Abstract
Objective
Interstitial lung disease (ILD) is associated with substantial morbidity in rheumatoid arthritis (RA), but very little is known about its long-term progression.
Methods
All patients with RA-ILD seen at Mayo Clinic in 1998-2014 with at least 4 weeks follow-up and at least 1 pulmonary function test (PFT) were identified and manually screened for study inclusion. Progression was defined as a diffusing capacity for carbon monoxide (DLCO) <40% predicted or too ill to perform, or a forced vital capacity (FVC) <50% predicted. Time to progression was analyzed using Kaplan-Meier methods.
Results
Of 167 included patients, 81 (49%) were female with mean age of 67 years (standard deviation: 10) at ILD diagnosis. Median follow-up time from ILD diagnosis was 3.3 (range: 0.01-14.8) years. A third of patients required supplemental oxygen, 40% developed DLCO <40% predicted and 22% developed FVC <50% predicted by 5 years after ILD diagnosis. Risk factors for DLCO progression were usual interstitial pneumonia (UIP) vs. nonspecific interstitial pneumonia (NSIP) (hazard ratio [HR]: 3.29; 95% confidence interval [CI]: 1.28, 8.41). Lower percent predicted DLCO and FVC at baseline increased the risk for progression to DLCO <40% and FVC<50% predicted, and higher rates of change in the first 6 months also increased the risk of progression.
Conclusion
Progressive loss of pulmonary function is common in RA-ILD and worse in patients with UIP than NSIP. Predictors of progression in patients with RA-ILD may aid clinicians in identifying patients at highest risk for progression of ILD.
Keywords: Interstitial Lung Disease, Rheumatoid Arthritis, Pulmonary Function, Progression
Introduction
Lung disease is a relatively common extra-articular manifestation of rheumatoid arthritis (RA), which can present with a restrictive and/or obstructive pattern, and is often clinically under recognized (1, 2). In patients with RA, significant restrictive lung disease, most commonly interstitial lung disease (ILD), occurs in 8 to 15 percent of patients (3, 4). Clinical symptoms are variable, most commonly dyspnea and cough, with some patients rapidly deteriorating while others remain relatively stable (5). The three main types of RA-ILD are usual interstitial pneumonia (UIP), non-specific interstitial pneumonia (NSIP), and organizing pneumonia (OP); of which UIP is the more common (6, 7).
Currently, there are only limited data on rates of change for lung disease in patients with RA-ILD, and uncertainty about which outcome measures are best to assess progression (8). Clinical evaluation and histopathological sampling along with high resolution computed tomography (HRCT) is sensitive for detection and classification of ILD, while pulmonary function testing (PFT) may be better suited for assessing progression (6, 9). Pulmonary physiology has recently been shown to independently predict mortality over HRCT pattern (10). Therefore, it is likely that evaluation of the rates of change by PFTs in this patient population will provide a needed tool in assessing progression.
In patients with idiopathic pulmonary fibrosis (IPF) forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO) are the most sensitive parameters for assessing the clinical course (11). Evaluation of pulmonary hypertension (PH) in patients with IPF is also important as it is relatively common in these patients and has been associated with more rapid progression to low DLCO and increased risk of death from pulmonary complications (12).
There is a strong correlation between progressively lower values of FVC and DLCO with significant morbidity and clinical disease severity from ILD, especially IPF (11). Consensus opinion from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation states that pulmonary testing parameters for candidate selection for lung transplantation in patients with ILD should focus on FVC, DLCO, and PH by right heart catheterization or echocardiography, as these measures are associated with poor quality of life and poor prognosis (13). They are also likely the parameters of most interest in assessing pulmonary decline in patients with RA-ILD.
The aim of the present study is to utilize a large single center cohort of patients with RA associated ILD to describe the progression of pulmonary disease using measures of pulmonary physiology.
Methods
Study Population
This retrospective cohort study was approved by the Mayo Clinic Institutional Review Board. Study subjects were identified through a unified single center electronic medical record system using international diagnosis codes (ICD9) for ILD with diagnosis occurring between January 1, 1998 and December 31, 2014. All identified cases that fulfilled the 1987 American College of Rheumatology criteria for RA, with a minimum of 4 weeks follow-up, chest HRCT, and at least 1 PFT were subsequently manually reviewed for ILD diagnosis verification (14, 15). Patients with concomitant rheumatologic disease other than RA-related secondary Sjögren's syndrome (such as systemic lupus erythematosus, vasculitis, etc.) were excluded. Individuals with ILD and concomitant obstructive lung disease, as defined as obstructive airway diseases, emphysema, chronic bronchitis, or asthma, were included. Only tests done at the study center were abstracted for analysis.
