Abstract
Aim
Diabetic foot ulcers are associated with an increased risk of death. We evaluated whether ulcer severity at presentation predicts mortality.
Methods
Patients from a national, retrospective, cohort of veterans with type 2 diabetes who developed incident diabetic foot ulcers between January 1, 2006 and September 1, 2010, were followed until death or the end of the study period, January 1, 2012. Ulcers were characterized as early stage, osteomyelitis, or gangrene at presentation. Cox proportional hazard regression identified independent predictors of death, controlling for comorbidities, laboratory parameters, and healthcare utilization.
Results
66,323 veterans were included in the cohort and followed for a mean of 27.7 months: 1-, 2-, and 5-year survival rates were 80.80%, 69.01% and 28.64%, respectively. Compared to early stage ulcers, gangrene was associated with an increased risk of mortality (HR 1.70, 95% CI 1.57 – 1.83, p<0.001). The magnitude of this effect was greater than diagnosed vascular disease, i.e., coronary artery disease, peripheral arterial disease, or stroke.
Conclusion
Initial diabetic foot ulcer severity is a more significant predictor of subsequent mortality than coronary artery disease, peripheral arterial disease, or stroke. Unrecognized or under-estimated vascular disease and/or sepsis secondary to gangrene should be explored as possible causal explanations.
Keywords: diabetes, foot ulcer, gangrene, mortality
1. INTRODUCTION
Half of patients who develop a diabetic foot ulcer will die within five years (Boyko et al., 1996; Martins-Mendes et al., 2014; Walsh et al., 2015). Most often, they succumb to vascular complications, namely heart attacks and strokes (Boyko et al., 1996; Moulik et al., 2003; Morbach et al., 2012). However, recent cohort studies concluded that foot ulcers alone remain a significant independent predictor of mortality, even after adjusting for known vascular disease and other comorbidities (Martins-Mendes et al., 2014; Walsh et al., 2015). We hypothesized that the subgroup of patients with severe diabetic foot ulcers, namely those complicated by osteomyelitis or gangrene, would have the highest associated mortality among patients with diabetic foot ulcers. This was based upon the finding that severe ulcers are associated with increased morbidity, especially major (above ankle) amputation (Prompers et al., 2008a; Helmer et al., 2011; Peters et al., 2001). Therefore, our aim was to determine whether the severity of incident diabetic foot ulcers at presentation predicts subsequent mortality.
2. SUBJECTS, MATERIALS AND METHODS
2.1 Study Design and Subjects
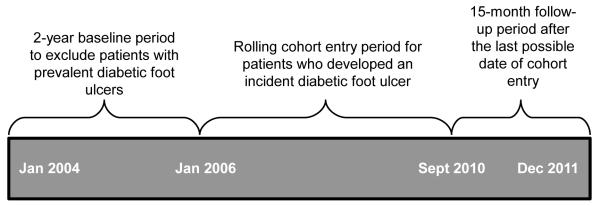
We constructed a retrospective, rolling cohort of all patients with type 2 diabetes and incident diabetic foot ulcers receiving care through the U.S. Department of Veterans Affairs (VA) national healthcare system. It included data from the VA National Patient Care Database, the VA Decision Support System Pharmacy Datasets, and the Centers for Medicare and Medicaid Services Medicare claims files, including Part D Event files, available through the VA Information Resources Center. International Statistical Classification of Disease and Related Health Problems, version 9, codes (ICD-9 codes) used to generate variables are listed in the Appendix. Patients were identified as having diabetes if they received at least one prescription for a diabetes medication in 2004 or if two or more ICD-9 codes for diabetes were present in VA in-patient or out-patient visits in 2004 or 2005, the two-year baseline period (Miller et al., 2004). To further increase specificity by excluding patients likely to have type 1 diabetes, those younger than 40 years or on insulin monotherapy were not included. Those with stage 5 chronic kidney disease (estimated glomerular filtration rate [eGFR] <15) were excluded because it falsely lowers hemoglobin A1C and cholesterol values. Finally, to ensure that the cohort was limited to patients with incident diabetic foot ulcers, we excluded patients with a foot ulcer diagnosis during the baseline period (Figure 1; Finke et al., 2010; Sohn et al., 2010; Dubskẏ et al., 2013).
Figure 1.
Timeline depicting rolling cohort study design.
