Abstract
Objectives
To determine the risk of incident cartilage damage in unaffected subregions when one tibiofemoral compartment has a full-thickness vs. partial-thickness focal defects in knees with and without radiographic osteoarthritis.
Methods
The Multicenter Osteoarthritis Study participants with semiquantitative MRI readings at baseline and 30-month were included. We estimated the risk of incident cartilage defects developing in tibiofemoral compartments with prevalent partial-thickness and full-thickness cartilage defects in a subregion within the compartment, using tibiofemoral compartments with no baseline cartilage defects as reference. Logistic regression with generalized estimating equations was used for all analyses with adjustments for confounders.
Results
374 compartments (359 knees) were included, of which 140 knees (39%) had radiographic osteoarthritis. Compared to compartments with no baseline cartilage defects, those with partial-thickness (aOR 1.62, 95%CI 1.06-2.47) and full-thickness cartilage defects (aOR 1.92, 95%CI 1.00-3.66) in a subregion had higher risk for incident cartilage defects in other subregions in the same compartment.
Conclusions
Prevalent focal cartilage defects, regardless of defect depth, in a single subregion within a tibiofemoral joint compartment increase the risk for development of new cartilage damage in other subregions of the same tibiofemoral joint compartment for middle-aged to elderly persons with or at high risk of knee osteoarthritis.
Keywords: osteoarthritis, knee, magnetic resonance imaging, epidemiology, cartilage
Prevalent cartilage defect is one of the risk factors for further cartilage loss [1,3]. For example, one study showed prevalent cartilage defect predicts patellofemoral and tibiofemoral cartilage loss in the same subregion over a 6-month period [1]. Two longitudinal studies with a 2-year follow-up period showed that the presence of partial thickness medial tibiofemoral cartilage defects identifies asymptomatic individuals at risk of knee cartilage loss in the absence of radiographic knee osteoarthritis, and that prevalent knee cartilage defects are predictive of compartment-specific cartilage volume loss over 2 years with a dose-response association [2,13]. Another recent study based on the Multicenter Osteoarthritis Study (MOST) cohort demonstrated prevalent small (<1 cm in width) focal cartilage defects at baseline was associated with increased risk of subsequent cartilage loss in subregions of the knee compared with subregions without any baseline cartilage defects [3]. However, what remains to be determined is the relevance of the depth of small focal cartilage defect in the setting of development of new cartilage damage in a knee compartment.
A small, focal cartilage defect does not cause joint space narrowing on radiography and therefore can be present in radiographically normal knees [4,5]. These small lesions can potentially be treated by cartilage repair surgery [6]. However, a question remains whether a focal partial-thickness cartilage defect is as relevant to osteoarthritis disease progression as a full-thickness lesion. A recent study using data from the Osteoarthritis Initiative and semiquantitative MRI analysis of cartilage defects showed the presence of full-thickness cartilage defects (as opposed to partial-thickness cartilage defects only) was associated with knee replacement surgery at a later time point [7].
Thus, we aimed to determine the risk of incident cartilage damage at follow-up in any non-damaged subregions at baseline, comparing tibiofemoral joint compartments with a baseline focal partial or full-thickness cartilage defect against compartments without any baseline cartilage damage as referent.
PATIENTS AND METHODS
Study subjects
Subjects were participants in the Multicenter Osteoarthritis Study (MOST), a prospective study of 3,026 persons aged 50-79 years with a goal of identifying risk factors for incident and progressive knee osteoarthritis in a sample either with osteoarthritis or at high risk of developing the disease. Subjects were recruited from two communities in the United States, Birmingham, Alabama and Iowa City, Iowa. Details of subject inclusion, exclusion and recruitment have been described previously [8,9]. The study protocol was approved by the institutional review boards at the University of Iowa, University of Alabama, Birmingham, University of California, San Francisco and Boston University Medical Campus, and written informed consent was obtained from all participants.
