Abstract
Perlecan, a large basement membrane heparan sulfate proteoglycan, is expressed in a wide array of tissues where it regulates diverse cellular processes including bone formation, inflammation, cardiac development, and angiogenesis. Here we provide a contemporary review germane to the biology of perlecan encompassing its genetic regulation as well as an analysis of its modular protein structure as it pertains to function. As perlecan signaling from the extracellular matrix converges on master regulators of autophagy, including AMPK and mTOR, via a specific interaction with vascular endothelial growth factor receptor 2, we specifically focus on the mechanism of action of perlecan in autophagy and angiogenesis and contrast the role of endorepellin, the C-terminal fragment of perlecan, in these cellular and morphogenic events.
Keywords: Angiogenesis, Autophagy, Endorepellin, Heparan sulfate, Proteoglycan
Introduction
Proteoglycans, ubiquitous residents of the extracellular matrix, participate in a range of both structural and signaling roles in order to maintain cellular homeostasis [1]. Proteoglycans participate in the genesis and maintenance of the extracellular milieu, the complex meshwork of proteins comprising multicellular organisms [2,3]. An example of proteoglycan versatility comes in the form of perlecan, an immense heparan sulfate proteoglycan primarily localized to basement membranes and pericellular spaces [4–9]. The modular structure of perlecan enables homeostatic regulation within a vast array of cellular processes including cell adhesion [10,11], endocytosis [12], bone and cartilage formation [13–16], lipid metabolism [17], peripheral node assembly [18], inflammation and wound healing [19,20], thrombosis [21], cancer angiogenesis [9,11,22–33], cardiovascular development [34], and autophagy [35,36]. Given the gargantuan structure and multitude of functions of perlecan, it is no surprise that its gene, HSPG2, is similarly large and transcriptionally controlled by complex promoter interactions.
In this review, we will provide a current analysis of the biology of perlecan focusing on its genetic regulation and protein structure as well as its functional role in physiological processes. In particular, we will examine the dichotomy between perlecan and its C-terminal fragment, endorepellin, in coordinating angiogenesis and autophagy as well as provide commentary on the emerging paradigm regarding the connection between these two vital cellular pathways.
Form follows function: Overview of the perlecan gene and protein structure
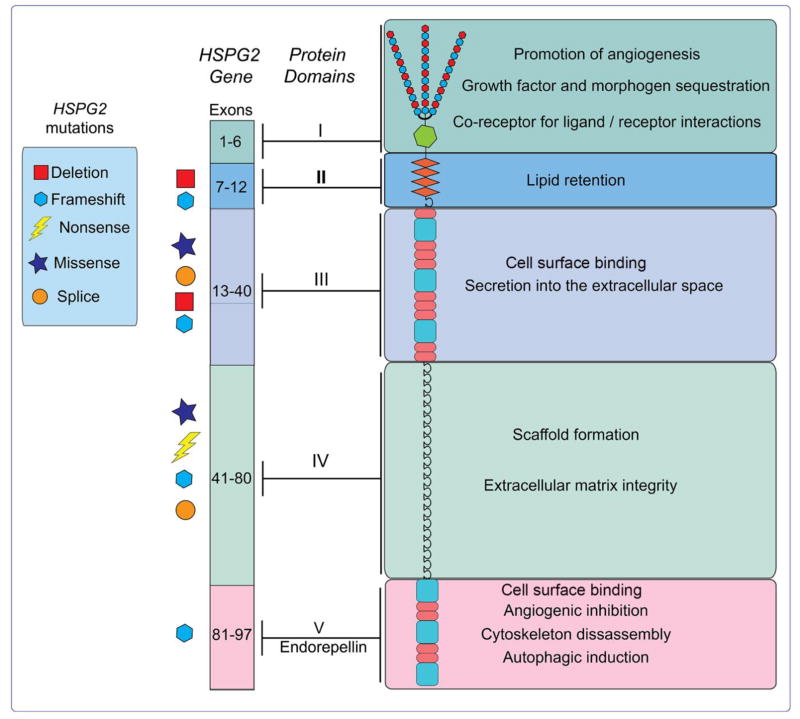
Perlecan is one of the largest proteoglycans discovered, possessing a protein core of approximately 500 kDa that can be modified by the addition of N-terminal heparan sulfate (HS) side chains, measuring ~65 kDa each [37]. The protein core is divided into several unique structural regions, each imparting distinct biofunctional diversity to perlecan [1,38]. The complexity and sheer size of the core protein is rivaled only by the modular nature [39] and tight regulation of the gene encoding this key proteoglycan. Here, we will discuss the genetics of perlecan as well as analyze the structure of each intra-protein domain and ascribed functions (Fig. 1). A more extensive description of the role of specific perlecan protein modules in angiogenesis and autophagy will be considered later.
Fig. 1.
Graphical representation of the human perlecan gene, HSPG2, and protein. Reported disease-causing mutations in the gene are symbolically depicted to the left of the exons. Individual domains of the protein are highlighted and grouped with their corresponding functions.
Genomic organization and transcriptional regulation of perlecan
The gene encoding perlecan, HSPG2, spans over 120 Kb and encompasses 97 exons [39,40] and is highly conserved across species [41]. The genomic DNA is organized in a modular fashion, with specific exons corresponding to distinct protein domains conventionally found in other basement membrane constituents (Fig. 1). Further, this exonic organization is evolutionarily conserved for the corresponding regions seen in homologous HSPG2 genes [40], suggesting a common ancestor arising from gene duplication and exon shuffling. Structurally, HSPG2 is one of a subset of so-called TATA-less driven-genes and is seemingly regulated by a variety of housekeeping transcription factors (e.g. Sp1 and ETF) while also being responsive to growth factor-controlled gene induction. This response is evidenced by HSPG2 transcriptional activation downstream of canonical TGF-β signaling via direct binding of nuclear factor-1 to its promoter [42] and transcriptional suppression mediated by IFN-γ [43]. Moreover, transcriptional activation by NFκB enhances HSPG2 gene expression in the desmoplastic prostate cancer microenvironment [44]. Due to this regulatory circuit, perlecan is considered an early response gene. Additionally, consistent with the lack of a traditional TATA-box, the perlecan promoter harbors multiple transcriptional start sites scattered over an 80-bp region of genomic DNA [40].
Complementing the transcriptional complexity mentioned above, the perlecan pre-mRNA is subject to alternative splicing, as splice variants occur in the HMC-1 human mast cell line [19]. Interestingly, these shorter perlecan isoforms encode biologically active endorepellin [20], an effective anti-angiogenic and pro-autophagic proteolytic cleavage product.