Patients entered the cohort at the time of incident foot ulcer diagnosis, beginning January 1, 2006, and ending September 1, 2010. Patients were followed until death or the end of the study period, January 1, 2012 (Figure 1). This study was approved by the institutional review boards of the Edward J. Hines Jr. and William S. Middleton Memorial Veterans Hospitals.
2.2 Independent Variables
Ulcer severity at the time of diagnosis was categorized as early stage, osteomyelitis, or gangrene based on validated ICD-9 codes (Finke et al., 2010; Sohn et al., 2010). Early stage ulcers were defined as uncomplicated or those with a diagnosis of infection limited to the skin and soft tissue, such as cellulitis. We chose not to separate skin and soft tissue infections into their own disease severity category due to concerns that this level of infection may be difficult to distinguish from non-infected wounds that were associated with bacterial colonization or dependent rubor in the outpatient setting. The following demographics, comorbidities, and healthcare utilization measures were assessed during the baseline period: age, sex, race, marital status, peripheral neuropathy, coronary artery disease, peripheral arterial disease and any previous vascular procedures, stroke, chronic kidney disease, eye disease, foot deformity, number of out-patient and emergency room visits, and number of hospitalizations (Newton et al., 1999). Hemoglobin A1C, total cholesterol, low density lipoprotein cholesterol (LDL-c) and out-patient systolic blood pressures were averaged over the 12-month period preceding the initial ulceration. Statin use, defined as possession of at least a 30-day supply, was also assessed in the 12-month period preceding the ulceration.
2.3 Dependent Variable
Time to death was measured as the number of days from incident diabetic foot ulcer to death (Sohn et al., 2006).
2.4 Statistical Analysis
Kaplan-Meier survival curves, stratified by ulcer severity, were used to compare unadjusted differences in survival. Differences in survival curves were assessed using the log rank test. To identify the most parsimonious model for predicting survival, stepwise Cox proportional hazard models were constructed using forward selection with a p ≤ 0.25 level for entry into the model and p > 0.15 level for exclusion. To model both stringent and lax control being associated with mortality, total cholesterol, LDL-c, hemoglobin A1C and systolic blood pressure were allowed into the model with the possibility of a squared term (Kontopantelis et al., 2015). A post-hoc sensitivity analysis was conducted, excluding 13,639 patients identified by ICD-9 codes 707.9 (chronic skin ulcer NOS) and 707.10 (lower limb ulcer, unspecified) due to concerns these codes may identify patient with calf ulcers rather than diabetic foot ulcers. SAS version 9.3 (SAS Institute Inc., Cary, NC) was used.
3. RESULTS
We identified 66,323 patients with type 2 diabetes and an incident foot ulcer. At the time of diagnosis, osteomyelitis and gangrene complicated 4.42% and 3.03% of the wounds. Nearly half the cohort had known coronary artery disease, and 15.33% were diagnosed with peripheral arterial disease. Ulcerated patients were followed from initial diagnosis for a mean of 27.72 months (Table 1). The 1-, 2-, and 5-year survival rates were as follows: 80.80%, 69.01%, and 28.94%.
Table 1.
Patient Characteristics, stratified by ulcer severity*
| Characteristic | Total cohort (n= 66,323) |
Early stage ulcer (n= 61,383) |
Osteomyelitis (n= 2,930) |
Gangrene (n= 2,010) |
|---|---|---|---|---|
| Age, years | 69 (10.38) |
69 (10.37) |
66 (10.45) |
69 (10.08) |
| Male | 65,280 (98.43) |
60,406 (98.41) |
2,888 (98.57) |
1,986 (98.81) |
| Race | ||||
| White | 52,837 (79.67) |
49,251 (80.24) |
2,163 (73.82) |
1,423 (70.80) |
| Black | 8,904 (13.43) |
7,952 (12.95) |
519 (17.71) |
433 (21.54) |
| Other | 4,582 (6.91) |
4,180 (6.81) |
