Abstract
We evaluated the association between two-dimensional (2D) echocardiography (echo) determined myocardial contraction fraction (MCF) and adverse cardiovascular outcomes including incident heart failure (HF), atherosclerotic cardiovascular disease (ASCVD) and mortality. The MCF, the ratio of left ventricular (LV) stroke volume (SV) to myocardial volume (MV), is a volumetric measure of myocardial shortening that can distinguish pathologic from physiologic hypertrophy. Using 2D-echo-guided M-mode data from the Cardiovascular Health Study, we calculated MCF among individuals with LV ejection fraction (EF)≥55%, and used Cox models to evaluate its association with incident HF, ASCVD, and all-cause mortality after adjusting for clinical and echo parameters. We assessed whether log2(SV) and log2(MV) were consistent with the expected 1:−1 ratio used in the definition of MCF. Among 2147 participants (age 72±5), average MCF was 59±13%. After controlling for clinical and echo variables, each 10% absolute increment in MCF was associated with lower risk of HF (HR=0.88; 95% CI=0.82, 0.94), ASCVD (HR=0.90; 95% CI=0.85, 0.95) and death (HR=0.93; 95% CI=0.89, 0.97). Moreover, the MCF was still significantly associated with ASCVD and mortality, but not HF, after adjustment for percent predicted LV mass. Significant departure from the 1:−1 ratio was not observed for ASCVD or death, but did occur for HF, driven by a stronger association for MV than SV. In conclusion, among older adults without CVD or low LVEF, 2D-echo-guided M-mode-derived MCF was independently associated with lower risk of adverse cardiovascular outcomes, but this ratiometric index may not capture the full relationship that is apparent when its components are modeled separately in the case of HF.
Keywords: myocardial contraction fraction, ejection fraction, aging, heart failure, cardiovascular disease
Left ventricular ejection fraction (LVEF) is the most commonly used measure of LV systolic function, but this measure has well-recognized disadvantages, including dependency on loading conditions1 and heart rate2. LVEF is also normal or preserved in many patients with heart failure regardless of the presence of LV hypertrophy.3 We previously defined a novel volumetric index, the myocardial contraction fraction (MCF), as the ratio of LV stroke volume (SV) to myocardial volume (MV), and demonstrated that measurement of the MCF by three-dimensional (3D) echocardiography (echo) could successfully distinguish patients with heart failure (HF) with preserved EF (HFpEF) from healthy athletes with LV hypertrophy and normal controls.4 More recently, MCF was determined by magnetic resonance imaging (MRI) in the Framingham study, where it was linked to higher incidence of a composite of atherosclerotic cardiovascular disease (ASCVD) and HF events.5 We leveraged a community-based older cohort, the Cardiovascular Health Study (CHS), who underwent standardized 2D echo, to further evaluate the association of MCF determined using this modality individually with HF, ASCVD, and death. We hypothesized that among older people with normal LVEF and no prevalent CVD, lower MCF would be independently associated with higher risk for these outcomes. We also tested the premise that this ratiometric measure adequately captures the predictive information contained by its individual components, SV and MV, modeled separately, a requirement for ratiometric measures that in the case of MCF has not to date been formally examined.
Methods
The overall design, objectives, and recruitment strategy of CHS have been reported in detail.6 Community-dwelling individuals 65 years of age or older were recruited from 4 geographically dispersed U.S. field centers. People were excluded from CHS if they were receiving active treatment for cancer, were wheelchair bound or institutionalized, or were unable to participate in the examination. The original cohort (recruited in 1989 to 1990; n=5,201) and a supplemental cohort of African Americans (recruited in 1992 to 1993; n=687) formed a total of 5,888 study participants. The present analyses focused on echocardiograms obtained at the baseline visit for the original cohort (1989–90) and at 2 years after the baseline visit (1994–95) for the supplemental cohort. Among the 5,888 total CHS participants, the current study excluded 754 with reduced LVEF (<55%); 1251 with normal LVEF having prevalent CVD; 1,237 with missing echo measures necessary to calculate MCF; and 504 with one or more missing covariates included in multivariable models. This left 2,147 participants eligible for analysis.