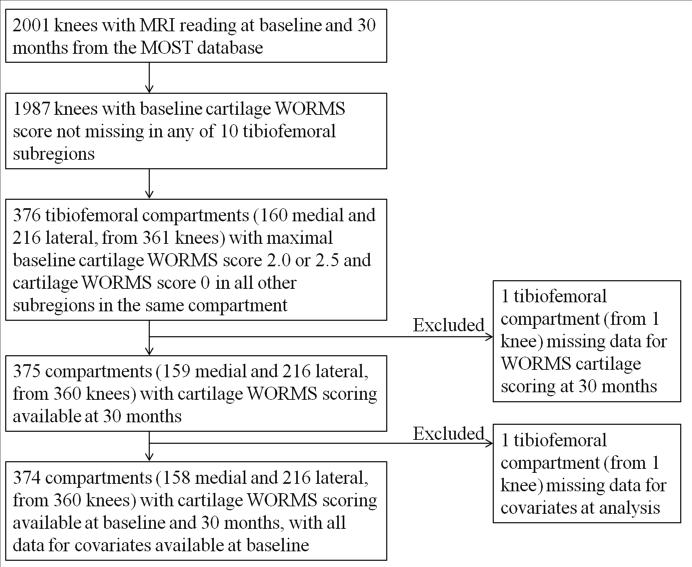
We included only tibiofemoral joint compartments that had one subregion with a small (<1cm in width) focal full thickness or partial thickness cartilage defect at baseline and all other subregions within the same compartment having no cartilage damage at baseline. The inclusion and exclusion process is summarized in Figure 1.
Figure 1.
Flowchart showing the subject inclusion and exclusion process for the compartment-based analysis.
Radiographs
All subjects underwent weight-bearing posteroanterior fixed flexion knee radiographs using a positioning frame (SynaFlexer™) [10] at baseline. A musculoskeletal radiologist (a non-author) and a rheumatologist (DTF), blinded to clinical data, graded radiographs according to the Kellgren-Lawrence (KL) grade [11], followed by an adjudication process (by two non-authors and DTF).
Full-limb radiographs of both legs were obtained at baseline. The mechanical axis was defined as the angle formed by the intersection of a line from the center of the head of the femur to the center of the tibial spines and a line from the center of the talus to the center of the tibial spines. Varus alignment was defined as a hip-knee-ankle (HKA) angle <179°; 179-181° was considered neutral and valgus alignment was defined as an HKA angle>181°.
MRI Acquisition
In the MOST parent study, MRI was performed using a 1.0 T extremity-based OrthOne scanner (Oni Inc, Wilmington, MA). Images were acquired using a circumferential extremity coil using fat-suppressed, fast spin echo, proton density-weighted sequence in two planes, sagittal (TR=4800 ms, TE=35 ms, 3.0mm slice thickness, 0mm interslice gap, FOV 14×14cm, matrix 288×192, NEX2); and axial (TR=4700 ms, TE=13.2 ms, 3.0mm slice thickness, 0mm interslice gap, FOV 14cm, matrix 288×192, NEX2) and a short tau inversion recovery sequence (STIR) in the coronal plane (TR=7820 ms, TE=14 ms, TI=100 ms, 3.0mm slice thickness, 0mm interslice gap, FOV 14cm, matrix 256×256, NEX2).
MRI Interpretation
MRI readings were performed independently by two musculoskeletal radiologists (AG, FWR), with 13 and 11 years of experience respectively in semiquantitative MRI assessment of knee OA. Images were assessed using eFilm™ software (Version 2.0.0, Merge Healthcare, Milwaukee, WI).
Baseline focal cartilage defects were defined according to the Whole Organ MRI Score (WORMS) system. Grade 2 represents focal partial-thickness cartilage defects <1cm in greatest width. Grade 2.5 represents focal full-thickness cartilage defects <1cm in greatest width.
