metastatic breast cancer osteolytic lesions (50).
A study analyzing human prostate cancer specimens by immunohistochemistry showed that sclerostin levels are reduced in prostate cancer as compared to nodular hyperplasia. Importantly, high BMP6 and low sclerostin and noggin protein expression predicted the development of distant metastases, suggesting a predictive value in monitoring these proteins in prostate cancer progression (51). This is similar to findings in non-small cell lung cancer, showing that elevated expression of the SOST/sclerostin paralog SOSTDC1 was associated with better prognosis over patients with lower SOSTDC1 expression (52). In a separate study, Hudson, et al., reported that sclerostin had an inhibitory effect on prostate cancer invasion by reducing Wnt signaling, and sclerostin deficient osteoblasts promoted prostate cancer migration. Consistent with this, overexpression of sclerostin by PC3 prostate cancer cells reduced metastasis and osteolysis in vivo (53). Together these studies suggest that low sclerostin promotes prostate cancer progression. In contrast, Yavropoulou, et al., found that patients with bone metastatic prostate cancer exhibited increased serum sclerostin (54). Increased serum sclerostin in prostate cancer was also reported by Garcia-Fontana, et al., who found that serum sclerostin was further increased by androgen deprivation therapy (55). While this is seemingly inconsistent to the findings by Hudson, et al., and Yuen, et al., it is likely that local tumor-derived sclerostin inhibits prostate cancer growth and progression at the primary site through inhibition of canonical Wnt signaling, whereas elevated serum sclerostin is reflective of the increased bone remodeling associated with cancer-induced bone disease. Indeed, prostate cancer-induced bone disease is commonly associated with osteoblastic lesions, and the increased sclerostin may result from increased osteoblast and osteocyte numbers. Further study of the role sclerostin at primary and metastatic tumor sites is required to fully elucidate the role of this protein in tumor progression and metastasis, as well as its contribution to cancer-induced bone disease (both osteolytic and osteoblastic metastases).
5. Other tissues and pathologies impacted by sclerostin
Sclerostin protein expression has also been reported in a variety of other soft tissue pathologies. SOST expression was induced, along with other osteocyte markers, in vascular smooth muscle cells cultured in calcifying medium (12) and sclerostin was reported in aortic valves in areas adjacent to calcification in hemodialysis patients (56). Serum sclerostin levels directly correlate with vascular calcification (VC) in renal transplant recipients (57) and chronic kidney disease (58); this correlation became inverse following multivariate analysis in these studies, suggesting that SOST/sclerostin expression by vascular cells may be a counter-regulatory mechanism to suppress VC progression (57, 58). Of interest, high to intermediate levels of serum sclerostin were associated with decreased short-term cardiovascular mortality in dialysis patients (59). Understanding the exact role for sclerostin in VC development and/or progression, as well as the effect of anti-sclerostin antibodies on this requires further study.
As discussed earlier in this review, SOST expression has been documented in liver (2). More recently, Guanabens, et al., found liver SOST expression in primary biliary cirrhosis (PBC) was upregulated 2.7 fold. PBC patients exhibited higher serum sclerostin that inversely correlated with markers of bone metabolism, and the authors suggest that the elevated sclerostin contributes to low bone formation in these patients. IHC confirmed sclerostin protein in seven out of the eleven PBC liver biopsies, with localization mainly in the cholangiocytes of the bile duct. Sclerostin staining was associated with cholangitis and early stage disease. However, serum sclerostin positively correlated with lumbar and hip BMD, suggesting that the increased sclerostin may be reflective of increased osteocyte number (10). Increased serum sclerostin has been reported in other liver conditions, including alcoholism (60) and advanced liver cirrhosis (61), although this appears to be due to impaired liver function and metabolism. It is unclear whether the sclerostin detected by IHC is reflective of cholangiocyte- or osteocyte-derived sclerostin, and whether the presence of sclerostin staining in the bile duct may be the result of impaired liver function in these patients; however, the fact that liver from these patients also exhibited increased SOST mRNA suggests the sclerostin is derived from a local site. It also remains unclear the extent to which the cholangiocyte-derived sclerostin contributes to overall serum sclerostin levels, and whether local sclerostin contributes to disease pathogenesis (10).