248 (8.46) |
154 (7.66) |
| Hispanic | 4,107 (6.19) |
3,734 (6.08) |
229 (7.82) |
144 (7.16) |
| Married | 41,305 (62.28) |
38,485 (62.70) |
1,667 (56.89) |
1,153 (57.36) |
| Comorbidities | ||||
| Peripheral neuropathy | 9,937 (14.98) |
9,291 (15.14) |
402 (13.72) |
244 (12.14) |
| Coronary artery disease | 31,998 (48.25) |
29,840 (48.61) |
1,185 (40.44) |
973 (48.41) |
| Peripheral arterial disease | 10,300 (15.53) |
9,455 (15.40) |
366 (12.49) |
479 (23.83) |
| History of stroke | 4,817 (7.26) |
4,448 (7.25) |
175 (5.97) |
194 (9.65) |
| History of vascular procedur | 508 (0.77) |
446 (0.73) |
16 (0.55) |
46 (2.29) |
| History of eye disease | 16,775 (25.29) |
15,464 (25.19) |
799 (27.27) |
512 (25.47) |
| Foot deformity | 1,445 (2.18) |
1,347 (2.19) |
69 (2.35) |
29 (1.44) |
| CKD stage 1a | 9,033 (15.31) |
8,277 (15.17) |
498 (18.74) |
258 (14.47) |
| CKD stage 2 | 28,161 (47.72) |
26,062 (47.75) |
1,317 (49.57) |
782 (43.86) |
| CKD stage 3 | 19,908 (33.73) |
18,512 (33.92) |
773 (29.09) |
623 (34.94) |
| CKD stage 4 | 1,916 (3.52) |
1,727 (3.16) |
69 (2.60) |
120 (6.73) |
| Healthcare utilization | ||||
| Outpatient visits | 18.01 (15.73) |
18.15 (15.68) |
15.33 (15.46) |
17.60 (17.13) |
| Emergency room visits | 1.49 (2.46) |
1.47 (2.42) |
1.55 (3.17) |
1.86 (2.43) |
| Hospitalizations | 0.71 (1.27) |
0.71 (1.27) |
0.63 (1.11) |
1.04 (1.53) |
| Statin use | 46,322 (69.84) |
42,987 (70.03) |
1,961 (66.93) |
1,374 (68.36) |
| Systolic blood pressure, mmHgb | 134 (15.38) |
134 (15.37) |
134 (14.72) |
135 (16.52) |
| Hemoglobin A1C, %c | 7.60 (1.60) |
7.58 (1.56) |
7.88 (1.74) |
7.76 (1.76) |
| Total cholesterol, mg/dLd | 159 (36.36) |
159 (36.18) |
163 (38.12) |
164 (39.42) |
| LDL cholesterol, mg/dLe | 86 (29.76) |
86 (29.62) |
89 (31.42) |
90 (32.01) |
| Follow-up, months | 27.72 (18.72) |
28.44 (18.72) |
27.36 (18.60) |
20.52 (18.60) |
| Died during follow-up | 33,554 (50.59) |
30,911 (50.36) |
1,332 (45.46) |
1,311 (65.22) |
Data are presented as counts (%) or means (SD).
Of the entire cohort, 7,305 (11.01%) patients did not have a serum creatinine measurement from which to calculate CKD stage. The percent of missing values for those presenting with early stage ulcers, osteomyelitis and gangrene were: 11.09%. 9.32%, and 11.29%, respectively.
Of the entire cohort, 4,916 (7.41%) patients did not have a systolic blood pressure measurement. The percent of missing values for those presenting with early stage ulcers, osteomyelitis and gangrene were: 7.31%, 7.34%, and 10.50%, respectively.
Of the entire cohort, 11,674 (17.60%) patients did not have a hemoglobin A1C value. The percent of missing values for those presenting with early stage ulcers, osteomyelitis, and gangrene were: 17.42%, 16.45%, and 24.93%, respectively.
Of the entire cohort, 14,244 (21.48%) patients did not have a total cholesterol value. The percent of missing values for those presenting with early stage ulcers, osteomyelitis and gangrene were: 21.25%, 20.92%, and 29.35%, respectively.
Of the entire cohort, 14,708 (22.18%) patients did not have a low density lipoprotein (LDL) cholesterol value. The percent of missing values for those presenting with early stage ulcers, osteomyelitis and gangrene were: 21.92%, 22.08%, and 30.00%, respectively.
Compared to the entire cohort, patients presenting with gangrene had a higher frequency of diagnosed peripheral arterial disease (15.53% versus 23.83%) and prior vascular procedures (0.77% versus 2.29%; Table 1). This subset also included a larger proportion of minorities. They were engaged in the healthcare system, with similar mean numbers of out-patient visits and slightly higher rates of emergency room utilization and hospitalizations. Despite healthcare engagement and similar proportions of statin use, a larger percentage of this subset had missing values for systolic blood pressures and laboratory parameters than the entire cohort (Table 1 footnote).