Data Collection
PFT results were recorded in both volume (liters) and percent predicted values and abstracted between 1 year prior to ILD diagnosis and 10 years after ILD diagnosis. All PFTs in the 12 months before or after ILD diagnosis and the first PFT for each subsequent follow-up year were included. Parameters included FVC, forced expiratory volume in 1 second (FEV1), total lung capacity (TLC), and DLCO; the DLCO results were corrected for hemoglobin level when appropriate for each individual pulmonary function test. If baseline DLCO values were unavailable, reasons for this were also manually abstracted. Transthoracic echocardiography examinations (TTE) were manually reviewed for the presence of PH with a definition of estimated right systolic ventricular pressure greater than 35 mmHg (12). Data for TTE recorded included date of first study, date when definition of PH was met (if available), and total number of TTEs per patient. Resting oxygen supplementation data collected included need at ILD diagnosis, date of first oxygen prescription (if available) and oxygen requirement at last follow-up in liters.
RA therapies and disease severity indicators were also collected by manual record review. Data abstracted on RA therapies at and after ILD diagnosis included prednisone, categorized as equal to or less than 10mg per day and greater than 10mg per day, as well as biologic and non-biologic immunosuppressive agents. Data abstracted on disease severity included rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA), presence of rheumatoid nodules, erosions, and any additional extra-articular manifestations other than ILD. Severe extra-articular manifestations were defined according to Malmö criteria and included pericarditis, pleuritis, Felty's syndrome, glomerulonephritis, vasculitis, peripheral neuropathy, scleritis and episcleritis (16). The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) values closest to and within 30 days of diagnosis of ILD were also recorded when available. Follow-up data was abstracted until death, 10 years follow-up, or December 31, 2015.
Statistical Analysis
Descriptive statistics (means, percentages, etc.) were used to summarize the data. Comparisons between groups were performed using Chi-square and rank sum tests. Progression was defined as the first time a DLCO less than 40 percent predicted (or those too ill to perform the DLCO) and/or those with FVC less than 50 percent predicted (severe restriction). Time to progression was analyzed using the Kaplan-Meier method and Cox models adjusted for age and sex. PH was modeled using time-dependent covariates. For data analysis purposes, patients were not at risk for PH until the time the first TTE was performed. Medications for RA were divided into distinct groups and were analyzed using time-dependent covariates that started when the medication was first used and stopped 30 days after the medication was discontinued to allow for a wash-out period.
Six month rate of change in DLCO and FVC was estimated using the PFT measures closest to ILD diagnosis (within ± 6 months) and closest to 6 months (up to one year) after ILD diagnosis. Six month rate of change was calculated as the absolute change in percent predicted DLCO or FVC between these 2 measures divided by the number of days between the measures and multiplied by the number of days in 6 months to ensure comparability across patients. Non-linear effects were examined using smoothing splines. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 167 patients were included of which 81 (49%) were female and the majority were Caucasian (97%) with a mean (±SD) age of 67 years (±10) at ILD diagnosis (table 1). Sixty-two (37%) were never smokers. At last follow-up 68 had died, 20 of which were directly attributable to pulmonary disease Eighty-nine (53%) had UIP, 70 (42%) had NSIP, and 8 (5%) had RA-related OP. The median ESR closest to ILD diagnosis was 34 mm/h (normal: 0-22 mm/h [men], 0-29 mm/h [women]); interquartile range: 19-53, (available for 120 patients); while the median CRP was 17 mg/L (normal: 0-8mg/dl), interquartile range 5-34; (available for 86 patients) with no significant differences between types of ILD. Erosive disease was documented in 41 patients (25%) and the most common additional extra-articular manifestations, apart from ILD, were subcutaneous nodules in 42 (25%) patients.
Table 1. Characteristics of 167 Patients with Interstitial Lung Disease and Rheumatoid Arthritis by Type of Interstitial Lung Disease.