SOST expression may also be impacted by aging. Several studies have noted that serum sclerostin increases with age (62–64). Roforth, et al., compared serum sclerostin in young (mean age of 30 years) and old (mean age 72.9 years) women and found a 46% increase with aging; however, bone needle biopsies isolated from these women revealed no change in bone SOST mRNA levels (62). Since these are whole bone biopsies, it is possible that differences were not detected due to the heterogeneity of the samples; however an alternative explanation may be that sclerostin expression by other tissues may contribute to elevated serum sclerostin with age.
Sclerostin protein has previously been reported to co-localize with MMP9, a marker of osteoclasts, in the developing embryonic skeleton (65). While osteoclasts derived from young C57Bl/6 and Balbc mice expressed low levels of Sost, we found that osteoclasts derived from the bone marrow of old mice produce significantly increased levels of sclerostin protein (66). The effect of age on SOST/sclerostin expression remains relatively under-investigated and further studies are required to determine the presence of non-osteocyte derived sclerostin in aging.
4. Conclusions
Although commonly referred to as an osteocyte-specific protein, it is clear that SOST/sclerostin is expressed by several other cell types and/or tissues in normal and pathogenic conditions, including osteoarthritis, rheumatic joint disease, cancers (bone and non-bone tumors), and vascular calcifications. Additionally, non-osteocyte sclerostin expression may be induced with age. Whether sclerostin plays a protective role in these conditions or contributes to disease pathogenesis remains unclear. Therefore, careful investigation is required to understand the implications of sclerostin neutralization in these conditions.
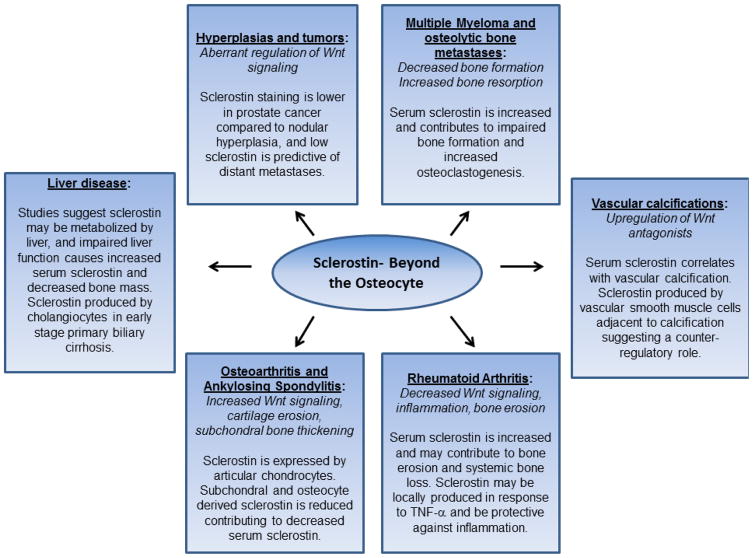
Figure 1.
Functions of sclerostin beyond the osteocyte. Typical characteristics of each disease/pathogenesis is italicized and the role of sclerostin is noted below.
Table 1.
SOST RNA and sclerostin protein expression in bone and non-bone tissues.