As covariates for the statistical analyses, the following MRI features were also scored using WORMS. Hoffa synovitis was defined as abnormal hyperintensity within the Hoffa's fat pad and graded 0-3 (0=normal, 1=mild, 2=moderate, 3=severe). Effusion-synovitis was graded 0-3 based on the degree of maximal distension of the synovial cavity (0=normal, 1= <33%, 2= 33-66%, 3= >66% of maximum potential distention). Meniscal damage was graded 0-1 according to severity (0=normal, 1=minor radial tear or parrot-beak tear, 2=non-displaced tear or prior surgical repair, 3=displaced tear or partial resection, 4=complete maceration/destruction). Meniscal extrusion was scored either present (grade 1) or absent (grade 0). Subchondral bone marrow lesions (BMLs) were scored from 0 to 3 based on the extent of subregional involvement (0 = none; 1 = <25% of the subregion; 2 = 25-50%; 3 = >50%).
Statistical analysis
We included tibiofemoral joint compartments with one subregion grade 2.0 or 2.5 cartilage damage at baseline and all other subregions within the same compartment having no morphologic cartilage damage (grade 0). Compartments with maximum cartilage damage grade greater than 2.5 at baseline were not included in our study. Risk of incident cartilage loss (=development of new cartilage damage grade ≥2.0) at follow-up in any non-damaged subregions at baseline was determined, comparing tibiofemoral joint compartments with a baseline grade 2.0 or 2.5 cartilage damage against compartments without any baseline cartilage damage (grade 0 and 1) as referent. We also directly compared risk of incident cartilage loss in any non-damaged subregions for tibiofemoral joint compartments with baseline focal full-thickness (grade 2.5) cartilage damage vs. compartments with baseline focal partial-thickness (grade 2.0) cartilage damage. The weighted kappa coefficients for interobserver reliability for readers who performed the MRI semiquantitative readings using WORMS were 0.78 for cartilage defect assessment in the MOST study, as reported previously [12].
For all analyses, we employed logistic regression with generalized estimating equations to account for correlations among multiple subregions/compartments within a knee. Adjustments were performed for potential confounders, including baseline body mass index (BMI), age, gender, radiographic KL grading, malalignment, effusion-synovitis, Hoffa-synovitis, meniscal damage and extrusion, and BMLs. We also performed an additional analysis excluding subjects with radiographic joint space narrowing (KL grade 3 and 4) to see if the presence of joint space narrowing affects the results of our analysis. All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC, USA).
RESULTS
Table 1 summarizes demographic characteristics of included subjects. 374 compartments (359 knees) were included. 140 knees (39%) had radiographic osteoarthritis (KL grade ≥2). Compared to compartments with no baseline cartilage damage (grade 0 or 1) as reference, those with both partial-thickness and full-thickness cartilage defects had significantly higher risk for incident cartilage damage in the other subregions at follow-up (aOR 1.62, 95%CI 1.06-2.47 for partial-thickness defects, and aOR 1.92, 95%CI 1.00-3.66 for full-thickness defects, Table 2). There was no significant difference between compartments that had partial-thickness or full-thickness cartilage defects at baseline for risk of incident cartilage damage in the other subregions in the same tibiofemoral joint compartment at follow-up (aOR 1.26, 95%CI 0.59-2.70 for full-thickness cartilage defects, compared against partial-thickness cartilage defects as reference). An additional analysis excluding subjects with KL grade 3 and 4 did not significantly change our results (Table 2).
Table 1.
Summary of demographic characteristics of included subjects in the compartment-based analysis.