This complex regulatory scheme underscores the dynamic expression profile for perlecan in various tissues throughout development [15,45–48] and in several pathological processes [29]. These areas include vascularized and non-vascularized regions as well as the connective tissue stroma [38,49]. This expression pattern leads to several downstream functional consequences. For example, as it is found ubiquitously within a diverse population of basement membranes [8,50], perlecan aids in the formation and maintenance of polarized epithelial cells, chiefly at the apical cell surface [27]. In addition, perlecan takes part in a variety of other functions such as the modulation of vascular endothelial cell adhesion [10] and proliferation [51], modulation of growth factor signaling [11,52–56], maintenance of biomechanical properties and cartilage homeostasis [57–59], skeletal muscle and cardiovascular development [34], and skin and endochondral bone formation [60,61]. These actions occur, in part, by the ability of perlecan to regulate the bioavailability of growth factors resulting in the modification of the bioactivity of various signaling pathways. Moreover, perlecan is involved in regulating solute transport and mechanosensing in the osteocyte lacunar-canalicular system [62], and its protein core can withstand over 100 pN of tension, suggesting that perlecan could act as an elastic tether in the lacunar-canalicular system [63]. Collectively, these properties culminate in a multifaceted biological agent that has been implicated in diseases such as osteoarthritis [64], atherosclerosis [65,66], muscle hypertrophy [67] cancer progression [22,24,27,68,69], aging [70], and pulmonary hypertension [71]
Domain I: An N-terminal region exclusive to perlecan
The N-terminus of perlecan possesses the primary location for HS side-chain attachment. Indeed, this region of perlecan contains three areas comprising the signature Ser-Gly-Asp (SGD) sequence [37], an amino acid arrangement that resembles the putative glycosaminoglycan (GAG) consensus site (Fig. 1). Structurally, domain I contains no cysteine residues, consists of many acidic amino acids, and, unlike the other perlecan domains, does not include any known sequential repeats, such as EGF-like repeats [37]. Surprisingly, following in-depth analysis utilizing several known protein sequence databases, no homology occurs between this domain and any recognized motif in other proteins [37], conferring specificity for this region to perlecan. The notable caveat to this statement is the Sea urchin sperm protein, Enterokinase, and Agrin (SEA) module of domain I, which is shared by some other extracellular matrix proteins from which the name originates.
The HS side chains of domain I allow it to sequester and present growth factors to their appropriate receptors [52,72–74]. This role is quite important for establishing morphogen gradients required for developmental processes and regulating angiogenesis, which will be discussed in more detail in a subsequent section.
Domain II: A pro-atherosclerotic domain
Unlike domain I, which is unique to perlecan, domain II contains four cysteine-rich repeats resembling the sequence of the LDL-binding region of the LDL receptor [37,39,75] (Fig. 1). As such, it is not surprising that this domain of perlecan interacts with LDL in a glycosylation-dependent manner [76]. This finding denotes a role for perlecan in the retention of LDL in the arterial sub-endothelium, where expression levels of perlecan correlate well with the progression of atherosclerosis [77]. Also worthy of mention is the single Ig-like repeat reminiscent of the repetitive structure of N-CAM that separates domain II from domain III. Notably, several of these repeats are prominently featured later in domain IV. However, there is currently no known function for this region in domain II.
Domain III: Homologue of the short arm of laminin A
Domain III is divided into several segments including three laminin globular domains interspersed among 11 laminin-like EGF repeats [37,39] (Fig. 1). Given this information, it is predictable that domain III shares homology with the short arm of laminin A [78–80] affording a high degree of probability that these two proteins descended from a shared ancestor over a common evolutionary period.
Functionally, domain III may modulate efflux of perlecan into the extracellular space as evidenced by a recent case study of a patient with Schwartz-Jampel syndrome [81], a disease known to be linked to altered perlecan expression and characterized by a short stature phenotype as well as myotonia and chondrodysplasia [82]. Expression studies utilizing perlecan harboring the reported domain III-specific missense mutation revealed impaired secretion of perlecan into cell culture media [81], suggesting the importance of this domain for proper localization of perlecan to enable its function in downstream signaling cascades. Additionally, in mice, perlecan domain III contains an RGD sequence, implicating it in cell attachment [39]. Indeed, recombinant murine domain III promotes adhesion of epithelial-like mouse mammary tumor cells, which can be inhibited upon introduction of a synthetic RGDS peptide [83].
Domain IV: N-CAM immunoglobulin stretch
Penultimate domain IV is the largest, encompassing over 2000 amino acid residues to form 21 Ig-like repeats in human perlecan that are homologous to those found in N-CAM [37,39,84] (Fig. 1). It is important to note that there is inter-species variation in the number of these Ig-like repeats [75]. Interestingly, though the most common GAG chain attachment sites are found in domain I, there exists a prospective GAG chain attachment sequence in the 14th Ig-like repeat of human perlecan [75]. With regard to function, this sizable domain is hypothesized to act as a scaffold, where it could mediate a constellation of heterotypic interactions to sustain extracellular matrix assembly and architecture. Indeed, domain IV binds several structural matrix components including nidogen, fibronectin, and fibulin-2 [85]. Recently, it has been shown that fragments of domain IV are markedly elevated in the blood of patients with advanced prostate carcinomas, but absent in normal sera, suggesting that domain IV might be linked to advanced prostate cancer [86]. These elevated levels of domain IV correlate with increased levels of MMP-7 [86], an enzyme known to cleave perlecan [29].
Domain V: An entity known as endorepellin
The final domain of perlecan, christened endorepellin due to its ability to act as an independent entity to inhibit endothelial cell motility [25], contains three laminin globular domains separated by dual EGF-like repeats [37,39] (Fig. 1). Like domain III, these globular domains share similarity with laminin A [78,79]. These sub-domains allow endorepellin to function in dual receptor antagonism (see below). These concurrent actions culminate in decreased endothelial cell migration and, consequently, angiogenesis. Intriguingly, recent studies also show soluble endorepellin as a key autophagic inducer in endothelial cells [35].
Endorepellin is found in vivo in the blood and in several body fluids where it is liberated from perlecan by partial proteolysis of the protein core [20] via matrix metalloproteinases, a family of enzymes involved in a multitude of biological processes [87–92]. In addition, the LG3 fragment is found in circulation as both Cathepsin L and BMP-1-Tolloid-like proteases catalyze proteolytic cleavage from the C-terminus of endorepellin [20,93]. LG3 is increased in the urine of women with premature rupture of fetal membranes [94], patients with end-stage renal failure and chronic allograft rejection [95,96], and in the amniotic fluid of women bearing fetuses with trisomy 21 [97]. Furthermore, LG3 may act as a useful biomarker for disease detection, as low levels of LG3 are associated with progression and increased invasion of breast cancer [98]. LG3 can also modulate homing of mesenchymal stem cells and neointima formation during vascular rejection [99], and can evoke auto-antibody production and allograft inflammation [100].
Defective perlecan is coupled with genetic conditions penetrant in multiple model organisms
As mentioned above, perlecan is present as a single, unique gene and is highly conserved across several organisms including C. elegans (unc-52, uncoordinated phenotype 52), D. melanogaster (trol, terribly reduced optical lobes), D. rerio (Hspg2), M. musculus (Hspg2), and H. sapiens (HSPG2) (Table 1). Predominantly, perlecan is expressed and functions, both structurally and as a signaling node, within developing and mature cartilages [48,73,101–103]. Cumulatively, these findings establish a pivotal role for perlecan in cartilage formation, making it indispensable for life. Given its importance in cartilage and other tissues, mutations in perlecan result in several distinct phenotypes observed across a spectrum of different organisms (Table 1).