| Cells/Tissue | Sample source | RNA/Protein |
|---|---|---|
| Musculoskeletal tissue | ||
| Bone | Whole long bone- human/mouse | RNA(2) |
| Mouse tibia, human hip bone, sclerosteosis patient mastoid | RNA(18) | |
| Osteocytes | Normal adult human bone | Protein(9) |
| E17.5 mouse mineralized bone, adult mouse tibia | RNA(18) | |
| Human bone | Protein(18) | |
| Osteoblasts | Primary human osteoblasts | RNA(9) |
| Human MSC derived osteoblasts | RNA(9) | |
| Human/mouse MSC derived mineralizing osteoblast cultures | RNA(18) | |
| Osteosarcoma | Human Saos2 cell line | RNA(43) |
| Human bone tumor biopsy | Protein(42) | |
| Osteoclasts | Mouse embryo | Protein(65) |
| Aged mouse bone marrow | RNA/Protein(66) | |
| Cartilage | Human/mouse | RNA(2) |
| Embryonic mouse (E15.5) ossifying cartilage | RNA(9) | |
| Hypertrophic chondrocytes | Human bone | Protein(9) |
| Human growth plate | Protein(19) | |
| Articular Cartilage-Osteoarthritis (OA) | Human OA cartilage | RNA/Protein(20) |
| Sheep, mouse surgery-induced OA cartilage | Protein(20) | |
| Human articular cartilage | RNA(21) | |
| Human cartilage | RNA/Protein(13) | |
| Human articular cartilage | RNA/Protein(22) | |
| STR/Ort OA mouse articular cartilage | RNA(30) | |
| Cementocytes | Human/mouse teeth | Protein(19) |
| IDG-CM6 mouse cementocyte cell line | RNA/Protein(11) | |
| Human/mouse teeth, mineralizing periodontal ligament cells | Protein(67) | |
| Multiple Myeloma | Patient CD138+ plasma cells and human cell lines H929, RPMI-8226, U266 and Karpas909 | RNA/Protein(48) |
| Soft tissue | ||
| Breast cancer | Human MDA-MB-231 cell line | RNA/Protein(50) |
| Prostate | Prostate nodular hyperplasia and prostate cancer | Protein(51) |
| Kidney | Human/mouse | RNA(2) |
| Liver | Human/mouse | RNA(2) |
| Human primary biliary cirrohsis biopsy | Protein(10) | |
| Heart | Mouse | RNA(2) |
| Aortic valves | Hemodialysis patient aortic valve | RNA/Protein |
| Brain | Mouse | RNA(2) |
| Thymus | Mouse | RNA(2) |
| Placenta | Human | RNA(2) |
| Fetal skin | Human | RNA(2) |
Highlights.
Sclerostin is produced by cells within mineralized matrices, including osteocytes, hypertrophic chondrocytes, and cementocytes
Sclerostin is expressed by non-mineralized cells in certain pathogenic conditions
Sclerostin expression is altered in osteoarthritis and rheumatic joint disease; it is unclear whether this is protective or pathogenic
Understanding the role for sclerostin beyond bone formation will have important implications for the use of anti-sclerostin neutralizing antibody treatments
Acknowledgments
Funding sources
This work was supported by the National Institutes of Health R01 AR067129, R01 AR65402, K01 AR070281; and Robert and Arlene Kogod Center on Aging Career Development Award.
Abbreviations
- OA
osteoarthritis
- AS
ankylosing spondylitis
- RA
rheumatoid arthritis
- MM
Multiple Myeloma
- ZA
zygapophyseal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Balemans W, et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum Mol Genet. 2001;10(5):537–43. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 2.Brunkow ME, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–89. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balemans W, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39(2):91–7. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Staehling-Hampton K, et al. A 52-kb deletion in the SOST-MEOX1 intergenic region on 17q12-q21 is associated with van Buchem disease in the Dutch population. Am J Med Genet. 2002;110(2):144–52. doi: 10.1002/ajmg.10401. [DOI] [PubMed] [Google Scholar]
- 5.Padhi D, et al. Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res. 2011;26(1):19–26. doi: 10.1002/jbmr.173. [DOI] [PubMed] [Google Scholar]
- 6.Ominsky MS, et al. Inhibition of sclerostin by monoclonal antibody enhances bone healing and improves bone density and strength of nonfractured bones. J Bone Miner Res. 2011;26(5):1012–21. doi: 10.1002/jbmr.307. [DOI] [PubMed] [Google Scholar]
- 7.McColm J, et al. Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res. 2014;29(4):935–43. doi: 10.1002/jbmr.2092. [DOI] [PubMed] [Google Scholar]