| Baseline Characteristics | Included in compartment-level analysis (N=359 knees) | ||
|---|---|---|---|
| TOTAL | Cartilage grade 2.0 (N=265) | Cartilage grade 2.5 (N=94) | |
| Age [Mean (SD)] | 61.3 (7.6) | 61.3 (7.7) | 61.3 (7.2) |
| Gender: Female [%] | 197 (55%) | 138 (52%) | 59 (63%) |
| Gender: Male [%] | 162 (45%) | 127 (48%) | 35 (37%) |
| Race [%White] | 318 (89%) | 239 (90%) | 79 (84%) |
| Clinic site: UAB [%] | 176 (49%) | 128 (48%) | 48 (51%) |
| Clinic site: Iowa [%] | 183 (51%) | 137 (52%) | 46 (49%) |
| BMI [Mean (SD)] | 29.8 (4.6) | 29.6 (4.5) | 30.4 (4.8) |
| KL grade | |||
| 0 | 162 (45%) | 122 (46%) | 40 (43%) |
| 1 | 57 (16%) | 44 (17%) | 13 (14%) |
| 2 | 83 (23%) | 56 (21%) | 27 (29%) |
| 3 | 39 (11%) | 27 (10%) | 12 (13%) |
| 4 | 18 (5%) | 16 (6%) | 2 (2%) |
| Any Effusion-synovitis (grade ≥1) | 231 (60%) | 175 (66%) | 56 (60%) |
| Any Hoffa-synovitis (grade ≥1) | 201 (56%) | 149 (56%) | 52 (55%) |
| Any meniscal damage and/or extrusion (grade ≥1) | 179 (50%) | 134 (51%) | 45 (48%) |
| Any bone marrow lesion (grade ≥1) | 294 (82%) | 214 (80.8%) | 80 (85%) |
| Malalignment | |||
| Varus (<179°) | 212 (59%) | 160 (60%) | 52 (55%) |
| Neutral (179-181°) | 43 (12%) | 27 (10%) | 16 (17%) |
| Valgus (>181°) | 104 (29%) | 78 (30%) | 26 (28%) |
Revised Table 2.
Risk for incident cartilage damage in the same tibiofemoral joint compartment at 30 months (including and excluding KL3 and KL4 subjects)
| Cartilage morphology status of a subregion within the same tibiofemoral joint compartment at baseline | Incident cartilage damage in the same tibiofemoral joint compartment at 30 months n/N (Number of compartments) | Crude odds ratio | Adjusted odds ratio | ||
|---|---|---|---|---|---|
| Odds ratio | 95% CI | Odds ratio | 95% CI | ||
| INCLUDING KL3 and KL4 subjects | |||||
| No focal cartilage defect (Grade 0) | 123/1668 (7%) | 1.00 (ref) | - | 1.00 (ref) | - |
| Partial-thickness cartilage defect (Grade 2.0) | 32/278 (12%) | 1.63 | (1.08, 2.48) | 1.62 | (1.06, 2.47) |
| Full-thickness cartilage defect (Grade 2.5) | 13/96 (14%) | 1.97 | (1.06, 3.64) | 1.92 | (1.00, 3.66) |
| EXCLUDING KL3 and KL4 subjects | |||||
| No focal cartilage defect (Grade 0) | 107/1485 (7%) | 1.00 (ref) | - | 1.00 (ref) | - |
| Partial-thickness cartilage defect (Grade 2.0) | 26/235 (11%) | 1.60 | (1.01-2.54) | 1.59 | (1.00-2.54) |
| Full-thickness cartilage defect (Grade 2.5) | 12/82 (15%) | 2.21 | (1.16-4.21) | 2.13 | (1.08-4.20) |
DISCUSSION
Our study showed that prevalent focal cartilage defects increased the risk for the development of incident cartilage damage in the same tibiofemoral joint compartment, regardless of defect depth. Partial-thickness and full-thickness defects are similarly relevant in regard to cartilage damage development in knee osteoarthritis.
Multiple studies have shown that prevalent cartilage damage is associated with worsening of existing cartilage damage assessed semiquantitatively and quantitatively [1,2,3,9,13,14]. However, it has not been well established if the depth of focal cartilage defect affects development of incidental cartilage damage in a knee compartment.
In osteoarthritis research, studies of focal cartilage defects are relevant as they are frequently present in knees with no or minimal radiographic evidence of knee osteoarthritis. A recent population based study showed that MRI-detected partial thickness (grade 2.0) and full thickness (grade 2.5) focal cartilage defects are present within the medial central subregion of the femur in 13.7% (95/696) of radiographically normal knees with KL grade 0 and 1 [4].