Table 1.
Tabulated snapshot of mutations of perlecan in various model organisms and resultant phenotypes and clinical manifestations
| Model organism | Gene symbol | Mutation | Phenotype | Reference |
|---|---|---|---|---|
| C. elegans | unc-52 | Chemical mutagenesis | Abnormal gonadal leader cell migration due to disruptions in the UNC-6/netrin system | [104] |
| Non-sense mutations in diverse splice variants | Systemic paralysis and alterations in muscle morphology | [106] | ||
| D. melanogaster | trol | P-element insertion | Neural proliferative failure and deficient neural stem cells deriving from disrupted FGF2 and Shh signaling pathways | [107,108] |
| D. rerio | Hspg2 | Morpholino-mediated knockdown | Myopathy, disorganized sarcomeres, reduced extension of vessel sprouts; mis- localized VEGFA | [34] |
| M. musculus | Hspg2 | Global knockout | Embryonic lethality due to respiratory failure, pericardial hemorrhage, transposition of great vessels, malformation of coronary arteries, chondrodysplasia | [112] |
| Hspg2 Δ3/Δ3 | Exon 3 deletion | Loss of proper corneal structure, arterial narrowing, delayed wound healing; impaired angiogenesis and tumorigenesis; reduced heparan sulfate content | [116–119] | |
| Hspg2−/−;Tg | Global knockout with re- introduction in cartilage to prevent lethality | Impaired vascular relaxation, enhanced unloading-induced atrophy, increased autophagy | [36,120] | |
| H. sapiens | HSPG2 | Duplicated exon 34 and frameshifts; functionally null | Dyssegmental dysplasia, Silver- Handmaker type due to altered FGF2 signaling leading to aberrant cartilage architecture | [121,123] |
| Missense and errors in alternative splicing resulting in truncated or ablated domain V | Schwartz-Jampel syndrome originating from disrupted neuromuscular junction arrangement and function | [82,122] |
In the most basic model organism, C. elegans, unc-52 mutations lead to aberrant gonadal leader cell migration via disrupted growth factor signaling (e.g. FGF, TGF-β, Wnt) operative during gonadogenesis [104]. As mentioned previously, there is evidence for alternative splicing of perlecan in mast cells [19]. Likewise, in C. elegans, unc-52 is heavily spliced and mediates spatiotemporal deposition and localization of perlecan [105]. Nonsense mutations occurring within these splice variants trigger progressive paralysis [106].
Moving to D. melanogaster, studies of trol reveal critical roles for perlecan in the proliferative activation of quiescent neuroblasts [107] and neural stem cell signaling [108] (Table 1). These functions seemingly depend on FGF and Shh pathways [109] inasmuch as reintroduction of FGF2 into trol mutants re-establishes the neural proliferative defect [109]. Therefore, trol may orchestrate key regulatory roles in neurogenesis and stem cell niches. This role is further reinforced by the requirement of trol in the proliferation of hematopoietic progenitor cells and with the prevention of premature hemocyte differentiation. Analogous to the FGF2 rescue, this phenotype can be reversed with exogenously expressed Hedgehog [110].
The comparatively more sophisticated model organism, D. rerio, also sustains the effects of aberrant perlecan expression (Table 1). Zebrafish morphants, created via morpholino-mediated knockdown of perlecan, demonstrate disorganized and reduced amounts of actin filaments leading to abnormal sarcomeres [34]. These structural alterations in the absence of perlecan result in severe myopathy. Furthermore, these same morphants do not demonstrate proper extension of aortic vessel sprouts resulting in abnormal angiogenesis and decreased circulation [34]. Moreover, morpholino-mediated knockdown of the major receptor for perlecan/endorepellin, the α2β1 integrin, causes an even more dramatic impairment of the intersegmental vessels which are normally generated by angiogenic sprouting from the dorsal aorta [111]. Taken together, the absence of perlecan and/or its cell surface receptor α2β1 in zebrafish demonstrate the necessity for this proteoglycan signaling axis in muscle development and angiogenesis.
The perlecan null mice (Hspg2−/−) are embryonic lethal [112]. Indeed, an estimated 50% of Hspg2−/− mice succumb to pericardial hemorrhage between embryonic days 10–12 [112] (Table 1). Due to the biomechanical properties ascribed to perlecan, it is thought that regions of the pericardial cavity basement membrane are significantly compromised, leading to deterioration and ultimately, mechanical breakdown [112]. As the perlecan null embryos reach more progressive stages of development, stark morphological complications of the cardiovascular system arise [113] including transposition of the great vessels, malformed coronary arteries, and irregular cardiac outflow tracts [113]. The few mice that do survive exhibit severe cartilage malformations and perish shortly after birth from respiratory failure [112,114]. Notably, some of these cardiac defects are similar to mutant mice lacking NDST-1, a key enzyme involved in the biosynthesis of heparan sulfate [115].
However, despite the embryonic lethality that follows from global perlecan deletion, there exist some viable mouse models that provide invaluable insight into the function of perlecan. The first transgenic model was generated by genomic deletion of exon 3 (designated as Hspg2 Δ3/Δ3), which encodes two out of the three SGD consensus sites for HS attachment in domain I [116]. These mice are fertile, despite having reduced amounts of HS, and show lens degeneration three weeks post birth [116], indicating a key role for the HS chains in lens capsule homeostasis [117]. Further, the HS-deficient mice have narrowing of an experimentally occluded carotid artery due to augmented intimal hyperplasia concomitant with smooth muscle cell proliferation [118], as well as impaired tumor angiogenesis, tumorigenesis, and delayed wound healing [119]. These latter deficits are consistent with the co-receptor roles perlecan plays within the tumor and stroma microenvironment. The second transgenic model features reconstituted Hspg2 expression specifically within the cartilage [120], obviating the lethality associated with cartilage failure in the Hspg2−/− mouse. The phenotype of these mice will be discussed in a later section due to its relevance to autophagy.
In humans, HSPG2 has been linked to two well-characterized autosomal recessive genetic diseases: lethal dyssegmental dysplasia, Silver-Handmaker type (DSSH) [121] and Schwartz-Jampel syndrome (SJS) [82,122] (Table 1). Clinically, DSSH patients exhibit anisospondyly, a marked difference in size and shape of the vertebral bodies, and micromelia, disproportionally short limbs. Genetically, truncated forms of perlecan, resulting from skipped exons caused by duplications in exon 34, frameshifts, and point mutations, are responsible for the clinical manifestations of this disease [123]. Intriguingly, these truncations generate a situation where perlecan is functionally null, as the shorter perlecan species are labile and quickly degraded. The end result is phenotypically akin to the Hspg2−/− mice [123] where loss of perlecan results in dysregulated FGF signaling, impaired cartilage architecture, and decreased chondrocyte proliferation [114,121,123].