- 8.Recker RR, et al. A randomized, double-blind phase 2 clinical trial of blosozumab, a sclerostin antibody, in postmenopausal women with low bone mineral density. J Bone Miner Res. 2015;30(2):216–24. doi: 10.1002/jbmr.2351. [DOI] [PubMed] [Google Scholar]
- 9.Winkler DG, et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. Embo J. 2003;22(23):6267–76. doi: 10.1093/emboj/cdg599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guanabens N, et al. Sclerostin Expression in Bile Ducts of Patients with Chronic Cholestasis May Influence the Bone Disease in Primary Biliary Cirrhosis. J Bone Miner Res. 2016 doi: 10.1002/jbmr.2845. [DOI] [PubMed] [Google Scholar]
- 11.Zhao N, et al. Isolation and Functional Analysis of an Immortalized Murine Cementocyte Cell Line, IDG-CM6. J Bone Miner Res. 2016;31(2):430–42. doi: 10.1002/jbmr.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu D, et al. The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS One. 2011;6(5):e19595. doi: 10.1371/journal.pone.0019595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roudier M, et al. Sclerostin is expressed in articular cartilage but loss or inhibition does not affect cartilage remodeling during aging or following mechanical injury. Arthritis Rheum. 2013;65(3):721–31. doi: 10.1002/art.37802. [DOI] [PubMed] [Google Scholar]
- 14.Appel H, et al. Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum. 2009;60(11):3257–62. doi: 10.1002/art.24888. [DOI] [PubMed] [Google Scholar]
- 15.van Bezooijen RL, et al. SOST expression is restricted to the great arteries during embryonic and neonatal cardiovascular development. Developmental dynamics: an official publication of the American Association of Anatomists. 2007;236(2):606–12. doi: 10.1002/dvdy.21054. [DOI] [PubMed] [Google Scholar]
- 16.Collette NM, et al. Sost and its paralog Sostdc1 coordinate digit number in a Gli3-dependent manner. Developmental Biology. 2013;383(1):90–105. doi: 10.1016/j.ydbio.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collette NM, et al. Genetic evidence that SOST inhibits WNT signaling in the limb. Dev Biol. 2010;342(2):169–79. doi: 10.1016/j.ydbio.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Bezooijen RL, et al. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med. 2004;199(6):805–14. doi: 10.1084/jem.20031454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Bezooijen RL, et al. Sclerostin in mineralized matrices and van Buchem disease. J Dent Res. 2009;88(6):569–74. doi: 10.1177/0022034509338340. [DOI] [PubMed] [Google Scholar]
- 20.Chan BY, et al. Increased chondrocyte sclerostin may protect against cartilage degradation in osteoarthritis. Osteoarthritis Cartilage. 2011;19(7):874–85. doi: 10.1016/j.joca.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 21.Karlsson C, et al. Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthritis Cartilage. 2010;18(4):581–92. doi: 10.1016/j.joca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Papathanasiou I, et al. DNA methylation regulates sclerostin (SOST) expression in osteoarthritic chondrocytes by bone morphogenetic protein 2 (BMP-2) induced changes in Smads binding affinity to the CpG region of SOST promoter. Arthritis Res Ther. 2015;17:160. doi: 10.1186/s13075-015-0674-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wehmeyer C, et al. Sclerostin inhibition promotes TNF-dependent inflammatory joint destruction. Sci Transl Med. 2016;8(330):330ra35. doi: 10.1126/scitranslmed.aac4351. [DOI] [PubMed] [Google Scholar]
- 24.Brabnikova-Maresova K, et al. Serum sclerostin in high-activity adult patients with juvenile idiopathic arthritis. Arthritis Res Ther. 2014;16(5):460. doi: 10.1186/s13075-014-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corr M. Wnt-beta-catenin signaling in the pathogenesis of osteoarthritis. Nat Clin Pract Rheumatol. 2008;4(10):550–6. doi: 10.1038/ncprheum0904. [DOI] [PubMed] [Google Scholar]
- 26.Usami Y, et al. Wnt signaling in cartilage development and diseases: lessons from animal studies. Lab Invest. 2016;96(2):186–96. doi: 10.1038/labinvest.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Velasco J, et al. Wnt pathway genes in osteoporosis and osteoarthritis: differential expression and genetic association study. Osteoporos Int. 2010;21(1):109–18. doi: 10.1007/s00198-009-0931-0. [DOI] [PubMed] [Google Scholar]