Echocardiographic methods in CHS have been published previously.7 In brief, standardized M-mode, 2-D, color Doppler, and spectral Doppler examinations with pre-specified sequence, technique and priorities were performed at each field site with a Toshiba SSH-160A ultrasound machine (Tustin, California) fitted with standard 2.5-MHz transducers. Studies were recorded onto super-VHS videotapes and batch-mailed to the echo reading center at the University of California Irvine, where images from each study were selected and digitized. Measurements were obtained using a digital image analysis system (Nova Microsonics). Two-dimensionally guided M-mode measurements of systolic and diastolic LV dimensions and wall thicknesses were obtained, and LV mass determined using a validated formula.8 LV mass was indexed to sex, height, and weight using a regression equation developed in a subset of healthy CHS participants, and the indexed value multiplied by 100 to yield percent predicted LV mass, as detailed previously.9 Determinations of LVEF, LV fractional shortening (FS), left atrial anteroposterior diameter and relative wall thickness (RWT) have been previously described, as have Doppler assessments of aortic and mitral valvular regurgitation and stenosis, and transmitral diastolic filling indices.7,10, 11
Using echocardiographic data (1989–90 for the original cohort, 1994–95 for the supplemental cohort), MCF was calculated as the ratio of LV stroke volume to myocardial volume. LV end-diastolic and -systolic volumes (EDV and ESV, respectively) were calculated from 2D-guided M-mode echo dimensions by a previously validated technique12, 13:
Because this technique has been shown to be reliable only in symmetrically contracting ventricles with normal LVEF, participants with reduced LVEF were excluded from this analysis. From EDV and ESV, LV SV was calculated as EDV – ESV. Myocardial volume was estimated from the measurement of LV myocardial mass, as determined by the ASE formula8, divided by the density of myocardial tissue (1.04 g/ml). As previously reported14, the inter-reader mean percent measure differences for LV mass, septal thickness and posterior wall thickness were 17%, whereas for LV internal diastolic dimension, the value was no higher than 6%.
Information on clinical covariates was obtained at each CHS examination through standardized questionnaires, anthropometric assessment, physical examination, blood collection, and electrocardiography, as reported previously.6 Laboratory methods and procedures have been detailed elsewhere.15,16 Diabetes was defined as fasting blood glucose ≥126 mg/dl, non-fasting blood glucose ≥200 mg/dl or use of anti-diabetes medication. Cystatin C was used to derive estimated glomerular filtration rate (eGFR).17 FEV1 was measured during pulmonary function testing.18 For the supplemental cohort, FEV1 was obtained in the year prior to echo and LDL cholesterol (LDLc), and HDL cholesterol (HDLc) and cystatin C from two years prior to echo. Missing values for income were imputed.
The primary outcomes were HF, ASCVD and all-cause mortality. Prevalent CVD was an exclusion criterion, comprising coronary heart disease (CHD), stroke, transient ischemic attack, peripheral arterial disease, HF, and atrial fibrillation; methods for ascertainment have been reported.19 Surveillance for incident CVD events, hospitalizations and mortality entailed semi-annual participant contacts through in-person visits or telephone calls. Incident CVD events, including HF and ASCVD, and deaths were investigated by review of medical records and adjudicated by CHS events committees.20,21 ASCVD was defined as nonfatal myocardial infarction, nonfatal stroke, and death due to CHD, stroke, or other atherosclerotic disease during follow up. HF was based on diagnosis by a physician and treatment for HF plus a constellation of symptoms, signs, and radiographic findings. Ascertainment of events was through June 30, 2013.
We detailed the levels of baseline covariates across quartiles of MCF using standard descriptive statistics and assessed for linear trends using linear regression or a chi-square test, as appropriate. Cox regression was used to analyze the association of MCF (for every 10% unit increment) with incident HF, ASCVD and mortality, accounting for clinical and echo covariates in a sequential manner. We first adjusted for age, sex, and race (Model 1); then additionally for income, body mass index, systolic blood pressure, anti-hypertensive medications, diabetes, LDLc, HDLc, smoking, alcohol intake, eGFR, self-reported health status, FEV1, and lipid-lowering therapy (Model 2); next also for echo covariates including LA diameter and transmitral E/A ratio10 (Model 3); and subsequently also for percent predicted LV mass (Model 4). An exploratory analysis of Model 3 with RWT was performed. Finally, since NT-proBNP is not obtained as part of routine care in community-dwelling individuals but is an important CVD predictor, we evaluated in an exploratory model whether the associations of MCF with primary outcomes persisted after further adjustment for the logarithm of NT-proBNP (Model 5). We further assessed the functional form of the associations of MCF with our outcomes of interest in Model 3 using penalized smoothing splines. These were consistent with a linear trend even after allowing for up to a fourth order polynomial. The proportional hazards assumption was assessed using Schoenfeld’s residuals, which revealed no meaningful violations.