In SJS, the prevalence of missense and alternative splicing mutations gives rise to a non-lethal muscle disorder [82] resulting in myotonia. These mutations either completely or partially (up to 64 amino acids) ablate domain V/endorepellin from the C-terminus of perlecan thereby rendering the secreted product non-functional or only partially functional [122]. Mechanistically, these deletions may affect the clustering and anchoring of multiprotein receptor complexes at neuromuscular junctions (i.e. acetylcholinesterase, acetylcholine receptor, MuSK [124]) and/or perturb association with adjacent proteoglycan scaffolds, such as agrin [125,126]. Loss of synaptic responsiveness, via defects in acetylcholine signaling or organization, could cause myotonia. Indeed, Hspg2−/− mice do not retain acetylcholinesterase at the neuromuscular junction [127].
Antithetic biological functions of perlecan and endorepellin
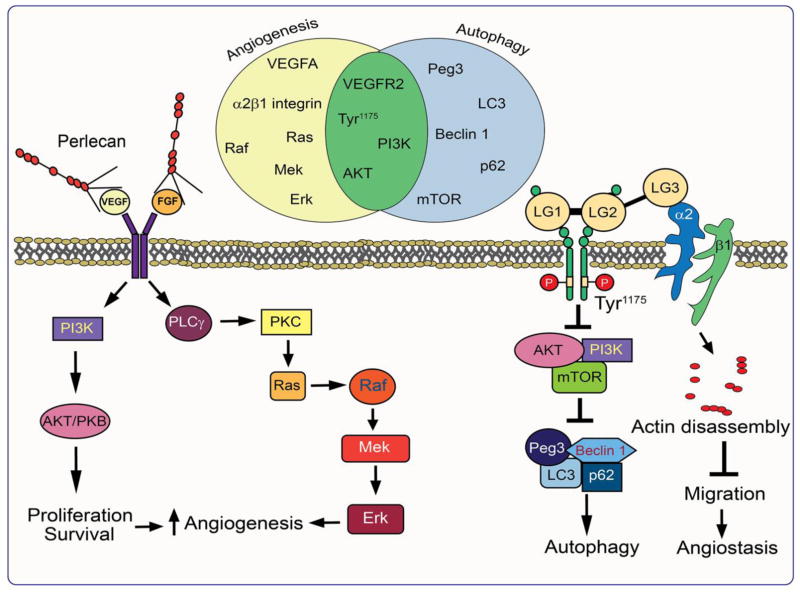
As perlecan is expressed in both vascular and avascular tissues, it follows that this proteoglycan is directly involved in a variety of cellular events including cell adhesion [10,11], thrombosis [21], and vascular development [34]. Though these examples emphasize cell signaling versatility, we will focus below on two hallmark processes in which the terminal domain of perlecan is strongly implicated: angiogenesis and autophagy (Fig. 2).
Fig. 2.
Schematic diagram demonstrating the mechanism of action of perlecan and endorepellin in angiogenesis and autophagy. Note the overlap between some of the signaling pathway intermediates as illustrated by the Venn diagram.
Perlecan is an archetypical HSPG required for bivalent angiogenic regulation
Perlecan intrinsically coordinates angiogenesis by harboring pro- and anti-angiogenic properties within the same molecule [128]. Most prominent are the intricate regulatory roles perlecan exerts over the VEGFA/VEGFR2 and α2β1 integrin signaling axes [69,129]. As a whole molecule, perlecan acts predominantly in a pro-angiogenic fashion (Fig. 2). The N-terminal HS side chains of domain I act as a reservoir for growth factors, which can then be presented in the proper arrangement to their respective cell surface receptors [52,72–74].
For example, perlecan induces neovascularization in a rabbit ear model of angiogenesis by promoting FGF2 binding to its receptor [130]. Furthermore, perlecan enhances VEGFA-mediated phosphorylation of VEGFR2 [131]. Additionally, mice lacking the HS attachment site of perlecan demonstrate compromised FGF2-mediated corneal angiogenesis [119], underscoring the importance of the N-terminal GAG chains for the regulation of this process. As mentioned above, morpholino-mediated knockdown of perlecan in zebrafish causes reduced extension of angiogenic sprouts from the dorsal aorta [34]. These perlecan morphants show aberrant distribution of pro-angiogenic VEGFA, which can be rescued by the addition of exogenous growth factor [131]. This information, combined with the knowledge that VEGF itself can stimulate the synthesis of perlecan [132], supports the view that there is an evolutionarily-conserved positive feedback loop for this proteoglycan in the angiogenic signaling cascade.
Given the aforementioned role of perlecan in developmental angiogenesis, it is not surprising that, in the setting of tumorigenesis, perlecan functions as a cornucopia of pro-angiogenic factors involved in the promotion of unrestrained neovascularization and tumorigenic growth [27]. Indeed, perlecan favors pathologic angiogenesis via direct interactions with a multitude of HS-binding angiokines such as progranulin, VEGFA, and PDGF, as well as several FGF superfamily members, including FGF2, FGF7, and FGF18 [55,133]. This mechanism allows perlecan to assume the role of co-receptor for presentation of the appropriate ligand to the cognate receptor for efficient and robust signaling responses, resulting in tumor progression [27].
In contrast to its N-terminus, the C-terminal domain V of perlecan, endorepellin, takes on an opposing role to the parent molecule: it inhibits angiogenesis, capillary morphogenesis, and endothelial cell migration [25,134,135] (Fig. 2). Specifically, soluble endorepellin is a dual receptor antagonist by acting as a molecular bridge for simultaneous ligation of the α2β1 integrin and VEGFR2 [136]. The discovery of “dual receptor antagonism” could be fundamental for understanding the mechanism through which endorepellin, and perhaps other angiostatic factors such as endostatin, would halt neovascularization.
At the receptor level, the N-terminal LG1/2 domains of endorepellin primarily interact with the VEGFR2 ectodomain in a region encompassing Ig3–5 domains. Meanwhile, the most C-terminal LG module, LG3, binds the α2 I-domain of the α2β1 integrin [129,137–139]. The formation of the heterotrimeric endorepellin-VEGFR2-α2β1 integrin complex brings the SHP-1 phosphatase, constitutively bound to the intracellular tail of α2 integrin subunit, in close functional juxtaposition with the cytoplasmic tails of VEGFR2 [140]. SHP-1-mediated dephosphorylation of VEGFR2 has dramatic consequences for the ternary complex including inactivation of downstream signaling effectors [141] and caveolin-mediated internalization [141]. Interestingly, the bioactivities of endorepellin can be physically separated insofar as the VEGFR2-binding domains (i.e. LG1/2) are sufficient and embody the properties necessary for attenuating downstream VEGFA signaling [138]. Likewise, purified LG3, the α2β1-binding region, is competent for dissolution of the actin cytoskeleton [128,138].
Mechanistically, dephosphorylation of VEGFR2, particularly at Tyr1175 [142], in a SHP-1-dependent manner removes a crucial recruitment site for Shb and PLC-γ nucleation [141], ultimately resulting in protracted angiogenic suppression. Downstream of VEGFR2 effector attenuation, pro-angiogenic HIF-1α/VEGFA signaling is potently abrogated in a non-canonical, oxygen-independent manner [141]. Further, NFAT nuclear translocation is significantly inhibited via PLC-γ attenuation, resulting in diminished calcineurin and PKC/JNK/AP1 activity, culminating in decreased VEGFA expression and secretion [136,141].