- 28.Zhu M, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24(1):12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Staines KA, et al. Endochondral Growth Defect and Deployment of Transient Chondrocyte Behaviors Underlie Osteoarthritis Onset in a Natural Murine Model. Arthritis Rheumatol. 2016;68(4):880–91. doi: 10.1002/art.39508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Florio M, et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat Commun. 2016;7:11505. doi: 10.1038/ncomms11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouaziz W, et al. Loss of sclerostin promotes osteoarthritis in mice via beta-catenin-dependent and -independent Wnt pathways. Arthritis Res Ther. 2015;17:24. doi: 10.1186/s13075-015-0540-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Power J, et al. Sclerostin and the regulation of bone formation: Effects in hip osteoarthritis and femoral neck fracture. J Bone Miner Res. 2010;25(8):1867–76. doi: 10.1002/jbmr.70. [DOI] [PubMed] [Google Scholar]
- 34.Mabey T, et al. Plasma and synovial fluid sclerostin are inversely associated with radiographic severity of knee osteoarthritis. Clin Biochem. 2014;47(7–8):547–51. doi: 10.1016/j.clinbiochem.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Dequeker J, et al. Hip Fracture and The MEDOS StudyOsteoarthritis protects against femoral neck fracture: The MEDOS study experience. Bone. 1993;14:51–6. doi: 10.1016/8756-3282(93)90350-j. [DOI] [PubMed] [Google Scholar]
- 36.Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;(213):34–40. [PubMed] [Google Scholar]
- 37.Sakellariou GT, et al. Circulating periostin levels in patients with AS: association with clinical and radiographic variables, inflammatory markers and molecules involved in bone formation. Rheumatology (Oxford) 2015;54(5):908–14. doi: 10.1093/rheumatology/keu425. [DOI] [PubMed] [Google Scholar]
- 38.Tsui FW, et al. Serum levels of novel noggin and sclerostin-immune complexes are elevated in ankylosing spondylitis. Ann Rheum Dis. 2014;73(10):1873–9. doi: 10.1136/annrheumdis-2013-203630. [DOI] [PubMed] [Google Scholar]
- 39.Vincent C, et al. Pro-Inflammatory Cytokines TNF-Related Weak Inducer of Apoptosis (TWEAK) and TNFα Induce the Mitogen-Activated Protein Kinase (MAPK)-Dependent Expression of Sclerostin in Human Osteoblasts. Journal of Bone and Mineral Research. 2009;24(8):1434–49. doi: 10.1359/jbmr.090305. [DOI] [PubMed] [Google Scholar]
- 40.Chen XX, et al. Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis. Ann Rheum Dis. 2013;72(10):1732–6. doi: 10.1136/annrheumdis-2013-203345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marenzana M, et al. Effect of sclerostin-neutralising antibody on periarticular and systemic bone in a murine model of rheumatoid arthritis: a microCT study. Arthritis Res Ther. 2013;15(5):R125. doi: 10.1186/ar4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inagaki Y, et al. Sclerostin expression in bone tumours and tumour-like lesions. Histopathology. 2016 doi: 10.1111/his.12953. [DOI] [PubMed] [Google Scholar]
- 43.Sevetson B, et al. Cbfa1/RUNX2 directs specific expression of the sclerosteosis gene (SOST) J Biol Chem. 2004;279(14):13849–58. doi: 10.1074/jbc.M306249200. [DOI] [PubMed] [Google Scholar]
- 44.Eda H, et al. Regulation of Sclerostin Expression in Multiple Myeloma by Dkk-1; A Potential Therapeutic Strategy for Myeloma Bone Disease. J Bone Miner Res. 2016 doi: 10.1002/jbmr.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roodman GD. Pathogenesis of myeloma bone disease. J Cell Biochem. 2010;109(2):283–91. doi: 10.1002/jcb.22403. [DOI] [PubMed] [Google Scholar]
- 46.Terpos E, et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: reduction post-bortezomib monotherapy. Int J Cancer. 2012;131(6):1466–71. doi: 10.1002/ijc.27342. [DOI] [PubMed] [Google Scholar]