| Characteristic | NSIP (n=70) | UIP (n=89) | OP (n=8) | Total* (n=167) |
|---|---|---|---|---|
| Age at ILD diagnosis, years | 67.3 (±9.9) | 68.2 (±9.7) | 56.2 (±8.9) | 67.3 (±10.0) |
| Age at RA diagnosis, years | 59.5 (±14.4) | 59.5 (±12.3) | 50.1 (±10.7) | 59.0 (±13.2) |
| Ethnicity, Caucasian (n, %) | 68 (97%) | 84 (97%) | 7 (88%) | 159 (97%) |
| Sex, female (n, %) | 37 (53%) | 40 (55%) | 4 (50%) | 81 (49%) |
| Smoking status | ||||
| Never (n, %) | 30 (43%) | 31 (35%) | 1 (13%) | 62 (37%) |
| Former (n, %) | 38 (54%) | 57 (64%) | 6 (75%) | 101 (60%) |
| Current (n, %) | 2 (3%) | 1 (1%) | 1 (13%) | 4 (2%) |
| Length of follow-up from ILD diagnosis, years, median (range) | 4.3 (0.1 – 13.4) | 3.7 (0.1 – 14.8) | 3.7 (0.1 – 8.8) | 3.3 (0.1 – 14.8) |
| RA disease duration at ILD diagnosis, years, median (range) | 3.6 (-10.9 – 48.1) | 5.4 (-9.8 – 43.0) | 2.4 (-1.7 – 19.1) | 4.2 (-10.9 – 48.1) |
| RF positive, n positive/ n tested (%) | 56 / 68 (82%) | 74 / 87 (85%) | 4 / 8 (100%) | 134 / 163 (82%) |
| ACPA positive, n positive/ n tested (%) | 51 / 64 (80%) | 47 / 59 (80%) | 3 / 6 (50%) | 101 / 129 (78%) |
| Erosive disease (n, %) | 16 (23%) | 22 (25%) | 3 (38%) | 41 (25%) |
| Extra-articular manifestations other than ILD (n, %) | 31 (44%) | 38 (43%) | 4 (50%) | 73 (44%) |
| Rheumatoid nodules | 16 (23%) | 25 (28%) | 1 (13%) | 42 (25%) |
| Severe extra-articular manifestations other than ILD (n, %) | 4 (6%) | 6 (7%) | 2 (25%) | 12 (7%) |
| Emphysema on chest HRCT | 8 (11%) | 13 (15%) | 1 (13%) | 22 (13%) |
| Required supplemental oxygen at ILD diagnosis (n, %) | 6 (9%) | 17 (19%) | 0 (0%) | 23 (14%) |
| Oxygen requirement at last visit (liters/minute) | 3.0 (±2.1) | 2.7 (±1.2) | 3.0 (±1.4) | 2.8 (±1.5) |
Values in table are mean (±standard deviation) or n (%) unless otherwise specified
Abbreviations: Anti-citrullinated protein antibody (ACPA), number (n), high-resolution computed tomography (HRCT), interstitial lung disease (ILD), rheumatoid arthritis (RA), rheumatoid factor (RF), nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), usual interstitial pneumonia (UIP)
Supplemental oxygen was used at ILD diagnosis in 23 (14%) patients. Another 54 patients required supplemental oxygen during follow-up. Including those on supplemental oxygen at ILD diagnosis, 33.0% (95% CI: 23.9%, 41.1%) of patients required supplemental oxygen by 5 years after ILD diagnosis. The requirement for supplemental oxygen was somewhat more common among patients with UIP than NSIP, but this difference did not reach statistical significance (hazard ratio [HR]: 1.58; 95% confidence interval [CI]: 0.90, 2.78).
A total of 564 PFTs were included in the analysis. Baseline PFTs at time of ILD diagnosis (within ± 6 months) included a median percent predicted FVC of 71%, FEV1 of 70%, TLC of 72% and DLCO of 54.5%. The median percent predicted DLCO for UIP was 51.5%, NSIP 57%, and OP 71% (P=0.006). There were no other significant differences in these PFT parameters at baseline between types of ILD (table 2). DLCO declined more in the first 6 months after ILD diagnosis among OP and UIP than NSIP (median change in percent predicted: -13.7 in OP, -2.3 in UIP and 2.2 in NSIP; p=0.04).
Table 2. Pulmonary Function in Percent Predicted by Type of Interstitial Lung Disease in 167 Patients with Rheumatoid Arthritis.