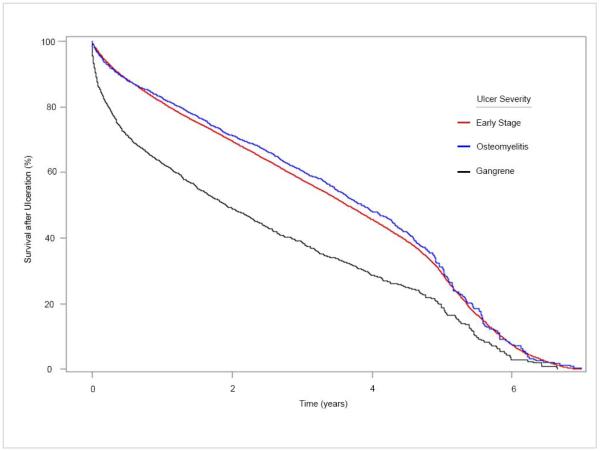
Of the 2,010 patients presenting with gangrene, the 1-, 2-, and 5-year survival rates were 62.67%, 48.95%, and 18.82%, respectively. The Kaplan-Meier survival curve for those presenting with gangrene was significantly steeper than for those with early stage ulcers or osteomyelitis (log rank p<0.001; Figure 2). This was particularly true for the first year following ulceration, after which the lines ran relatively parallel to one another.
Figure 2.
Kaplan-Meier survival curves following incident diabetic foot ulcer diagnosis, stratified by ulcer severity.
In a multivariate stepwise Cox proportional hazard model, the hazard ratio for death among patients with gangrene was 1.70 (95% CI 1.57 – 1.83, p<0.001; Table 2) compared to those with early stage ulcers. This effect was higher than that for known coronary artery disease, stroke, or peripheral arterial disease (Table 2). The hazard ratio for osteomyelitis was also elevated at 1.09 (95% CI 1.02 – 1.17, p = 0.014) compared to early stage ulcers. Seventy percent of the cohort used statins in the year prior to ulceration, which was protective (HR 0.89, 95% CI 0.86 – 0.92, p<0.001). Neither total cholesterol nor LDL-c were included in the final model after eliminating variables. Hemoglobin A1C and systolic blood pressure were adjusted for in the final analysis and modeled with a U-shaped curve, although their associations with death were not statistically significant (Table 2 footnote). In the sensitivity analysis, the hazard ratio for death among patients with gangrene remained robust (HR 1.67 with 95% CI 1.55 – 1.81, p<0.001) compared to those with early stage ulcers. The hazard ratio for osteomyelitis bordered on statistical significance, with a hazard ratio of 1.07 (95% CI 0.999 – 1.15, p=0.055).
Table 2.
Adjusted hazard ratios for death following incident diabetic foot ulcer diagnosis, modeled using stepwise Cox proportional hazards*
| Characteristic | Hazard Ratio [95% CI] | p value |
|---|---|---|
| Foot ulcer severity (reference= early stage ulcer) | ||
| Osteomyelitis (reference = early stage ulcer) | 1.09 (1.02 – 1.17) | 0.014 |
| Gangrene (reference = early stage ulcer) | 1.70 (1.57 – 1.83) | <0.0001 |
| Covariates | ||
| Additional year of age | 1.04 (1.04 – 1.04) | <0.0001 |
| Male | 1.45 (1.26 – 1.66) | <0.0001 |
| Black (reference = White) | 0.97 (0.92 – 1.01) | 0.150 |
| Other race (reference = White) | 0.92 (0.87 – 0.97) | 0.003 |
| Married | 0.98 (0.97 – 0.99) | 0.004 |
| Peripheral neuropathy | 0.94 (0.91 – 0.98) | 0.002 |
| Coronary artery disease | 1.14 (1.11 – 1.18) | <0.0001 |
| Peripheral arterial disease | 1.09 (1.05 – 1.13) | <0.0001 |
| History of stroke | 1.14 (1.09 – 1.20) | <0.0001 |
| Foot deformity | 0.87 (0.79 – 0.95) | 0.003 |
| Additional unit increase in eGFRa | 0.99 (0.99 – 0.99) | <0.0001 |
| Additional outpatient visit | 1.01 (1.01 – 1.01) | <0.0001 |
| Additional emergency room visit | 1.02 (1.02 – 1.03) | <0.0001 |
| Additional hospitalization | 1.12 (1.11 – 1.14) | <0.0001 |
| Statin use | 0.89 (0.86 – 0.92) | <0.0001 |
Hazard ratios were also adjusted for systolic blood pressure and hemoglobin A1C, both of which were best modeled using base and square terms, generating a U-shaped relationship with time-to-death. The mathematical model for systolic blood pressure (SBP) was as follows: ln (HRSBP) = −0.0000106(SBP – 144) + 0.0002577(SBP – 144)2. The standard error for the base term was 0.0005777, and p-value was 0.9854. The standard error for the squared term was 0.0000172, and p-value was <0.0001. The mathematical model for hemoglobin A1C (A1C) was as follows: ln (HRA1C) = −0.00167(A1C – 5.9) + 0.00935(A1C – 5.9)2. The standard error for the base term was 0.01073, and p-value was 0.8765. The standard error for the squared term was 0.00191, and p-value was <0.0001.