It is potentially important to detect focal cartilage defects early as there are various options for repair of focal cartilage defects [6]. MRI is an imaging tool that can noninvasively detect focal partial-thickness and full-thickness cartilage defects [5]. This opens up a possibility that MRI can be used to screen persons with early stage osteoarthritis or those at high risk of osteoarthritis and initiate treatment for focal cartilage defects. It has been shown that the presence of MRI-detected partial-thickness medial tibiofemoral cartilage defects identifies healthy individuals most likely to lose knee cartilage in the absence of radiographic knee osteoarthritis and that interventions aimed at reducing or reversing cartilage defects may reduce the risk of subsequent knee osteoarthritis [2]. Evaluation of focal cartilage defects is not possible with radiography, the current gold standard for osteoarthritis imaging.
Our study focused on semiquantitative assessment of MRI-detected cartilage damage. Various studies have shown quantitative cartilage morphometry is an important alternative to semiquantitative analysis and it is not yet known if depth of focal cartilage damage at baseline affects cartilage volume or thickness loss over time. It is important to note that quantitative MRI technique is limited in its ability to assess focal cartilage defects, and thus semiquantitative analysis is the method of choice for partial-thickness and full-thickness focal cartilage defect evaluation on MRI [15,16].
Our study population consisted of those who had very little cartilage damage based on MRI findings. However 39% of subjects had radiographic osteoarthritis, implying those participants either had definite osteophytes (giving rise to KL grade 2, 23% of subjects) without joint space narrowing, or had joints space narrowing secondary to meniscal damage/extrusion without much cartilage damage (KL grade 3 and 4, total 16% of subjects) [17]. Lack of arthroscopic confirmation of focal cartilage defect is a limitation of our study, however due to epidemiological nature of the MOST study, it is not feasible to obtain arthroscopic images.
In conclusion, prevalent focal cartilage defects increase the risk for development of incident cartilage damage in the same tibiofemoral joint compartment regardless of defect depth in middle-aged to elderly persons who have or are at high risk of knee osteoarthritis. Our findings imply that even sub-centimeter partial-thickness cartilage defects are as relevant as full-thickness defects for development of new cartilage defects in osteoarthritis disease process.
Acknowledgments
Funding: The MOST study is supported by National Institutes of Health (NIH) grants from the National Institute on Aging to Drs Lewis (U01-AG-18947), Torner (U01-AG-18832), Nevitt (U01-AG-19069), and Felson (U01-AG-18820) and NIH AR47785.
Footnotes
Authorship criteria:
Criteria 1a – AG, DH, FWR, JN, DTF
Criteria 1b – AG, FWR, MDC, MCN, JT, CEL, DTF
Criteria 1c – AG, DH, FWR, JN, EKQ, MCN, DTF
Criteria 2 – AG, DH, FWR, JN, EKQ, MDC, MCN, JT, CEL, DTF
Criteria 3 - AG, DH, FWR, JN, EKQ, MDC, MCN, JT, CEL, DTF
Competing Interests: Ali Guermazi is the president of Boston Imaging Core Lab, LLC (BICL), Boston, MA, a company providing radiological image assessment services. He is a consultant to MerckSerono, Astra Zeneca, OrthoTrophix, and TissueGene. Frank W Roemer and Michel D Crema are shareholders of BICL. None of the other authors have declared any possible conflict of interest.
Ethics approval: This study was conducted with the approval of the institutional review board at Boston University School of Medicine.