We also investigated the associations of MCF components, SV and MV, with outcomes, and evaluated the premise that MCF adequately captures the predictive information of these components modeled separately. To this end, we logarithmically transformed (base 2) SV and MV to assess their associations with outcomes of interest in Models 1–3 using a multiplicative model22 to allow for potential heteroskedasticity of each individual component. We then evaluated whether the regression coefficients of log2SV and log2MV are consistent with the hypothesized 1:−1 by constructing a 95% CI on the linear sum of the regression coefficients of log2SV and log2MV. Significant departure from a linear sum of 0 would indicate departure of the expected ratio used in the computation of MCF. All statistical analyses were performed with STATA 11 and R 3.1.1 and two-sided p<0.05 was considered statistically significant.
Results
As compared with the 1737 participants missing clinical and echo measures, the 2147 participants having such information were younger and tended to have fewer cardiovascular risk factors. The average MCF in the study sample was 59±13% (range: 13–107%). The demographic, clinical, and echo characteristics of the study cohort, both overall and stratified by quartiles of MCF, are shown in Table 1. Participants were similar in age across quartiles of MCF. A higher proportion of male participants and African Americans were in the lower quartiles of MCF relative to the higher quartiles of MCF. At the lower quartiles of MCF, average eGFR measurements were lower and average systolic blood pressure was higher relative to other quartiles of MCF. There was a higher prevalence of diabetes, higher alcohol consumption, higher HDLc and higher use of antihypertensive medication across decreasing MCF quartiles. Lower MCF quartiles were associated with lower SV and higher MV, percent predicted LV mass, and RWT. Lower quartiles of MCF were also associated with lower FS, higher left atrial diameter and NT-proBNP, and higher proportions of transmitral E/A <0.7.
Table 1.
Demographic, Clinical and Echocardiographic Parameters by Quartile of Myocardial Contraction Fraction
| All | Quartile 1 (13.3 – 50.1%) |
Quartile 2 (50.1 – 57.8%) |
Quartile 3 (57.8 – 66.8%) |
Quartile 4 (66.8 – 106.8%) |
P value | |
|---|---|---|---|---|---|---|
| (N=2147) | (n=536) | (n=537) | (n=537) | (n=537) | ||
| Age (years) | 72.2±4.8 | 73.1±5.0 | 72.2±4.8 | 71.9±4.7 | 71.5±4.5±) | <0.001 |
| Men | 663 (31%) | 253 (47%) | 177 (33%) | 128 (24%) | 105 (20%) | <0.001 |
| Black | 286 (13%) | 99 (18%) | 68 (13%) | 67 (12%) | 52 (10%) | <0.001 |
| BMI (kg/m2) | 26.3 ±4.3 | 27.3±4.5 | 26.5±4.2 | 26.2±4.3 | 25.1±4.1 | <0.001 |
| Systolic BP (mm Hg) | 135±21 | 140±23 | 134±21 | 133±19 | 131±21 | <0.001 |
| Prevalent Diabetes | 230 (11%) | 86 (16%) | 60 (11%) | 46 (9%) | 38 (7%) | <0.001 |
| Alcohol use (drinks/week) | 2.5 (6.2%) | 3.1 (7.1%) | 2.5 (6.4%) | 2.2 (5.3%) | 2.4 (5.8%) | 0.03 |
| Smoker | 0.11 | |||||
| Never | 1051 (49.0%) | 235 (43.8%) | 272 (50.7%) | 273 (50.8%) | 271 (50.5%) | |