eGFR= estimated glomerular filtration rate, measured in mL/min/1.73m2
4. DISCUSSION
Diabetic foot ulcers are increasingly recognized as a marker for significantly elevated mortality. Half the cohort died during a mean follow-up of more than two years, similar to previous reports (Boyko et al., 1996; Martins-Mendes et al., 2014; Walsh et al., 2015). In our cohort, roughly 5% presented with osteomyelitis and 3% had gangrene upon initial diagnosis. The proportion with osteomyelitis is on par or lower than previous reports (Sohn et al., 2013; Lavery et al., 2009). One reason it may be lower than other estimates is that we assessed severity at the time of initial diagnosis only rather than following patients over time and recording the maximal disease severity. Our results show that advanced ulceration, especially gangrene, was associated with an increased hazard ratio for death. This association remained after controlling for known vascular disease, which is associated with both diabetic foot ulcers and death, and other risk factors for mortality. Our study expands upon a previous finding that foot ulceration was an independent predictor of death after controlling for known vascular disease (Walsh, et al., 2015).
The Kaplan-Meier curves can be used to generate hypothesis as to why gangrene was such a strong predictor of death. The immediate decline in the Kaplan-Meier curve following ulcer diagnosis suggested a possible role for acute sepsis secondary to wet gangrene. Prior studies examining cause of death substantiated this possibility, with approximately 14% of deaths in patients with diabetic foot ulcers attributed to sepsis (Brownrigg et al., 2014; Morbach et al., 2012). The gangrene curve (black) continued to decline more precipitously than that for early stage ulcers (red) or osteomyelitis (blue) after these initial months and up to approximately one year (Figure 2). We hypothesized that this continued decline is due to deaths from vascular disease, including heart attacks and strokes. The hypothesis was based upon 1) previous cohorts identifying vascular disease as the most common cause of death in patients with diabetic foot ulcers, 2) the fact that gangrene is often an advanced manifestation of peripheral arterial disease which, in turn, has been linked to advanced cardiovascular disease, and 3) known under-diagnosis of vascular disease in patients with advanced diabetic foot ulcers (Boyko et al., 1996; Moulik et al., 2003; Morbach et al., 2012; Prompers et al., 2007; Prompers et al., 2008b). Clinically unrecognized disease would not be measured when vascular disease is determined by diagnostic codes. This suggests an important gap in care, rather than a study limitation alone.
Our patient characteristic data support the concern for under-diagnosis of peripheral arterial disease. Less than a quarter of those presenting with gangrene were previously identified as having peripheral arterial disease. Furthermore, these patient’s records often lacked laboratory measurements needed to optimize medical management of vascular disease and diabetes: 30% did not have an LDL-c value, and 25% lacked a hemoglobin A1C. This gap cannot be entirely explained by a lack of healthcare utilization, as these patients engaged with the VA healthcare system about 20 times a year. A substantial group of patients presenting with gangrene are missing quality metrics of care, despite seeing physicians prior to presenting with gangrene. Reasons for this discrepancy need to be explored. While there may be residual confounding resulting from these missing values and under-diagnosis of vascular disease, the current model is still useful because it estimates mortality based on data available to clinicians and researchers at the time of ulcer diagnosis. The discrepancy in missing data compounds that of under-diagnosed vascular disease, suggesting the gap in care for these patients is substantial.