Cumulatively, these studies acknowledge contrasting roles for perlecan, whereby its N-terminus sequesters and presents growth factors to specific receptors to promote angiogenesis, while its C-terminus possesses a starkly anti-angiogenic function through its intrinsic property of dual receptor antagonism.
Divergent roles of perlecan and endorepellin in autophagy
In analogy with their roles in angiogenesis, whole perlecan and endorepellin once again find themselves in opposition in the regulation of autophagy, an essential cellular process encompassing the lysosomal breakdown of cellular components following isolation in double-membraned autophagosomes [143]. Studies utilizing transgenic mice that harbor expression of Hspg2 only in cartilage to ensure viability [112,114] depict perlecan as an autophagic inhibitor [36]. Indeed, perlecan-null soleus muscle demonstrates increased numbers of autophagosomes when compared to wild-type muscle [36]. Mechanistically, absence of perlecan results in inhibition of the canonical mTOR pathway, causing increased autophagic flux vis-a-vis wild-type mice [36]. Physiologically, perlecan expression is necessary to maintain muscle mass following tenotomy as its absence results in atrophy due to improper autophagic regulation [36].
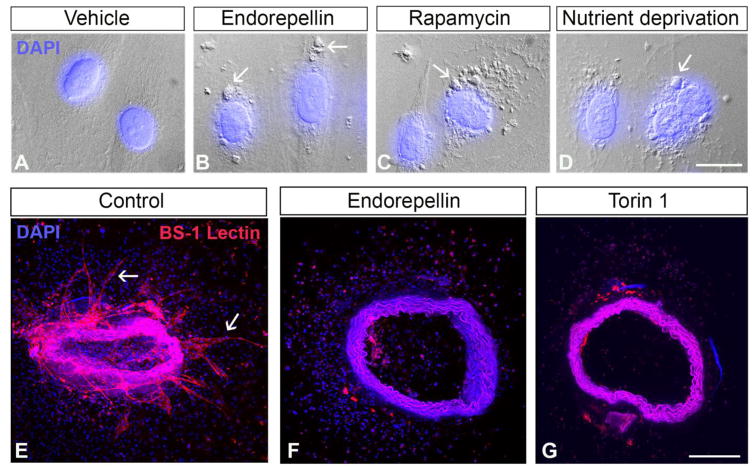
As in the case of angiogenesis, endorepellin takes on a contrasting role to perlecan as an autophagic inducer. Exposure to nanomolar concentrations of endorepellin causes the formation of prominent autophagosomes in endothelial cells (Fig. 3B), similar to those evoked by canonical autophagic stimuli such as rapamycin, which inhibits the mTOR pathway (Fig. 3C), or nutrient deprivation (Fig. 3D). Operating as a partial agonist to VEGFR2 under nutrient-rich conditions, endorepellin evokes a pro-autophagic cascade composed of Peg3, Beclin 1, p62, and LC3 [35] (Fig. 2). This action inhibits mTOR signaling in a manner similar to that of the gold standard, rapamycin [143]. Curiously, though endorepellin typically serves to inhibit VEGFR2 signaling, its ability to induce autophagy in endothelial cells relies on the phosphorylation of the VEGFR2 residue Tyr1175 as the tyrosine kinase inhibitor, SU5416, abrogates this autophagic effect [35]. The autophagic induction by endorepellin is completely independent of its interaction with α2β1 integrin [136] as endothelial cells treated with LG3 do not undergo autophagy [35]. In fact, LG3 quells autophagic gene expression, suggesting that, like the different domains of the parent proteoglycan, individual regions of the same domain may also partake in disparate activities [35].
Fig. 3.
Autophagy may be a mechanism by which endorepellin curtails angiogenesis. (A–D) Differential interference contrast microscopy images of HUVEC under specified conditions. Note the presence of autophagosome-like structures (white arrows) in the endorepellin, rapamycin, and nutrient-deprived cells Bar ~ 15 μm. (E–G) Representative images of murine aortic rings treated with vehicle, endorepellin, or Torin 1. Note the sprouts in the control ring (white arrows) and the absence of sprouts following endorepellin and Torin 1 treatment. Bar ~ 100 μm.
Future work will likely deconstruct the link between angiogenesis and autophagy. It appears that a pattern is emerging in that pro-angiogenic perlecan is anti-autophagic whereas the converse holds true for endorepellin. Intriguingly, recent work in our laboratory [144], has demonstrated that endorepellin and Torin 1, a potent autophagic inducer [145], both inhibit vessel sprouting in an ex vivo model of angiogenesis (Fig. 3F,G), suggesting that autophagy may be one mechanism by which endorepellin inhibits neovascularization.
In our study, we discovered that protracted endothelial cell autophagy evoked by endorepellin can curtail neovascularization, as both endorepellin and Torin 1 reduce sprouting in ex vivo aortic ring assays [144]. Notably, the angiostatic activity of endorepellin could be blocked using Compound C, an inhibitor of AMPKα (Fig. 2). Interestingly, the synthesis of hyaluronan, a major constituent of the provisional angiogenic matrix whose biosynthetic pathway is under complex metabolic control [146–148] is seemingly inhibited by AMPK [149]. Mechanistically, AMPK directly phosphorylates hyaluronan synthase 2 at Thr110, a post-translational modification that blocks its enzymatic activity [149]. Thus, together with autophagic induction, endorepellin could also modify the tumor microenvironment to favor angiostasis by reducing the expression of this pro-angiogenic glycosaminoglycan. These new findings, together with previous in vivo data depicting endorepellin as a powerful means to curtail tumor growth and angiogenesis [150], could potentially yield unique therapeutic targets for novel drug design.
Interestingly, other matrix components, most soluble, follow this same paradigm. For example, endostatin, the C-terminal fragment of the HSPG collagen XVIII and an established inhibitor of angiogenesis, induces autophagy in endothelial cells via α5β1 integrin signaling to increase Beclin 1 expression [151]. Additionally, decorin, an anti-angiogenic small, leucine-rich proteoglycan (SLRP) [152], correspondingly induces autophagy in HUVEC using a mechanism almost identical to that of endorepellin [153–155]. Decorin also promotes mitochondrial autophagy within the tumor proper [156]. Notably, decorin is an autophagy-inducible proteoglycan and its absence results in diminished cardiac autophagy [157]. Therefore, as perlecan deficiency results in enhanced autophagic activity, an intricate in vivo signaling network could take place whereby proteoglycans and other matrix residents would ensure appropriate flux through this pathway under different cellular conditions [158].
It is also worthy to mention a recent study which demonstrates that biglycan, an extracellular matrix SLRP and close relative to decorin, can counteract the anti-angiogenic effects of endostatin [159]. As endostatin is pro-autophagic, it is conceivable that future studies may show that biglycan is yet another matrix component that follows the aforementioned model where it could potentially inhibit autophagy to promote angiogenesis.