| Former | 839 (39.1%) | 227 (42.4%) | 209 (38.9%) | 194 (36.1%) | 209 (38.9%) | |
| Current | 257 (12.0%) | 74 (13.8%) | 56 (10.4%) | 70 (13.0%) | 57 (10.6%) | |
| Good/Excellent Health | 1829 (85.2%) | 446 (83.2%) | 463 (86.2%) | 456 (84.9%) | 464 (86.4%) | 0.43 |
| Income > $25,000 | 972 (45%) | 237 (44%) | 245 (46%) | 242 (45%) | 248 (46%) | 0.93 |
| Anti-hypertensive | 785 (37%) | 246 (46%) | 197 (37%) | 190 (35%) | 152 (28%) | <0.001 |
| Any lipid medication | 101 (5%) | 24 (4%) | 19 (4%) | 25 (5%) | 33 (6%) | 0.24 |
| LDLc (mg/dl) | 131±35 | 131± 33 | 134±37 | 130±36 | 130±34 | 0.22 |
| HDLc (mg/dl) | 57±16 | 54±15 | 56±16 | 59±15 | 61±16 | <0.001 |
| eGFR (ml/min/1.73 m2) | 82±19 | 77±19 | 83±18 | 83±17 | 84±19 | <0.001 |
| FEV1(liters) | 2.1±0.6 | 2.1±0.7 | 2.1±0.6 | 2.0±0.6 | 2.1±0.6 | 0.07 |
| Transmitral E/A ratio | <0.001 | |||||
| <0.7 | 301 (14%) | 107 (20%) | 82 (15.3%) | 65 (12.1%) | 47 (8.8%) | |
| 0.7 – 1.5 | 1772 (82.5%) | 413 (77.1%) | 440 (81.9%) | 458 (85.3%) | 461 (85.8%) | |
| >1.5 | 74 (3.5%) | 16 (2.9%) | 15 (2.8%) | 14 (2.6%) | 29 (5.4%) | |
| Fractional Shortening, %† | 43.0±7.5 | 39.3±8.0 | 42.5±7.1 | 43.9±6.6 | 46.1±6.7 | <0.001 |
| LA diameter (cm) | 3.8±0.6 | 3.9±0.6 | 3.8±0.6 | 3.8±0.6 | 3.7±0.6 | <0.001 |
| Percent predicted LV mass, | 109±30 | 128±30 | 112±20 | 104±20 | 92± 20 | <0.001 |
| Stroke volume (ml) | 77.0±19.0 | 70.9±19.1 | 76.4±18.8 | 78.8±17.3 | 82.2±18.9 | <0.001 |
| Myocardial volume (ml) | 136.0±42.0 | 168.8±50.6 | 141.4±34.7 | 127.1±28.0 | 108.7±26.0 | <0.001 |
| Valvular disease | 188 (8.8%) | 47 (8.8%) | 55 (10.2%) | 46 (8.6%) | 40 (7.4%) | 0.45 |
| Relative wall thickness | 0.35 (0.08) | 0.42 (0.09) | 0.36 (0.05) | 0.33 (0.05) | 0.29 (0.05) | <0.001 |
| NT-proBNP (pg/ml)† | 93.2 (49.3 – 176) | 107.4 (50.6 – 199.7) | 85.8 (40.8 – 175.1) | 88.19 (52.1 – 146.9) | 95.2 (53.8 – 168.8) | 0.004 |
Data were available in 2,143 subjects,
Data was available in 1,683 subjects and data is median (IQR).
Over a median follow-up of 14.6 years, there were 638 incident cases of HF, 832 incident cases of ASCVD, and 1658 deaths in the study population. Table 2 presents the risk estimates associated with higher MCF for each of these outcomes for each model. Higher MCF was associated with significantly lower risk of HF, ASCVD and all-cause mortality after adjustment for demographic factors (Model 1). These associations were attenuated after further adjustment for clinical (Model 2), as well as echo covariates (Model 3), but remained statistically significant (p<0.001 for each outcome), such that every 10% absolute increase in MCF imparted 12% (95% CI: 18%, 6%), 10% (95% CI: 15%, 5%) and 7% (95% CI: 11%, 3%) lower risks of HF, ASCVD, and death, respectively. In exploratory analyses, MCF remained significantly associated with all three outcomes after adjustment for RWT, and with ASCVD and death, but not HF, after further adjustment for percent predicted LV mass (Model 4). MCF was significantly associated with all outcomes upon adjustment for NT-proBNP in addition to Model 3 covariates (Model 5).
Table 2.