The association between gangrene and death is important for at least two reasons. First, it offers a potential causal link between foot ulcers and death. Second, it allows clinicians and researchers to readily identify a subset of patients with diabetic foot ulcers who are at a high risk of early death. On an individual patient-provider level, this allows clinicians to better prognosticate and proactively address impending mortality (Robbins JM J Am Pod Med Ass 2008). If our hypothesis regarding residual confounding by unrecognized or underestimated vascular disease is substantiated, it suggests that this sub-population may benefit from aggressive diagnosis and management of vascular disease. Efforts to predict who will develop gangrenous foot ulcers, and address these problems prior to ulceration, would be ideal.
Our finding that statin use was protective against death warrants further exploration as a possible way to curb the mortality associated with diabetic foot ulcers. This drug class has already been recommended for all patients with diabetes given the disease is a myocardia infarct equivalent (Stone et al., 2014). However, only 70% of our cohort used statins in the year preceding foot ulceration, and other studies have also reported suboptimal use (Sohn et al., 2016; Gyberg et al., 2015; Lin et al., 2016). A single center study comparing the survival of patients with diabetic foot ulcers before and after implementing an aggressive cardiovascular management program, including statin use, demonstrated a significant decrease in four-year mortality from 43% to 22% (Young et al., 2008). Statins have also been associated with a reduced risk of major amputation in a large cohort study (Sohn et al., 2013). These findings suggest initiating statins at the time of foot ulcer diagnosis may have a positive effect on both morbidity and mortality; despite the high associated mortality with foot ulceration, it may not be too late in the disease course to improve both outcomes.
In our model, a foot deformity was protective. While counter-intuitive, a possible explanation is that patients with these diagnoses were receiving higher quality care than those without (e.g., they were referred to podiatrists for diabetic foot care, who subsequently coded for these problems). Alternatively, they could represent patients with few serious comorbidities, as their clinicians had time to address foot deformity and peripheral neuropathy at clinic visits rather than higher priority issues. For example, a physician caring for a patient with serious multimorbidity may not be able to address preventative foot care in a brief clinic visit because they were focused on managing active COPD, cancer, and anticoagulation issues. When prospectively assessed, Charcot foot deformity was not found to be independently associated with death (Morbach et al., 2012).
Our study has a number of strengths. First, we were able to study the full spectrum of diabetic foot ulcer disease from the VA national population with diabetes and avoid the selection bias inherent in cohorts generated from referral centers specializing in advanced diabetic foot ulcer care. Second, we controlled for statin use, systolic blood pressure, and hemoglobin A1C, an unusual ability in large, claims-based data. Third, we included measures of healthcare utilization. To our knowledge, this is the first multivariate model predicting mortality among patients with diabetic foot ulcers and controlling for healthcare utilization. As expected, patients with more hospitalizations and emergency room visits had an increased risk of death. Including this covariate was particularly important when studying incident ulcer severity: it decreased the possibility that gangrene was associated with death solely because of confounding by a lack of medical care.
Our study also has limitations. We were unable to control for smoking, a factor linked to both diabetic foot ulcers and mortality. It is possible that unmeasured smoking biased our estimated hazard ratio for ulcer severity. However, because a previous model found foot ulceration to be an independent predictor of death after controlling for smoking, residual confounding due to smoking is unlikely to fully explain the measured association between foot ulcer severity and mortality (Walsh et al., 2015). Second, we were unable to study the cause of death among our cohort. This would have provided additional insight regarding the role of sepsis and vascular disease in the causal pathways between gangrene and subsequent death. Third, claims data limits the ability to characterize the severity of coronary artery disease, peripheral arterial disease, and stroke. Without this detail, we may have overstated the relative strength of association between gangrene and death compared to diagnosed vascular disease. Lastly, although reflective of the veteran population, our cohort was predominantly male. This may impact the generalizability of our findings to women with diabetic foot ulcers. However, it is unlikely that the association between ulcer severity and death would be influenced by sex, especially in post-menopausal women.
4.1 Conclusion
In conclusion, ulcer severity can be used to predict subsequent mortality associated with diabetic foot ulcers. Those presenting with gangrene are at particularly high risk of death. This may be due to a combination of immediate complications from sepsis, as well as unrecognized or underestimated vascular disease. Further studies should explore ways to reduce this risk, specifically focusing on the potential protective role of statins in this population.
ACKNOWLEDGEMENTS AND FUNDING
The authors wish to thank Dr. Laura Hogan for her editorial assistance.
The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. Additional funding was through the NIH grant numbers KL2TR000428, K24DK105340, and P30DK092949] and AHRQ grants R01 HS018542 and R01 HS018368. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH, AHRQ, or VA.