REFERENCES
- 1.Roemer FW, Kwoh CK, Hannon MJ, et al. Risk factors for magnetic resonance imaging-detected patellofemoral and tibiofemoral cartilage loss during a six-month period: the joint on glucosamine study. Arthritis Rheum. 2012;64:1888–98. doi: 10.1002/art.34353. [DOI] [PubMed] [Google Scholar]
- 2.Cicuttini F, Ding C, Wluka A, Davis S, Ebeling PR, Jones G. Association of cartilage defects with loss of knee cartilage in healthy, middle-age adults: a prospective study. Arthritis Rheum. 2005;52:2033–9. doi: 10.1002/art.21148. [DOI] [PubMed] [Google Scholar]
- 3.Roemer FW, Felson DT, Wang K, et al. Co-localisation of non-cartilaginous articular pathology increases risk of cartilage loss in the tibiofemoral joint--the MOST study. Ann Rheum Dis. 2013;72:942–8. doi: 10.1136/annrheumdis-2012-201810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi D, Felson DT, Niu J, Hunter DJ, Roemer FW, Aliabadi P, et al. Pre-radiographic osteoarthritic changes are highly prevalent in the medial patella and medial posterior femur in older persons: Framingham OA study. Osteoarthritis Cartilage. 2014;22:76–83. doi: 10.1016/j.joca.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guermazi A, Niu J, Hayashi D, Roemer FW, Englund M, Neogi T, et al. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: population based observational study (Framingham Osteoarthritis Study). BMJ. 2012;345:e5339. doi: 10.1136/bmj.e5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guermazi A, Roemer FW, Alizai H, Winalski CS, Welsch G, Brittberg M, et al. State of the Art: MR Imaging after Knee Cartilage Repair Surgery. Radiology. 2015;277:23–43. doi: 10.1148/radiol.2015141146. [DOI] [PubMed] [Google Scholar]
- 7.Roemer FW, Kwoh CK, Hannon MJ, Hunter DJ, Eckstein F, Wang Z, et al. Can structural joint damage measured with MRI imaging be used to predict knee replacement in the following year? Radiology. 2015;274:810–20. doi: 10.1148/radiol.14140991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–2992. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 9.Roemer FW, Zhang Y, Niu J, Lynch JA, Crema MD, Marra MD, et al. Tibiofemoral joint osteoarthritis: risk factors for MR-depicted fast cartilage loss over a 30-month period in the multicenter osteoarthritis study. Radiology. 2009;252:772–780. doi: 10.1148/radiol.2523082197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterfy CG, Guermazi A, Zaim PF. Non-fluoroscopic method for flexed radiography of the knee that allows reproducible joint-space width measurement. Arthritis Rheum. 1998;41:S361. [Google Scholar]
- 11.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guermazi A, Hayashi D, Roemer F, Felson DT, Wang K, Lynch J, et al. Severe radiographic knee osteoarthritis – does Kellgren and Lawrence grade 4 represent end stage disease? – the MOST study. Osteoarthritis Cartilage. 2015;23:1499–505. doi: 10.1016/j.joca.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding C, Cicuttini F, Scott F, Boon C, Jones G. Association of prevalent and incident knee cartilage defects with loss of tibial and patellar cartilage: a longitudinal study. Arthritis Rheum. 2005;52:3918–27. doi: 10.1002/art.21474. [DOI] [PubMed] [Google Scholar]
- 14.Carnes J, Stannus O, Cicuttini F, Ding C, Jones G. Knee cartilage defects in a sample of older adults: natural history, clinical significance and factors influencing change over 2.9 years. Osteoarthritis Cartilage. 2012;20:1541–7. doi: 10.1016/j.joca.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 15.Hunter DJ, Zhang W, Cohaghan P G, Hirko K, Menashe L, et al. Responsiveness and reliability of MRI in knee osteoarthritis: a meta-analysis of published evidence. Osteoarthritis Cartilage. 2011;19:589–605. doi: 10.1016/j.joca.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guermazi A, Roemer FW, Haugen IK, Crema MD, Hayashi D. MRI-based semiquantitative scoring of joint pathology in osteoarthritis. Nat Rev Rheumatol. 2013;9:236–51. doi: 10.1038/nrrheum.2012.223. [DOI] [PubMed] [Google Scholar]
- 17.Hunter DJ, Zhang YQ, Tu X, Lavalley M, Niu JB, Amin S, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54:2488–95. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]