Finally, in this section we have focused primarily on the action of the N- and C-termini of perlecan in angiogenesis and autophagy. It is possible that the three internal domains of perlecan may also contribute to one or both of these processes. For example, laminin-α2 knockout mice, which exhibit a congenital muscular dystrophy phenotype, display increased muscle autophagy vis-à-vis wild-type mice [160]. Moreover, as in the case of the perlecan knockout, there is increased atrophy in the Lama2−/− model, which can be rescued with autophagic abatement [160]. Thus, domain III, which shares homology with laminin, may possess the anti-autophagic properties conferred to perlecan and could possibly act independently as an inhibitor of this catabolic process.
Final considerations
The long-standing dogma of large, HS-substituted matrix constituents acting strictly as structural components is rapidly evolving. The emergent paradigm involves proteoglycans as central signaling hubs, coordinating and integrating a multitude of signals for maintaining proper cellular homeostasis. Perlecan exemplifies this concept via strategic localization in various basement membranes and subsequent homo- and heterotypic interactions with adjacent matrix molecules and vectorial juxtaposition with diverse subsets of cell surface receptors. The continued study of perlecan genetics, particularly of mutations of the gene that lead to defective or absent protein, is fundamental for advances in disease management. Furthermore, though the functions of perlecan are broad in scope, its key roles in angiogenesis and autophagy are most interesting, especially as they pertain to tissue homeostasis and dysregulation of normal cellular signaling, and thus should be the paramount focus of future work in order to understand better the mechanisms of disease progression.
Highlights.
A modern view of the biological properties of perlecan, an archetypal basement membrane proteoglycan
The complex genomic organization of perlecan translates into a large, modular proteoglycan capable of regulating numerous cellular pathways
Mutations in the perlecan gene across multiple organisms result in diverse phenotypes
Perlecan and its C-terminal fragment, endorepellin, display antagonistic roles in angiogenesis and autophagy
Acknowledgments
We thank the past and present members of the Iozzo laboratory for their contributions to this body of knowledge. The original research was supported in part by the National Institutes of Health grants RO1 CA39481, RO1 CA47282, and RO1 CA164462 (to RVI). M.A. Gubbiotti was supported in part by NIH training grant T32 AA07463. T. Neill was supported in part by NIH training grant 5T32 AR060715-05.
Abbreviations used
- Akt/PKB
protein kinase B
- AP1
activator protein 1
- BMP
bone morphogenetic protein
- DSSH
dyssegmental dysplasia Silver-Handmaker type
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- ETF
Tef-1 and abaA domain family member 2
- FGF
fibroblast growth factor
- GAG
glycosaminoglycan
- HIF-1α
hypoxia-inducible factor-1α
- HMC-1
human mast cell line 1
- HS
heparan sulfate
- HSPG
heparan sulfate proteoglycan
- HUVEC
human umbilical vein endothelial cell
- IFN-γ
interferon gamma
- Ig
immunoglobulin
- JNK
Jun N-terminal kinase
- LC3
microtubule-associated protein light chain 3
- LDL
low-density lipoprotein
- LG
laminin globular domain
- MEK
mitogen-activated protein kinase kinase
- mTOR
mammalian target of rapamycin
- MuSK
muscle-specific kinase
- N-CAM
neural cell adhesion molecule
- NFAT
nuclear factor of activated T-cells
- p62
sequestosome 1
- PDGF
platelet-derived growth factor
- Peg3
paternally expressed gene 3
- PI3K
phosphoinositide 3-kinase
- PKC
protein kinase C
- PLC-γ
phospholipase C gamma
- Raf
rapidly accelerated fibrosarcoma
- Ras
rat sarcoma
- SEA
Sea urchin sperm protein, enterokinase, and agrin domain
- Shh
Sonic hedgehog
- SHP-1
Src homology phosphatase-1
- SJS
Schwartz-Jampel Syndrome
- SLRP
small, leucine-rich proteoglycan
- Sp1
specificity protein 1
- TGF-β
transforming growth factor β
- Unc
uncoordinated phenotype
- VEGFA
vascular endothelial growth factor A
- VEGFR2
vascular endothelial growth factor receptor 2
- Wnt
wingless-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 3.Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. The extracellular matrix: Tools and insights for the “omics” era. Matrix Biol. 2016;49:10–24. doi: 10.1016/j.matbio.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hassell JR, Leyshon WC, Ledbetter SR, Tyree B, Suzuki S, Kato M, et al. Isolation of two forms of basement membrane proteoglycans. J Biol Chem. 1985;260:8098–8105. [PubMed] [Google Scholar]
- 5.Iozzo RV, Hassell JR. Identification of the precursor protein for the heparan sulfate proteoglycan of human colon carcinoma cells and its post-translational modifications. Arch Biochem Biophys. 1989;269:239–249. doi: 10.1016/0003-9861(89)90105-7. [DOI] [PubMed] [Google Scholar]
- 6.Iozzo RV. Biosynthesis of heparan sulfate proteoglycan by human colon carcinoma cells and its localization at the cell surface. J Cell Biol. 1984;99:403–417. doi: 10.1083/jcb.99.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yurchenco PD, Cheng YS, Ruben GC. Self-assembly of a high molecular weight basement membrane heparan sulfate proteoglycan into dimers and oligomers. J Biol Chem. 1987;262:17668–17676. [PubMed] [Google Scholar]
- 8.Murdoch AD, Liu B, Schwarting R, Tuan RS, Iozzo RV. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against domain III and by in situ hybridization. J Histochem Cytochem. 1994;42:239–249. doi: 10.1177/42.2.7507142. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy KJ. The Basement Membrane Proteoglycans Perlecan and Agrin: Something Old, Something New. Curr Top Membr. 2015;76:255–303. doi: 10.1016/bs.ctm.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Whitelock JM, Graham LD, Melrose J, Murdoch AD, Iozzo RV, Underwood PA. Human perlecan immunopurified from different endothelial cell sources has different adhesive properties for vascular cells. Matrix Biol. 1999;18:163–178. doi: 10.1016/s0945-053x(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 11.Lord MS, Chuang CY, Melrose J, Davies MJ, Iozzo RV, Whitelock JM. The role of vascular-derived perlecan in modulating cell adhesion, proliferation and growth factor signaling. Matrix Biol. 2014;35:112–122. doi: 10.1016/j.matbio.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christianson HC, Belting M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 2014;35:51–55. doi: 10.1016/j.matbio.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Hassell JR, Yamada Y, Arikawa-Hirasawa E. Role of perlecan in skeletal development and diseases. Glycoconj J. 2003;19:263–267. doi: 10.1023/A:1025340215261. [DOI] [PubMed] [Google Scholar]