| UIP | NSIP | OP | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | Value* | N | Value* | N | Value* | p-value |
| Baseline (±6 months) | |||||||
| TLC | 56 | 70.0 (62.5, 79.0) | 40 | 72.0 (63.0, 81.0) | 5 | 94.0 (88.0, 97.0) | 0.021 |
| FVC | 83 | 69.0 (59.0, 85.0) | 65 | 75.0 (59.0, 91.0) | 6 | 69.5 (57.0, 75.0) | 0.700 |
| FEV1 | 82 | 70.0 (59.0, 84.0) | 65 | 71.0 (54.0, 91.0) | 6 | 51.5 (35.0, 69.0) | 0.140 |
| DLCO | 78 | 51.5 (38.0, 63.0) | 63 | 57.0 (45.0, 71.0) | 5 | 71.0 (67.0, 79.0) | 0.006 |
| Change in first 6 months | |||||||
| TLC | 33 | -0.8 (-7.1, 5.0) | 13 | 1.8 (-5.2, 5.4) | 1 | -10.8 | 0.318 |
| FVC | 53 | 1.5 (-3.9, 5.6) | 40 | -0.3 (-7.8, 5.2) | 2 | -9.1 (-13.5, -4.6) | 0.163 |
| FEV1 | 53 | -0.7 (-4.4, 4.4) | 39 | 1.0 (-7.5, 6.7) | 2 | -9.8 (-22.8, 3.1) | 0.759 |
| DLCO | 47 | -2.2 (-7.0, 3.8) | 36 | 2.3 (-3.6, 8.1) | 2 | -13.7 (-21.5, -5.9) | 0.040 |
| Overall Change | |||||||
| Years from first to last PFT, mean (±SD) | 3.6 (±3.5) | 3.6 (±3.1) | 3.3 (±3.1) | 0.840 | |||
| TLC | 34 | -0.1 (-0.6, 0.1) | 25 | 0.0 (-0.1, 0.5) | 2 | -0.2 (-0.2, 0.1) | 0.204 |
| FVC | 58 | -0.2 (-0.5, 0.1) | 59 | -0.2 (-0.5, 0.2) | 4 | 0.0 (0.0, 0.2) | 0.367 |
| FEV1 | 58 | -0.2 (-0.4, 0.0) | 59 | -0.2 (-0.4, 0.1) | 4 | (-0.1, 0.2) | 0.185 |
| DLCO | 48 | -2.1 (-4.5, -0.9) | 51 | -0.9 (-2.7, 0.9) | 3 | -0.3 (-3.3, 1.2) | 0.017 |
Values in table are median (interquartile range)
Abbreviations: Diffusing capacity for carbon monoxide (DLCO),), forced expiratory volume in 1 second (FEV1), forced vital capacity (FVC), number (N), nonspecific interstitial pneumonia (NSIP), organizing pneumonia (OP), pulmonary function test (PFT), standard deviation (SD), total lung capacity (TLC), usual interstitial pneumonia (UIP)
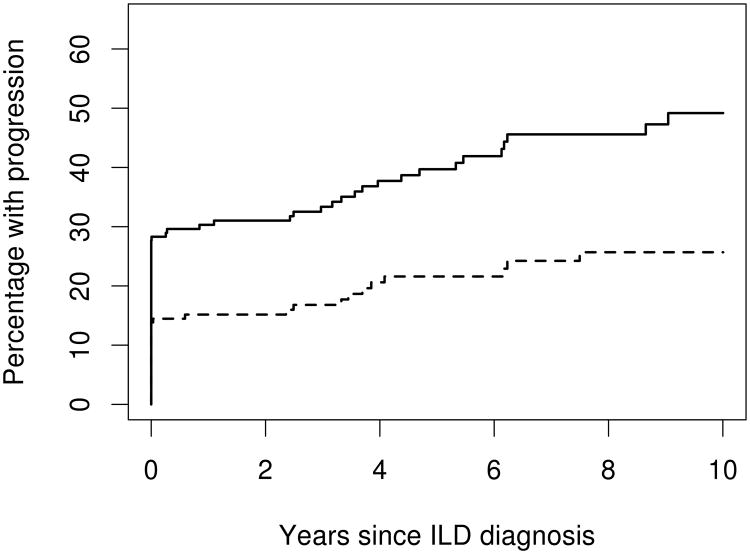
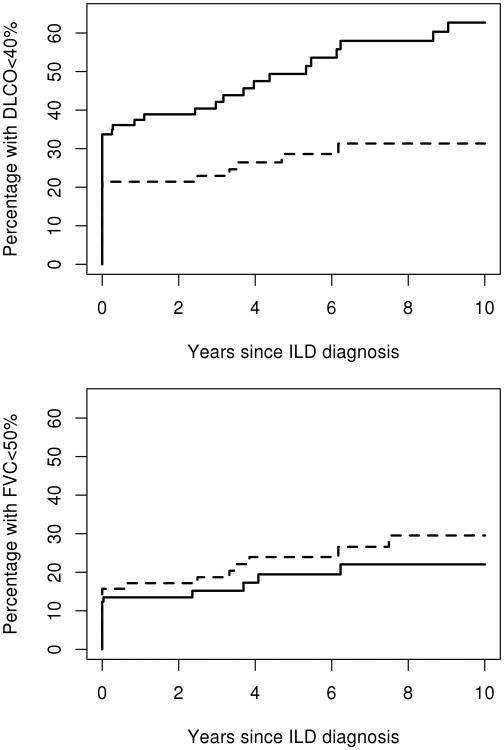
At ILD diagnosis (including PFTs up to 6 months after ILD diagnosis), 48 (29%) patients had DLCO <40% predicted and 23 (14%) patients had FVC <50% predicted. During follow-up, DLCO declined to <40% predicted (or were too ill to perform the test) in 22 additional patients and 13 additional patients developed an FVC <50% indicating severe restriction. Combining data at ILD diagnosis and during follow-up, by 5 years after ILD diagnosis, 39.7% (95% CI: 31.0%, 47.3%) of patients reached a DLCO <40% predicted (or were too ill to perform the test) and 21.6% (95% CI: 14.4%, 28.2%) of patients reached FVC <50% predicted (figure 1). Among those who did not meet the definitions of progression at ILD diagnosis, those with UIP were more likely to progress to DLCO <40% than those with NSIP (HR: 3.29; 95% CI: 1.28, 8.41; figure 2 upper panel). This association persisted after additional adjustment for baseline DLCO (HR: 3.04; 95% CI: 1.04, 8.89). Ever smoking had a protective effect on progression to a DLCO <40% predicted (HR: 0.35; 95% CI: 0.14, 0.85). However, this association did not persist after adjustment for percent predicted DLCO (p=0.52). Other factors analyzed, including PH, did not reach statistical significance (table 3).