APPENDIX
International Statistical Classification of Disease and Related Health Problems, version 9, codes (ICD-9 codes) used to generate variables for the current study
| Variable | ICD-9 codes |
|---|---|
| Diabetes | 250.xx |
| Foot ulcer severity | |
| Early stage foot ulcer | 440.23, 707.1x, 707.9 |
| Osteomyelitis | 730.07, 730.17, 730.27, 730.97 |
| Gangrene | 0.40.0, 440.24, and 785.4 but only if at least one of the following vascular disease codes is also present: 250.7, 440.2, 440.21, 440.22, 440.23 |
| Comorbidities | |
| Peripheral neuropathy | 250.6x, 355.xx, 337.1, 357.2 |
| Coronary artery disease | 410.xx, 411.1x, 427.4, 427.5 |
| Peripheral arterial disease | 250.7, 443.9 |
| History of stroke | 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.1, 434.11, 434.9, 434.91, 436, 431, 430 |
| History of vascular procedure | 440.20 – 440.24 |
| History of eye disease | 250.x, 362.01 – 362.067, 362.83, 366.41, 369.xx |
| Foot deformity | 94.0, 713.5, 727.1, 735.0, 735.2, 735.4 – 735.9 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Boyko EJ, Ahroni JH, Smith DG, Davignon D. Increased mortality associated with diabetic foot ulcer. Diabet Med. 1996;13(11):967–72. doi: 10.1002/(SICI)1096-9136(199611)13:11<967::AID-DIA266>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Brownrigg JR, Griffin M, Hughes CO, Jones KG, Patel N, Thompson MM, Hinchliffe RJ. Influence of foot ulceration on cause-specific mortality in patients with diabetes mellitus. J Vasc Surg. 2014;60(4):982–6. doi: 10.1016/j.jvs.2014.04.052. [DOI] [PubMed] [Google Scholar]
- Dubskẏ M, Jirkovská A, Bern R, Fejfarová V, Skibová J, Schaper NC, Lipsky BA. Risk factors for recurrence of diabetic foot ulcers: prospective follow-up analysis in the Eurodiale subgroup. Int Wound J. 2013;10(5):555–61. doi: 10.1111/j.1742-481X.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finke BG, Miller DR, Turpin R. A classification of diabetic foot infections using ICD-9-CM codes: application to a large computerized medical database. BMC Health Serv Res. 2010;10:192–200. doi: 10.1186/1472-6963-10-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyberg V, Kotseva K, Dallongeville J, Backer GD, Mellbin L, Rydén L, Wood D, Bacquer DD. Euroaspire Study Group. Does pharmacologic treatment in patients with established coronary artery disease and diabetes fulfill guideline recommended targets? Eur J Prev Cardiol. 2015;22(6):753–61. doi: 10.1177/2047487314529353. [DOI] [PubMed] [Google Scholar]
- Helmer D, Tseng CL, Wrobel J, Tiwari A, Rajan M, Pogach L, Sambamoorthi U, Feinglass J. Assessing the risk of lower extremity amputations using an administrative data-based foot risk index in elderly patients with diabetes. J Diabetes. 2011;3(3):248–55. doi: 10.1111/j.1753-0407.2011.00135.x. [DOI] [PubMed] [Google Scholar]
- Kontopantelis E, Springate DA, Reeves D, Ashcroft DM, Rutter M, Buchan I, Doran T. Glucose, blood pressure and cholesterol levels and their relationship to clinical outcomes in type 2 diabetes: a retrospective cohort study. Diabetologia. 2015;58(3):505–18. doi: 10.1007/s00125-014-3473-8. [DOI] [PubMed] [Google Scholar]
- Lavery LA, Peters EJG, Armstrong DG, Wendel CS, Murdoch DP, Lipsky BA. Risk factors for developing osteomyelitis in patients with diabetic foot wounds. 2009;83(3):347–52. doi: 10.1016/j.biabres.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Lin I, Sung J, Sanchez RJ, Mallya UG, Friedman M, Panaccio M, Koren A, Neumann P, Menzin J. Patterns of statin use in a real-world population of patients at high cardiovascular risk. J Manag Care Spec Pharm. 2016;22(6):685–98. doi: 10.18553/jmcp.2016.22.6.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins-Mendes D, Monteiro-Soares M, Boyko EJ, Ribeiro M, Barata P, Lima J, Soares R. The independent contribution of diabetic foot ulcer on lower extremity amputation and mortality risk. J Diabetes Complications. 2014;28(5):632–8. doi: 10.1016/j.jdiacomp.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- Morbach S, Furchert H, Gröblinghoff U, Hoffmeier H, Kersten K, Klauke GT, Klemp U, Roden T, Icks A, Haastert B, Rümenapf G, Abbas ZG, Bharara M, Armstrong DG. Long-term prognosis of diabetic foot patients and their limbs: amputation and death over the course of a decade. Diabetes Care. 2012;35(10):2021–7. doi: 10.2337/dc12-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulik PK, Mtonga R, Gill GV. Amputation and mortality in new-onset diabetic foot ulcers stratified by etiology. Diabetes Care. 2003;26(2):491–9. doi: 10.2337/diacare.26.2.491. [DOI] [PubMed] [Google Scholar]