Association between Myocardial Contraction Fraction and incident Heart Failure, Atherosclerotic Coronary Vascular Disease and Death
| Outcome (Cumulative incidence) |
HF (n=638) |
ASCVD (n=823) |
Death (n=1658) |
|---|---|---|---|
| HR* (95% CI) | HR* (95% CI) | HR* (95% CI) | |
| Models | |||
| Unadjusted | 0.79 (0.74, 0.84) | 0.83 (0.78, 0.87) | 0.86 (0.82, 0.89) |
| Model 1 | 0.82 (0.76, 0.87) | 0.86 (0.81, 0.91) | 0.91 (0.87, 0.94) |
| Model 2 | 0.87 (0.82, 0.93) | 0.89 (0.84, 0.95) | 0.92 (0.89, 0.96) |
| Model 3 | 0.88 (0.82, 0.94) | 0.90 (0.85, 0.95) | 0.93 (0.89, 0.97) |
| Model 4 | 0.93 (0.86, 1.01) | 0.92 (0.86, 0.99) | 0.94 (0.90, 0.99) |
| Model 5 | 0.88 (0.82, 0.95) | 0.90 (0.84, 0.96) | 0.92 (0.88, 0.97) |
per 10% absolute increase in MCF
Model 1: adjusted for age, sex, and race
Model 2: adjusted for Model 1 + income, BMI, SBP, diabetes, LDLc, HDLc, eGFR, smoking, alcohol use, anti-hypertensive medications, self-reported health status, FEV1, and lipid-lowering therapy
Model 3: adjusted for Model 2+ valve disease, transmitral E/A ratio, left atrial dimension
Model 4 (exploratory): adjusted for Model 3+ percent predicted left ventricular mass index
Model 5 (exploratory): adjusted for Model 3+ log NT-proBNP
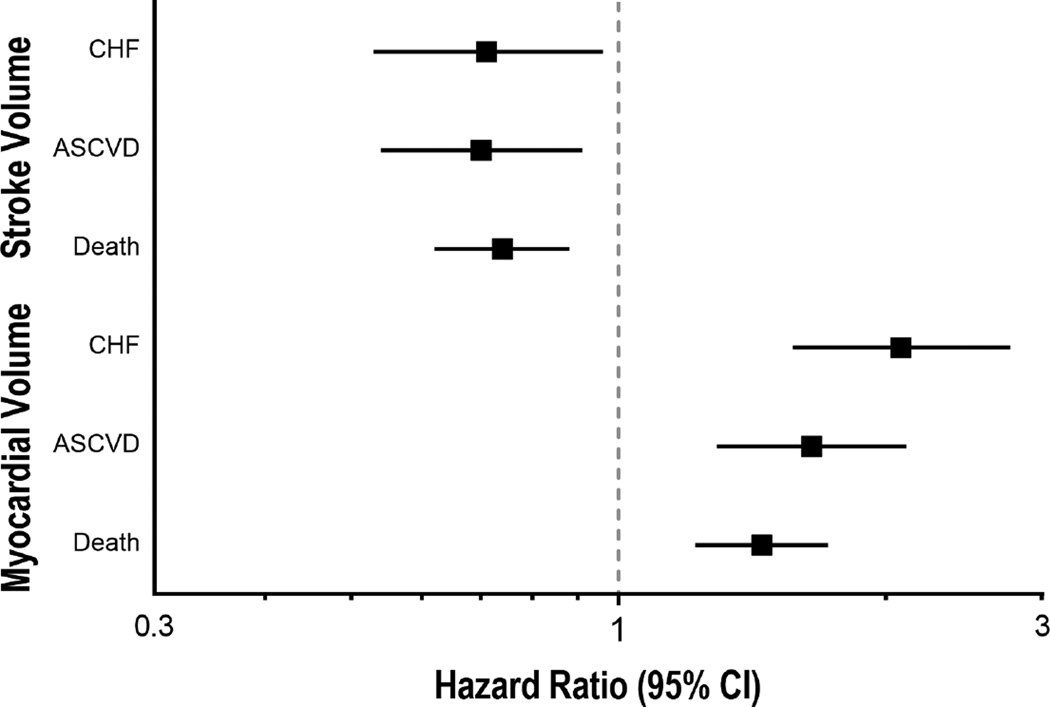
Both MV and SV were associated with the outcomes of interest after mutual adjustment. Specifically, in our final model (Model 3), log2(SV) was inversely associated with HF (HR=0.73; 95% CI=0.54, 0.98; p=0.036), ASCVD (HR=0.69; 95% CI=0.54, 0.89; p=0.005) and mortality (HR=0.75; 95% CI: 0.63 - 0.90; p=0.002). In contrast, log2(MV) in Model 3 was directly associated with HF (HR=2.07; 95% CI=1.56, 2.74; p<0.001), ASCVD (HR=1.67; 95% CI=1.31, 2.14; p<0.001) and mortality (HR=1.43; 95% CI=1.20, 1.70; p<0.001) (see Figure 1). We next assessed whether the coefficients of log2SV and log2MV departed from the 1:−1 ratio inherent in the computation of MCF. In the case of HF, the sum of the coefficients was significantly different from 0 (0.41 [95% CI: 0.14, 0.68]; p=0.003), indicating deviation from the 1:−1 ratio. For ASCVD and death, the sums of the observed estimates of 0.15 (95% CI: −0.09, 0.39) and 0.07 (95% CI: −0.10, 0.24), respectively, are consistent with the hypothesis that the coefficients for the individual components of MCF occur in the expected 1:−1 ratio.
Figure 1.
Hazard ratios and 95% CI’s for associations of components of MCF, specifically log2 (stroke volume) and log2(Myocardial Volume), with HF, ASCVD and death in a model controlling for demographic, clinical and echocardiographic variables (Model 3 in Table 2).
Discussion
The present study shows that among participants without prevalent CVD or reduced LVEF from a population-based cohort of older adults, higher MCF was significantly associated with lower risk of HF, ASCVD and death after adjustment for clinical risk factors and echo measures, which persisted even after additional adjustment for NT-proBNP. Moreover, in additional exploratory analyses, the MCF was still significantly associated with ASCVD and mortality but not HF after adjustment for percent predicted LV mass. Corresponding regression coefficients did not deviate from the expected 1:−1 ratio inherent in MCF for ASCVD or all-cause mortality, but there was evidence of such a departure for the HF outcome.