Figure 1.
Estimated percentage of patients with diffusing capacity for carbon monoxide (DLCO) <40% (or too ill to perform the test) (solid line) and with forced vital capacity (FVC)<50% (dashed line) according to time since diagnosis of interstitial lung disease (ILD) in 167 patients with rheumatoid arthritis and ILD.
Figure 2.
Estimated percentage of patients with diffusing capacity for carbon monoxide (DLCO) <40% (or too ill to perform the test) (upper panel) and with forced vital capacity (FVC) <50% (lower panel) according to time since diagnosis of interstitial lung disease (ILD) in 167 patients with rheumatoid arthritis and ILD subdivided by ILD type (solid line is usual interstitial pneumonia; dashed line is non-specific interstitial pneumonia).
Table 3. Risk Factors at Baseline for Progressive Lung Function Decline in Patients with Rheumatoid Arthritis associated Interstitial Lung Disease.
| DLCO < 40% predicted | FVC < 50% | |||
|---|---|---|---|---|
| Risk Factor | Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value |
| Age * | 1.26 (0.79, 1.98) | 0.33 | 1.06 (0.61, 1.82) | 0.84 |
| Male sex | 1.75 (0.74, 4.10) | 0.20 | 0.98 (0.33, 2.92) | 0.97 |
| Ever smoker | 0.35 (0.14, 0.85) | 0.021 | 0.45 (0.15, 1.47) | 0.20 |
| Emphysema | 1.34 (0.39, 4.57) | 0.64 | 0.52 (0.07, 4.04) | 0.53 |
| RF or ACPA positive | 2.61 (0.35, 19.57) | 0.35 | 0.51 (0.11, 2.33) | 0.38 |
| ESR (mm/1hr) * | 1.04 (0.86, 1.25) | 0.71 | 0.83 (0.58, 1.17) | 0.28 |
| CRP (mg/L) * | 0.98 (0.79, 1.21) | 0.82 | 0.74 (0.37, 1.49) | 0.40 |
| Rheumatoid nodules | 1.00 (0.40, 2.53) | 0.99 | -- | 0.99 |
| Severe extra-articular manifestations | -- | 0.99 | -- | 0.99 |
| UIP vs NSIP | 3.29 (1.28, 8.41) | 0.013 | 0.86 (0.27, 2.73) | 0.79 |
| FVC percent predicted† | 1.25 (0.97, 1.64) | 0.087 | 2.38 (1.37, 4.17) | 0.002 |
| DLCO percent predicted† | 3.74 (1.96, 7.14) | <0.001 | 1.67 (1.16, 2.44) | 0.005 |
| Pulmonary hypertension** | 2.65 (0.93, 7.58) | 0.069 | 1.26 (0.32, 5.04) | 0.74 |
| Ever having had a TTE†† | 1.56 (0.48, 5.10) | 0.46 | 1.49 (0.40, 5.61) | 0.55 |
| Years from RA to ILD Dx* | 1.35 (0.74, 2.46) | 0.32 | 1.14 (0.56, 2.34) | 0.72 |
| FVC rate of change*** | 3.42 (1.61, 7.24) | 0.001 | ||
| FVC rate of decrease ≥ 10% | 7.53 (1.81, 31.27) | 0.005 | ||
| DLCO rate of change*** | 1.72 (1.07, 2.74) | 0.024 | ||
| DLCO rate of decrease ≥ 15% | 1.52 (0.19, 12.40) | 0.69 | ||
| SSZ/HCQ alonex | 1.0 (Reference) | 1.0 (Reference) | ||
| MTX/LEF alone | 1.42 (0.15, 13.76) | 0.76 | -- | 0.99 |
| TNFi (with anyxx) | 1.60 (0.18, 14.31) | 0.68 | 1.04 (0.09, 11.72) | 0.97 |
| non-TNFi biologic (with any) | -- | 0.99 | 1.67 (0.14, 19.60) | 0.68 |
| Prednisone ≤ 10 mg per day alonex | 2.60 (0.26, 25.77) | 0.41 | 1.78 (0.11, 29.00) | 0.69 |
| Prednisone > 10 mg per day (with anyxx) | 5.32 (0.60, 47.11) | 0.13 | 15.04 (1.50, 150.8) | 0.021 |
| Other DMARD or combination | 1.57 (0.16, 15.45) | 0.70 | 0.60 (0.04, 10.20) | 0.73 |
| No therapy | 2.33 (0.24, 22.56) | 0.46 | 1.42 (0.09, 23.14) | 0.81 |
All models adjusted for age at ILD diagnosis and sex.