- Newton KM, Wagner EH, Ramsey SD, McCulloch D, Evans R, Sandhu N, Davis C. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol. 1999;52(3):199–207. doi: 10.1016/s0895-4356(98)00161-9. [DOI] [PubMed] [Google Scholar]
- Peters EJ, Childs MR, Wunderlich RP, Harkless LB, Armstrong DG, Lavery LA. Functional status of persons with diabetes-related lower extremity amputations. Diabetes Care. 2001;24(10):1799–804. doi: 10.2337/diacare.24.10.1799. [DOI] [PubMed] [Google Scholar]
- Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Dauricio D, Ragnerson Tennvall G, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, van Baal J, van Merode F, Schaper N. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia. 2007;50(1):18–25. doi: 10.1007/s00125-006-0491-1. [DOI] [PubMed] [Google Scholar]
- a.Prompers L, Schaper N, Apelqvist J, Edmonds M, Jude E, Mauricio D, Uccioli L, Urbancic V, Bakker K, Holstein P, Jirkovska A, Piaggesi A, Ragnarson-Tennvall G, Reike H, Spraul M, Van Acker K, Van Baal J, Van Merode F, Ferreira I, Huijberts M. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The Eurodiale Study. Diabetologia. 2008;51(5):747–55. doi: 10.1007/s00125-008-0940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- b.Prompers L, Huijberts M, Apelqvist J, Jude E, Piaggesi A, Bakker K, Edmonds M, Holstein P, Jirkovska A, Mauricio D, Tennvall GR, Reike H, Spraul M, Uccioli L, Urbancic V, Van Acker K, van Baal J, van Merode F, Schaper N. Delivery of care to diabetic patients with foot ulcers in daily practice: results of the Eurodiale Study, a prospective cohort study. Diabet Med. 2008;25(6):700–7. doi: 10.1111/j.1464-5491.2008.02445.x. [DOI] [PubMed] [Google Scholar]
- Robbins JM, Strauss G, Aron D, Long J, Kuba J, Kaplan Y. Mortality rates and diabetic foot ulcers: is it time to communicate mortality risk to patients with diabetic foot ulceration? J Am Podiatr Med Assoc. 2008;98(6):489–93. doi: 10.7547/0980489. [DOI] [PubMed] [Google Scholar]
- Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Pop Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M, Budiman-Mak E, Stuck RM, Siddiqui F, Lee TA. Diagnostic accuracy of existing methods for identifying diabetic foot ulcers from inpatient and outpatient datasets. J Foot Ankle Res. 2010;3:27–32. doi: 10.1186/1757-1146-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MW, Meadows JL, Oh EH, Budiman-Mak E, Lee TA, Stone NJ, Pearce WB. Statin use and lower extremity amputation risk in nonelderly diabetic patients. J Vasc Surg. 2013;58(6):1578–1585. doi: 10.1016/j.jvs.2013.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn MW, Cooper J, Brennan M, Kang H, Mason-Lobo J, Basu A, Huang ES. Compliance with ADA recommended statin use among persons with diabetes. Presented at the American Diabetes Association 76th scientific sessions; New Orleans, LA. June 13, 2016. [Google Scholar]
- Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr., Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovasc RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr., Tomaselli GF, American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2015 doi: 10.1111/dme.13054. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Young MJ, McCardle JE, Randall LE, Barclay JI. Improved survival of diabetic foot ulcer patients 1995-2008: possible impact of aggressive cardiovascular risk management. Diabetes Care. 2008;31(11):2143–7. doi: 10.2337/dc08-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]