- 14.Jochmann K, Bachvarova V, Vortkamp A. Heparan sulfate as a regulator of endochondral ossification and osteochondroma development. Matrix Biol. 2014;34:55–63. doi: 10.1016/j.matbio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsueh MF, Onnerfjord P, Kraus VB. Biomarkers and proteomic analysis of osteoarthritis. Matrix Biol. 2014;39:56–66. doi: 10.1016/j.matbio.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 17.Fuki I, Iozzo RV, Williams KJ. Perlecan heparan sulfate proteoglycan. A novel receptor that mediates a distinct pathway for ligand catabolism. J Biol Chem. 2000;275:25742–25750. doi: 10.1074/jbc.M909173199. [DOI] [PubMed] [Google Scholar]
- 18.Colombelli C, Palmisano M, Eshed-Eisenbach Y, Zambroni D, Pavoni E, Ferri C, et al. Perlecan is recruited by dystroglycan to nodes of Ranvier and binds the clustering molecule gliomedin. J Cell Biol. 2015;208:313–329. doi: 10.1083/jcb.201403111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lord MS, Jung M, Cheng B, Whitelock JM. Transcriptional complexity of the HSPG2 gene in the human mast cell line, HMC-1. Matrix Biol. 2014;35:123–131. doi: 10.1016/j.matbio.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Jung M, Lord MS, Cheng B, Lyons JG, Alkhouri H, Hughes JM, et al. Mast cells produce novel shorter forms of perlecan that contain functional endorepellin: A role in angiogenesis and wound healing. J Biol Chem. 2013;288:3289–3304. doi: 10.1074/jbc.M112.387811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nugent MA, Nugent HM, Iozzo RV, Sanchack K, Edelman ER. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc Natl Acad Sci USA. 2000;97:6722–6727. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen IR, Murdoch AD, Naso MF, Marchetti D, Berd D, Iozzo RV. Abnormal expression of perlecan proteoglycan in metastatic melanomas. Cancer Res. 1994;54:5771–5774. [PubMed] [Google Scholar]
- 23.Iozzo RV. Proteoglycans and neoplasia. Cancer Metastasis Rev. 1988;7:39–50. doi: 10.1007/BF00048277. [DOI] [PubMed] [Google Scholar]
- 24.Mathiak M, Yenisey C, Grant DS, Sharma B, Iozzo RV. A role for perlecan in the suppression of growth and invasion in fibrosarcoma cells. Cancer Res. 1997;57:2130–2136. [PubMed] [Google Scholar]
- 25.Mongiat M, Sweeney S, San Antonio JD, Fu J, Iozzo RV. Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan. J Biol Chem. 2003;278:4238–4249. doi: 10.1074/jbc.M210445200. [DOI] [PubMed] [Google Scholar]
- 26.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349–355. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iozzo RV, Zoeller JJ, Nyström A. Basement membrane proteoglycans: Modulators par excellence of cancer growth and angiogenesis. Mol Cells. 2009;27:503–513. doi: 10.1007/s10059-009-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iozzo RV, Sanderson RD. Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J Cell Mol Med. 2011;15:1013–1031. doi: 10.1111/j.1582-4934.2010.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grindel BJ, Martinez JR, Pennington CL, Muldoon M, Stave J, Chung LW, et al. Matrilysin/matrix metalloproteinase-7(MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol. 2014;36:64–76. doi: 10.1016/j.matbio.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiang B, Lim SY, Lekas M, Kuliszewski MA, Wolff R, Osherov AB, et al. Perlecan heparan sulfate proteoglycan is a critical determinant of angiogenesis in response to mouse hind-limb ischemia. Can J Cardiol. 2014;30:1444–1451. doi: 10.1016/j.cjca.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Poluzzi C, Iozzo RV, Schaefer L. Endostatin and endorepellin: A common route of action for similar angiostatic cancer avengers. Adv Drug Deliv Rev. 2016;97:156–173. doi: 10.1016/j.addr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Theocharis AD, Gialeli C, Bouris P, Giannopoulou E, Skandalis SS, Aletras AJ, et al. Cell-matrix interactions: focus on proteoglycan-proteinase interplay and pharmacological targeting in cancer. FEBS J. 2014;281:5023–5042. doi: 10.1111/febs.12927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theocharis AD, Skandalis SS, Neill T, Multhaupt HA, Hubo M, Frey H, et al. Insights into the key roles of proteoglycans in breast cancer biology and translational medicine. Biochim Biophys Acta. 2015;1855:276–300. doi: 10.1016/j.bbcan.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoeller JJ, McQuillan A, Whitelock J, Ho SY, Iozzo RV. A central function for perlecan in skeletal muscle and cardiovascular development. J Cell Biol. 2008;181:381–394. doi: 10.1083/jcb.200708022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poluzzi C, Casulli J, Goyal A, Mercer TJ, Neill T, Iozzo RV. Endorepellin evokes autophagy in endothelial cells. J Biol Chem. 2014;289:16114–16128. doi: 10.1074/jbc.M114.556530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ning L, Xu Z, Furuya N, Nonaka R, Yamada Y, Arikawa-Hirasawa E. Perlecan inhibits autophagy to maintain muscle homeostasis in mouse soleus muscle. Matrix Biol. 2015;48:26–35. doi: 10.1016/j.matbio.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Murdoch AD, Dodge GR, Cohen I, Tuan RS, Iozzo RV. Primary structure of the human heparan sulfate proteoglycan from basement membrane (HSPG2/perlecan). A chimeric molecule with multiple domains homologous to the low density lipoprotein receptor, laminin, neural cell adhesion molecules, and epidermal growth factor. J Biol Chem. 1992;267:8544–8557. [PubMed] [Google Scholar]
- 38.Farach-Carson MC, Warren CR, Harrington DA, Carson DD. Border patrol:Insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol. 2014;34:64–79. doi: 10.1016/j.matbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noonan DM, Fulle A, Valente P, Cai S, Horigan E, Sasaki M, et al. The complete sequence of perlecan, a basement membrane heparan sulfate proteoglycan, reveals extensive similarity with laminin A chain, low density lipoprotein-receptor, and the neural cell adhesion molecule. J Biol Chem. 1991;266:22939–22947. [PubMed] [Google Scholar]
- 40.Cohen IR, Grässel S, Murdoch AD, Iozzo RV. Structural characterization of the complete human perlecan gene and its promoter. Proc Natl Acad Sci USA. 1993;90:10404–10408. doi: 10.1073/pnas.90.21.10404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warren CR, Kassir E, Spurlin J, Martinez J, Putnam NH, Farach-Carson MC. Evolution of the perlecan/HSPG2 gene and its activation in regenerating Nematostella vectensis. PLoS One. 2015;10:e0124578. doi: 10.1371/journal.pone.0124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iozzo RV, Pillarisetti J, Sharma B, Murdoch AD, Danielson KG, Uitto J, et al. Structural and functional characterization of the human perlecan gene promoter. Transcriptional activation by transforming factor-β via a nuclear factor 1-binding element. J Biol Chem. 1997;272:5219–5228. doi: 10.1074/jbc.272.8.5219. [DOI] [PubMed] [Google Scholar]