- 47.Delgado-Calle J, et al. Bidirectional Notch Signaling and Osteocyte-Derived Factors in the Bone Marrow Microenvironment Promote Tumor Cell Proliferation and Bone Destruction in Multiple Myeloma. Cancer Res. 2016;76(5):1089–100. doi: 10.1158/0008-5472.CAN-15-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Colucci S, et al. Myeloma cells suppress osteoblasts through sclerostin secretion. Blood Cancer J. 2011;1(6):e27. doi: 10.1038/bcj.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunetti G, et al. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Ann N Y Acad Sci. 2011;1237:19–23. doi: 10.1111/j.1749-6632.2011.06196.x. [DOI] [PubMed] [Google Scholar]
- 50.Mendoza-Villanueva D, et al. Metastatic breast cancer cells inhibit osteoblast differentiation through the Runx2/CBFbeta-dependent expression of the Wnt antagonist, sclerostin. Breast Cancer Res. 2011;13(5):R106. doi: 10.1186/bcr3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuen HF, et al. The prognostic significance of BMP-6 signaling in prostate cancer. Mod Pathol. 2008;21(12):1436–43. doi: 10.1038/modpathol.2008.94. [DOI] [PubMed] [Google Scholar]
- 52.Liu L, et al. SOSTDC1 is down-regulated in non-small cell lung cancer and contributes to cancer cell proliferation. Cell & bioscience. 2016;6:24. doi: 10.1186/s13578-016-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hudson BD, et al. SOST Inhibits Prostate Cancer Invasion. PLoS One. 2015;10(11):e0142058. doi: 10.1371/journal.pone.0142058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yavropoulou MP, et al. Serum sclerostin levels in Paget’s disease and prostate cancer with bone metastases with a wide range of bone turnover. Bone. 2012;51(1):153–7. doi: 10.1016/j.bone.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 55.Garcia-Fontana B, et al. Sclerostin serum levels in prostate cancer patients and their relationship with sex steroids. Osteoporos Int. 2014;25(2):645–51. doi: 10.1007/s00198-013-2462-y. [DOI] [PubMed] [Google Scholar]
- 56.Brandenburg VM, et al. Relationship between sclerostin and cardiovascular calcification in hemodialysis patients: a cross-sectional study. BMC Nephrol. 2013;14:219. doi: 10.1186/1471-2369-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Evenepoel P, et al. Sclerostin Serum Levels and Vascular Calcification Progression in Prevalent Renal Transplant Recipients. J Clin Endocrinol Metab. 2015;100(12):4669–76. doi: 10.1210/jc.2015-3056. [DOI] [PubMed] [Google Scholar]
- 58.Claes KJ, et al. Sclerostin: Another vascular calcification inhibitor? J Clin Endocrinol Metab. 2013;98(8):3221–8. doi: 10.1210/jc.2013-1521. [DOI] [PubMed] [Google Scholar]
- 59.Drechsler C, et al. High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrology Dialysis Transplantation. 2014 doi: 10.1093/ndt/gfu301. [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez-Reimers E, et al. Serum sclerostin in alcoholics: a pilot study. Alcohol and alcoholism (Oxford, Oxfordshire) 2013;48(3):278–82. doi: 10.1093/alcalc/ags136. [DOI] [PubMed] [Google Scholar]
- 61.Rhee Y, et al. Effect of liver dysfunction on circulating sclerostin. Journal of bone and mineral metabolism. 2014;32(5):545–9. doi: 10.1007/s00774-013-0524-z. [DOI] [PubMed] [Google Scholar]
- 62.Roforth MM, et al. Effects of age on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in humans. Bone. 2014;59:1–6. doi: 10.1016/j.bone.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Modder UI, et al. Relation of age, gender, and bone mass to circulating sclerostin levels in women and men. J Bone Miner Res. 2011;26(2):373–9. doi: 10.1002/jbmr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amrein K, et al. Sclerostin and its association with physical activity, age, gender, body composition, and bone mineral content in healthy adults. J Clin Endocrinol Metab. 2012;97(1):148–54. doi: 10.1210/jc.2011-2152. [DOI] [PubMed] [Google Scholar]
- 65.Kusu N, et al. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem. 2003;278(26):24113–7. doi: 10.1074/jbc.M301716200. [DOI] [PubMed] [Google Scholar]
- 66.Ota K, et al. Sclerostin is expressed in osteoclasts from aged mice and reduces osteoclast-mediated stimulation of mineralization. J Cell Biochem. 2013;114(8):1901–7. doi: 10.1002/jcb.24537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jager A, et al. Localization of SOST/sclerostin in cementocytes in vivo and in mineralizing periodontal ligament cells in vitro. J Periodontal Res. 2010;45(2):246–54. doi: 10.1111/j.1600-0765.2009.01227.x. [DOI] [PubMed] [Google Scholar]