Measures of LV chamber performance are based on the premise that the myocardium is nearly incompressible and does not change volume significantly from end-diastole to end-systole. Evidence for the incompressibility of the myocardium comes from tomographic imaging,23,24 and eliminates the need to account for myocardial deformations in measuring ventricular performance, making governing equations much less complicated.25,26 Capitalizing on this fundamental principle by indexing SV to MV, the MCF is an index of the volumetric shortening of the myocardium that is independent of chamber size and geometry. As a measure of myocardial shortening, SV is most appropriately assessed relative to the myocardium, and specifically to MV, because it is the myocardium that shortens. Thus, MCF is a measure of the amount by which the myocardium contracts during systole relative to total MV, although the myocardium itself has not undergone a reduction in volume. MCF, though operationalized prior to the widespread use of strain in echo,4 is highly correlated with global longitudinal strain.
To our knowledge, MCF has only been evaluated in relation to outcomes by a single prospective, population-based study. Using cardiac MRI to quantify MCF in a subset of participants (n=318) with normal EF, the Framingham study5 demonstrated that the lowest quartile of MCF was associated with a ~7-fold increased relative risk of a composite CVD endpoint as compared with the remaining quartiles, whereas EF was not. The number of incident composite CVD events was modest (n=31), but the association persisted after adjustment for Framingham risk score and even LV mass. Our findings extend these observations to a large subset of participants from a population-based study of older adults having normal EF and no clinically overt CVD at baseline. The present analyses demonstrate that MCF estimated from 2D-guided M-mode echo measures routinely obtained in clinical practice is associated with HF, ASCVD and death independent of clinical risk factors and other echo variables in this population. Notably, the associations of MCF with ASCVD and death persisted after additional adjustment for percent predicted LV mass, as it did for composite CVD events in Framingham, but not with HF individually. Moreover, the larger number of incident events in the current study allowed us to test whether combining the individual components of the MCF into a ratio compromises the predictive information obtained when these components are modeled separately. Such potential loss of predictive information is a well-described pitfall of ratiometric measures, which carry widespread appeal in clinical practice.27
Our analyses suggest that in the case of incident HF, though not for ASCVD and mortality, MCF performed less well than its individual components modeled separately. Specifically, MV was more strongly associated with HF than SV, with regression coefficients of SV to MV approximating a 1:−2 ratio on the logarithmic scale. This dominant relationship for MV relative to SV for HF, but not ASCVD or death, is consistent with the greater importance of adverse LV remodeling as a determinant of HF risk as compared with these other major outcomes. The observed relationship of log2SV and log2MV with HF, however, would translate to a ratiometric variable of SV/MV2 on the original scale, having units of ml−1, which would lose the appeal of the unitless MCF index. Hence, our findings support an approach that considers SV and MV individually, rather than as a ratiometric variable, in relation to incident HF. Future studies will need to examine how MCF, SV and MV individually, and SV/MV2 compare to LV mass index in formal analyses of risk prediction of HF and other major cardiovascular outcomes.