Per 10-unit increase.
Per 10-unit decrease
Developed pulmonary hypertension during follow-up.
Per 10% decrease in first 6 months of follow-up.
Having TTE related to suspicion for pulmonary hypertension, not causation.
Alone: without other antirheumatic drug or glucocorticoid.
with any: alone or in combination with any other antirheumatic drug. Patients taking both TNFi (or non-TNFi) biologic and prednisone >10mg are in the TNFi (or non-TNFi) biologic group.
Abbreviations: Anti-citrullinated protein antibody (ACPA), C - reactive protein (CRP), diagnosis (Dx), diffusing capacity for carbon monoxide (DLCO), erythrocyte sedimentation rate (ESR), forced vital capacity (FVC), nonspecific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), rheumatoid factor (RF), transthoracic echo (TTE), sulfasalazine (SSZ), hydroxychloroquine (HCQ), methotrexate (MTX), leflunomide (LEF), tumor necrosis factor inhibitor (TNFi), rituximab and abatacept (non TNFi), disease modifying anti-rheumatic drug (DMARD)
There was no difference between UIP and NSIP in progression to FVC <50% (HR: 0.86; 95% CI: 0.27, 2.73; figure 2 lower panel). A lower percent predicted DLCO and FVC at ILD diagnosis increased the risk for progression to a DLCO <40% predicted (HR: 3.74 per 10 unit decrease; 95% CI: 1.96, 7.14; HR: 1.25 per 10 unit decrease; 95% CI: 0.97, 1.64; respectively) and an FVC <50% predicted (HR: 1.67 per 10 unit decrease; 95% CI: 1.16, 2.44; HR: 2.38 per 10 unit decrease; 95% CI: 1.37, 4.17; respectively). However, the association between FVC at ILD diagnosis and progression to DLCO<40% predicted did not reach statistical significance.
Estimated rates of change in the first 6 months for FVC was a median increase of 1.0% predicted (interquartile range: -5.0, 7.0) and for DLCO was a median loss of 1.0% (interquartile range: -6.0, 5.0). There was a significant association between the DLCO 6 month rate of change and progression to a DLCO <40% predicted (HR: 1.72 per 10 unit decrease; 95% CI: 1.07, 2.74) and between the FVC 6 month rate of change and progression to a FVC < 50% (HR: 3.42 per 10 unit decrease; 95% CI: 1.61, 7.24). Analyses of potential non-linear effects of 6 month DLCO rate of change on the development of progression to DLCO <40% and of 6 month rate of change in FVC on the development of progression to FVC<50% predicted did not reveal any significant non-linear effects (p=0.31 and p=0.29, respectively).
A total of 106 patients had at least one TTE performed as part of routine care with a median of 2 TTEs per patient. Fourteen patients had the diagnosis of PH prior to diagnosis of ILD and an additional 47 patients developed PH during follow-up. While the hazard ratios were elevated for PH on the development of DLCO <40% predicted and FVC <50% predicted, these associations did not reach statistical significance (HR: 2.65; 95% CI: 0.93, 7.58 and HR: 1.26; 95% CI: 0.32, 5.04, respectively).
RA therapies during follow-up were categorized into distinct groups including sulfasalazine and/or hydroxychloroquine alone (n=34, 68.3 person-years [py]), methotrexate and/or leflunomide alone (n=51, 107.3 py), tumor necrosis factor inhibitor (TNFi) alone or combined with any other therapy (n=55, 156.3 py), non-TNFi biologic response modifier alone or combined with any other therapy (n=37, 81.2 py), prednisone ≤10 mg/day alone (n=53, 54.3 py), prednisone >10mg/day alone or with any other non-biologic therapy (n=83, 69.5 py), other disease modifying anti-rheumatic drugs (DMARDs) alone or in combination (n=77, 118.2 py) and no therapy (n=46, 55.1 py). Only glucocorticoid use at doses of prednisone >10 mg/day was significantly associated with progression to FVC <50% predicted (HR: 15.0; 95% CI: 1.5, 151). No other significant associations were found due to the relatively low number of patients progressing within each medication group and the potential of confounding by indication (Table 3).