Several imitations to our work should be noted. The MCF could not be calculated in 21% of the entire CHS cohort, but was available in the healthier subset of participants with available echo measurements. Moreover, we focused the current analysis on participants having no baseline CVD, and particularly normal EF, given that the techniques employed to estimate ventricular volumes are accurate only in symmetrically contracting ventricles. Hence, these findings are not generalizable to elders with poorer health status, prevalent CVD or low EF. Our estimates of LV chamber and myocardial volumes, contrary to the original description of MCF which used 3D echo,4 were derived from linear dimensions obtained by 2D-guided M-mode, which may have introduced random misclassification from oblique LV orientations. Moreover, the corresponding derivations of chamber volumes are influenced by chamber dilation and sphericity, producing overestimation of LV and particularly myocardial volumes.28 The resulting bias, however, would be to underestimate the true association of higher MV’s with outcomes, suggesting that more accurate imaging modalities would yield stronger relationships for MCF, but perhaps also compound the loss of information associated with this ratiometric index by further strengthening the association for MV relative to SV with outcomes. Further study of this question by various imaging approaches, in different populations, and with statistical techniques for evaluating prediction will provide insights into the usefulness of MCF and its components for improving prediction of the outcomes of interest.
Acknowledgments
None.
Sources of Funding
This work was supported by R01 HL-094555, as well as by contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. Dr. Maurer is supported by a grant from the NIA (K24-AG036778).
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
None.
References
- 1.Wisenbaugh T, Booth D, DeMaria A, Nissen S, Waters J. Relationship of contractile state to ejection performance in patients with chronic aortic valve disease. Circulation. 1986;73:47–53. doi: 10.1161/01.cir.73.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Maurer MS, Sackner-Bernstein JD, El-Khoury Rumbarger L, Yushak M, King DL, Burkhoff D. Mechanisms underlying improvements in ejection fraction with carvedilol in heart failure. Circ Heart Fail. 2009;2:189–196. doi: 10.1161/CIRCHEARTFAILURE.108.806240. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL Cardiovascular Health Study Research G. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 4.King DL, El-Khoury Coffin L, Maurer MS. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40:325–329. doi: 10.1016/s0735-1097(02)01944-7. [DOI] [PubMed] [Google Scholar]
- 5.Chuang ML, Gona P, Salton CJ, Yeon SB, Kissinger KV, Blease SJ, Levy D, O'Donnell CJ, Manning WJ. Usefulness of the left ventricular myocardial contraction fraction in healthy men and women to predict cardiovascular morbidity and mortality. Am J Cardiol. 2012;109:1454–1458. doi: 10.1016/j.amjcard.2012.01.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 7.Gardin JM, Wong ND, Bommer W, Klopfenstein HS, Smith VE, Tabatznik B, Siscovick D, Lobodzinski S, Anton-Culver H, Manolio TA. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 8.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 9.Karas MG, Benkeser D, Arnold AM, Bartz TM, Djousse L, Mukamal KJ, Ix JH, Zieman SJ, Siscovick DS, Tracy RP, Mantzoros CS, Gottdiener JS, deFilippi CR, Kizer JR. Relations of plasma total and high-molecular-weight adiponectin to new-onset heart failure in adults >/=65 years of age (from the Cardiovascular Health study) Am J Cardiol. 2014;113:328–334. doi: 10.1016/j.amjcard.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aurigemma GP, Gottdiener JS, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–1048. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 11.Gottdiener JS, McClelland RL, Marshall R, Shemanski L, Furberg CD, Kitzman DW, Cushman M, Polak J, Gardin JM, Gersh BJ, Aurigemma GP, Manolio TA. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 12.de Simone G, Devereux RB, Ganau A, Hahn RT, Saba PS, Mureddu GF, Roman MJ, Howard BV. Estimation of left ventricular chamber and stroke volume by limited M-mode echocardiography and validation by two-dimensional and Doppler echocardiography. Am J Cardiol. 1996;78:801–807. doi: 10.1016/s0002-9149(96)00425-0. [DOI] [PubMed] [Google Scholar]
- 13.Maurer MS, Burkhoff D, Fried LP, Gottdiener J, King DL, Kitzman DW. Ventricular structure and function in hypertensive participants with heart failure and a normal ejection fraction: the Cardiovascular Health Study. J Am Coll Cardiol. 2007;49:972–981. doi: 10.1016/j.jacc.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 14.Gardin JM, Siscovick D, Anton-Culver H, Lynch JC, Smith VE, Klopfenstein HS, Bommer WJ, Fried L, O'Leary D, Manolio TA. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–1748. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 15.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 16.Erlandsen EJ, Randers E, Kristensen JH. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 17.Newman DJ, Thakkar H, Edwards RG, Wilkie M, White T, Grubb AO, Price CP. Serum cystatin C measured by automated immunoassay: a more sensitive marker of changes in GFR than serum creatinine. Kidney Int. 1995;47:312–318. doi: 10.1038/ki.1995.40. [DOI] [PubMed] [Google Scholar]
- 18.Jiang R, Burke GL, Enright PL, Newman AB, Margolis HG, Cushman M, Tracy RP, Wang Y, Kronmal RA, Barr RG. Inflammatory markers and longitudinal lung function decline in the elderly. Am J Epidemiol. 2008;168:602–610. doi: 10.1093/aje/kwn174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 20.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 21.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 22.Karas MG, Yee LM, Biggs ML, Djousse L, Mukamal KJ, Ix JH, Zieman SJ, Siscovick DS, Gottdiener JS, Rosenberg MA, Kronmal RA, Heckbert SR, Kizer JR. Measures of Body Size and Composition and Risk of Incident Atrial Fibrillation in Older People: The Cardiovascular Health Study. Am J Epidemiol. 2016;183:998–1007. doi: 10.1093/aje/kwv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King DL, Coffin Lel K, Maurer MS. Noncompressibility of myocardium during systole with freehand three-dimensional echocardiography. J Am Soc Echocardiogr. 2002;15:1503–1506. doi: 10.1067/mje.2002.126418. [DOI] [PubMed] [Google Scholar]
- 24.Gopal AS, Schnellbaecher MJ, Shen Z, Boxt LM, Katz J, King DL. Freehand three-dimensional echocardiography for determination of left ventricular volume and mass in patients with abnormal ventricles: comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 1997;10:853–861. doi: 10.1016/s0894-7317(97)70045-2. [DOI] [PubMed] [Google Scholar]
- 25.Yin FC, Chan CC, Judd RM. Compressibility of perfused passive myocardium. Am J Physiol. 1996;271:H1864–H1870. doi: 10.1152/ajpheart.1996.271.5.H1864. [DOI] [PubMed] [Google Scholar]
- 26.Mirsky I. Effects of anisotropy and nonhomogeneity on left ventricular stresses in the intact heart. Bull Math Biophys. 1970;32:197–213. doi: 10.1007/BF02476885. [DOI] [PubMed] [Google Scholar]
- 27.Kronmal RA. Spurious Correlation and the Fallacy of the Ratio Standard Revisited. Journal of the Royal Statistical Society Series A (Statistics in Society) 1993;156:379–392. [Google Scholar]
- 28.Stewart GA, Foster J, Cowan M, Rooney E, McDonagh T, Dargie HJ, Rodger RS, Jardine AG. Echocardiography overestimates left ventricular mass in hemodialysis patients relative to magnetic resonance imaging. Kidney Int. 1999;56:2248–2253. doi: 10.1046/j.1523-1755.1999.00786.x. [DOI] [PubMed] [Google Scholar]