Discussion
In this study of the clinical course of pulmonary disease in a large single-center cohort of patients with RA-associated ILD, progressive decline of pulmonary function is common and typically worse in patients with UIP than those with NSIP. Patients are more likely to progress to severe pulmonary function impairment if they suffer a greater decline of pulmonary function in the first six months after ILD diagnosis when compared to patients whose pulmonary function remains relatively stable during this time. A lower pulmonary reserve at time of ILD diagnosis as measured by DLCO and FVC increased the risk of progression. A third of patients required supplemental oxygen by 5 years after ILD diagnosis.
PFT measures at baseline were similar among the subtypes of ILD with the notable exception of DLCO which was lowest in UIP when compared to both NSIP and OP. Previous studies have shown that DLCO is the most sensitive PFT parameter for both early detection of ILD as well as an independent predictor of mortality (13, 17). In this cohort, percent predicted DLCO both at baseline and change at 6 months was significantly associated with progression for both UIP and NSIP.
At time of ILD diagnosis there were no meaningful differences in FVC between NSIP and UIP. During the first 6 months following diagnosis, the FVC increased modestly in over half the patients; the median change was an increase of 1.0 percent predicted. Whether this is a true and physiologically meaningful change or an artifact of testing is not certain. Interestingly, having ever smoked was associated with lack of progression to a DLCO of less than 40 percent predicted but not lack of progression or decline in FVC. The reason for this is uncertain. In contrast, DLCO is usually lower in patients with ILD and concomitant emphysema, a condition which has been termed combined pulmonary fibrosis and emphysema (18). While the hazard ratio for association between emphysema and DLCO progression was elevated, it did not reach statistical significance, likely related to the relatively small number of patients with RA-ILD and emphysema.
Overall, the present findings suggest that baseline and 6 month changes in pulmonary function in FVC are sensitive predictors for progressive and significant loss of pulmonary function. In the current study, patients with UIP were more likely to progress to a DLCO lower than 40 percent predicted than those with NSIP, complementing previous research findings that patients with UIP tend to have worse overall outcomes than those with NSIP (17). Previous studies on patients who suffer from IPF show that a UIP pattern as well as the extent of pulmonary fibrosis on chest HRCT are associated with more rapidly progressing disease [11]. Similarly, the current study suggests that a more rapid decline in pulmonary function in RA-ILD patients is also associated with a UIP pattern on HRCT. Prednisone at greater than 10 mg a day and DMARD use were associated with progression, which likely reflect overall disease severity. Data were insufficient to draw conclusions about their impact on treatment of ILD overall or to assess the relative impact of specific categories of traditional DMARDs, biologics and combinations on pulmonary outcomes.
Elevation of pulmonary artery pressure by echocardiography can be seen in as many as 28% of patients with RA even in the absence of clinically evident cardiopulmonary disease, although the measured pressures are generally only moderately elevated, corresponding to mild PH (19). In contrast, a larger proportion of patients with IPF had PH and higher pressures by TTE (12). The presence of PH in patients with ILD is associated with a more rapid deterioration and worse outcomes than those without PH (19). In the current study using information obtained at routine clinic visits rather than by protocol, the presence of PH in RA-ILD was marginally significantly associated with progressive loss of pulmonary function.
This study focused on patients encountered in clinical practice referred for multispecialty evaluation in a tertiary care center. This is both strength and a limitation of the current study. Only patients who presented with clinically evident ILD and RA were captured; routine screening for subclinical disease is not performed for the majority of patients with RA. This was not a prospective study. Pulmonary function testing and transthoracic echocardiography were performed and interpreted according to standard procedures and techniques during patient follow-up by expert specialists, which should reduce intraoperative variability. High resolution CT scans included were at initial diagnosis and last available for all patients and were evaluated by expert thoracic radiologists with subsequent review and management by expert subspecialty pulmonologists. The small number of patients who met the threshold for progression during follow-up limited the statistical power to examine risk factors for progression. The absence of ethnic diversity may hinder the generalizability to other populations, although there is little known about RA-ILD in other ethnic populations.
Conclusion
In this largest ever study of progression of pulmonary disease as assessed by both FVC and DLCO pulmonary function measures in patients with RA-ILD in long term follow-up, progressive loss of pulmonary function was common and generally worse in patients with UIP than NSIP. By 5 years after ILD diagnosis, 40% of patients progressed to severe pulmonary impairment by DLCO, 22% by FVC and a third of patients required supplemental oxygen at rest. A lower baseline in both DLCO and FVC increased the risk of progression and higher rates of change in the first 6 months increased the risk of severe pulmonary impairment over time. Predictors of progression in patients with RA-ILD may be used to aid clinicians in identifying that subset of patients who are at highest risk for progression of ILD and aiding management decisions, and serve as a tool for counseling both patients and their families.
Acknowledgments
Grants/Financial Supports: This study was made possible by the CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: The authors have no relevant conflicts of interest to disclose
References
- 1.Suzuki A, Ohosone Y, Obana M, Mita S, Matsuoka Y, Irimajiri S, et al. Cause of death in 81 autopsied patients with rheumatoid arthritis. J Rheumatol. 1994;21(1):33–6. [PubMed] [Google Scholar]
- 2.Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken) 2013;65(8):1243–50. doi: 10.1002/acr.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle JJ, Eliasson AH, Argyros GJ, Dennis GJ, Finger DR, Hurwitz KM, et al. Prevalence of pulmonary disorders in patients with newly diagnosed rheumatoid arthritis. Clin Rheumatol. 2000;19(3):217–21. doi: 10.1007/s100670050160. [DOI] [PubMed] [Google Scholar]
- 4.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62(6):1583–91. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee HK, Kim DS, Yoo B, Seo JB, Rho JY, Colby TV, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127(6):2019–27. doi: 10.1378/chest.127.6.2019. [DOI] [PubMed] [Google Scholar]
- 7.Nannini C, Ryu JH, Matteson EL. Lung disease in rheumatoid arthritis. Curr Opin Rheumatol. 2008;20(3):340–6. doi: 10.1097/BOR.0b013e3282f798ed. [DOI] [PubMed] [Google Scholar]
- 8.Saketkoo LA, Mittoo S, Huscher D, Khanna D, Dellaripa PF, Distler O, et al. Connective tissue disease related interstitial lung diseases and idiopathic pulmonary fibrosis: provisional core sets of domains and instruments for use in clinical trials. Thorax. 2014;69(5):428–36. doi: 10.1136/thoraxjnl-2013-204202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assayag D, Elicker BM, Urbania TH, Colby TV, Kang BH, Ryu JH, et al. Rheumatoid arthritis-associated interstitial lung disease: radiologic identification of usual interstitial pneumonia pattern. Radiology. 2014;270(2):583–8. doi: 10.1148/radiol.13130187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solomon JJ, Chung JH, Cosgrove GP, Demoruelle MK, Fernandez-Perez ER, Fischer A, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J. 2016;47(2):588–96. doi: 10.1183/13993003.00357-2015. [DOI] [PubMed] [Google Scholar]
- 11.Ley B, Collard HR, King TE., Jr Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183(4):431–40. doi: 10.1164/rccm.201006-0894CI. [DOI] [PubMed] [Google Scholar]
- 12.Nadrous HF, Pellikka PA, Krowka MJ, Swanson KL, Chaowalit N, Decker PA, et al. Pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Chest. 2005;128(4):2393–9. doi: 10.1378/chest.128.4.2393. [DOI] [PubMed] [Google Scholar]
- 13.Weill D, Benden C, Corris PA, Dark JH, Davis RD, Keshavjee S, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Phillips K, Flaherty KR, Matteson EL, Bongartz T, Bathon J, Brown KK, et al. Interstitial Lung Disease in Rheumatoid Arthritis. Current Rheumatology Reviews. 2010;6:120–6. [Google Scholar]
- 16.Turesson C, Jacobsson L, Bergstrom U. Extra-articular rheumatoid arthritis: prevalence and mortality. Rheumatology (Oxford) 1999;38(7):668–74. doi: 10.1093/rheumatology/38.7.668. [DOI] [PubMed] [Google Scholar]
- 17.Tsuchiya Y, Takayanagi N, Sugiura H, Miyahara Y, Tokunaga D, Kawabata Y, et al. Lung diseases directly associated with rheumatoid arthritis and their relationship to outcome. Eur Respir J. 2011;37(6):1411–7. doi: 10.1183/09031936.00019210. [DOI] [PubMed] [Google Scholar]
- 18.Cottin V, Le Pavec J, Prevot G, Mal H, Humbert M, Simonneau G, et al. Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35(1):105–11. doi: 10.1183/09031936.00038709. [DOI] [PubMed] [Google Scholar]
- 19.Keser G, Capar I, Aksu K, Inal V, Danaoglu Z, Savas R, et al. Pulmonary hypertension in rheumatoid arthritis. Scand J Rheumatol. 2004;33(4):244–5. doi: 10.1080/03009740410005809. [DOI] [PubMed] [Google Scholar]