- 43.Sharma B, Iozzo RV. Transcriptional silencing of perlecan gene expression by interferon-γ. J Biol Chem. 1998;273:4642–4646. doi: 10.1074/jbc.273.8.4642. [DOI] [PubMed] [Google Scholar]
- 44.Warren CR, Grindel BJ, Francis L, Carson DD, Farach-Carson MC. Transcriptional activation by NFkappaB increases perlecan/HSPG2 expression in the desmoplastic prostate tumor microenvironment. J Cell Biochem. 2014;115:1322–1333. doi: 10.1002/jcb.24788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Melrose J, Smith S, Whitelock J. Perlecan immunolocalizes to perichondrial vessels and canals in human fetal cartilaginous primordia in early vascular and matrix remodeling events associated with diarthrodial joint development. J Histochem Cytochem. 2004;52:1405–1413. doi: 10.1369/jhc.4A6261.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith SM, Whitelock JM, Iozzo RV, Little CB, Melrose J. Topographical variation in the distribution of versican, aggrecan and perlecan in the foetal human spine reflects their diverse functional roles in spinal development. Histochem Cell Biol. 2009;132:491–503. doi: 10.1007/s00418-009-0623-z. [DOI] [PubMed] [Google Scholar]
- 47.Handler M, Yurchenco PD, Iozzo RV. Developmental expression of perlecan during murine embryogenesis. Dev Dyn. 1997;210:130–145. doi: 10.1002/(SICI)1097-0177(199710)210:2<130::AID-AJA6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 48.French MM, Smith SE, Akanbi K, Sanford T, Hecht J, Farach-Carson MC, et al. Expression of the heparan sulfate proteoglycan, perlecan, during mouse embryogenesis and perlecan chondrogenic activity in vitro. J Cell Biol. 1999;145:1103–1115. doi: 10.1083/jcb.145.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farach-Carson MC, Carson DD. Perlecan - a multifunctional extracellular proteoglycan scaffold. Glycobiology. 2007;17:897–905. doi: 10.1093/glycob/cwm043. [DOI] [PubMed] [Google Scholar]
- 50.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 51.Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mongiat M, Otto J, Oldershaw R, Ferrer F, Sato JD, Iozzo RV. Fibroblast growth factor-binding protein is a novel partner for perlecan protein core. J Biol Chem. 2001;276:10263–10271. doi: 10.1074/jbc.M011493200. [DOI] [PubMed] [Google Scholar]
- 53.Mongiat M, Fu J, Oldershaw R, Greenhalgh R, Gown A, Iozzo RV. Perlecan protein core interacts with extracellular matrix protein 1 (ECM1), a glycoprotein involved in bone formation and angiogenesis. J Biol Chem. 2003;278:17491–17499. doi: 10.1074/jbc.M210529200. [DOI] [PubMed] [Google Scholar]
- 54.Ghiselli G, Eichstetter I, Iozzo RV. A role for the perlecan protein core in the activation of the keratinocyte growth factor receptor. Biochem J. 2001;359:153–163. doi: 10.1042/0264-6021:3590153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez EM, Mongiat M, Slater SJ, Baffa R, Iozzo RV. A novel interaction between perlecan protein core and progranulin: Potential effects on tumor growth. J Biol Chem. 2003;278:38113–38116. doi: 10.1074/jbc.C300310200. [DOI] [PubMed] [Google Scholar]
- 56.Kerever A, Mercier F, Nonaka R, de VS, Oda Y, Zalc B, et al. Perlecan is required for FGF-2 signaling in the neural stem cell niche. Stem Cell Res. 2014;12:492–505. doi: 10.1016/j.scr.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilusz RE, DeFrate LE, Guilak F. A biomechanical role for perlecan in the pericellular matrix of articular cartilage. Matrix Biol. 2012;31:320–327. doi: 10.1016/j.matbio.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muller C, Khabut A, Dudhia J, Reinholt FP, Aspberg A, Heinegard D, et al. Quantitative proteomics at different depths in human articular cartilage reveals unique patterns of protein distribution. Matrix Biol. 2014;40:34–45. doi: 10.1016/j.matbio.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 59.Sadatsuki R, Kaneko H, Kinoshita M, Futami I, Nonaka R, Culley KL, et al. Perlecan is required for the chondrogenic differentiation of synovial mesenchymal cells through regulation of Sox9 gene expression. J Orthop Res. 2016 doi: 10.1002/jor.23318. [DOI] [PubMed] [Google Scholar]
- 60.Sher I, Zisman-Rozen S, Eliahu L, Whitelock JM, Maas-Szabowski N, Yamada Y, et al. Targeting perlecan in human keratinocytes reveals novel roles for perlecan in epidermal formation. J Biol Chem. 2006;281:5178–5187. doi: 10.1074/jbc.M509500200. [DOI] [PubMed] [Google Scholar]
- 61.Ishijima M, Suzuki N, Hozumi K, Matsunobu T, Kosaki K, Kaneko H, et al. Perlecan modulates VEGF signaling and is essential for vascularization in endochondral bone formation. Matrix Biol. 2012;31:234–245. doi: 10.1016/j.matbio.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang B, Lai X, Price C, Thompson WR, Li W, Quabili TR, et al. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J Bone Miner Res. 2014;29:878–891. doi: 10.1002/jbmr.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wijeratne SS, Martinez JR, Grindel BJ, Frey EW, Li J, Wnag K, et al. Single molecule force measurements of perlecan/HSPG2: A key component of the osteocyte pericellular matrix. Matrix Biol. 2016;50:27–38. doi: 10.1016/j.matbio.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaneko H, Ishijima M, Futami I, Tomikawa-Ichikawa N, Kosaki K, Sadatsuki R, et al. Synovial perlecan is required for osteophyte formation in knee osteoarthritis. Matrix Biol. 2013;32:178–187. doi: 10.1016/j.matbio.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vikramadithyan RK, Kako Y, Chen G, Hu Y, Arikawa-Hirasawa E, Yamada Y, et al. Atherosclerosis in perlecan heterozygous mice. J Lipid Res. 2004;45:1806–1812. doi: 10.1194/jlr.M400019-JLR200. [DOI] [PubMed] [Google Scholar]
- 66.Tran-Lundmark K, Tannenberg P, Rauch BH, Ekstrand J, Tran PK, Hedin U, et al. Perlecan Heparan Sulfate Is Required for the Inhibition of Smooth Muscle Cell Proliferation by All-trans-Retinoic Acid. J Cell Physiol. 2015;230:482–487. doi: 10.1002/jcp.24731. [DOI] [PubMed] [Google Scholar]
- 67.Xu Z, Ichikawa N, Kosaki K, Yamada Y, Sasaki T, Sakai LY, et al. Perlecan deficiency causes muscle hypertrophy, a decrease in myostatin expression, and changes in muscle fiber composition. Matrix Biol. 2010;29:461–470. doi: 10.1016/j.matbio.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma B, Handler M, Eichstetter I, Whitelock J, Nugent MA, Iozzo RV. Antisense targeting of perlecan blocks tumor growth and angiogenesis in vivo. J Clin Invest. 1998;102:1599–1608. doi: 10.1172/JCI3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Douglass S, Goyal A, Iozzo RV. The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy. Connect Tissue Res. 2015;19:1–11. doi: 10.3109/03008207.2015.1045297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dos SM, Michopoulou A, Andre-Frei V, Boulesteix S, Guicher C, Dayan G, et al. Perlecan expression influences the keratin 15-positive cell population fate in the epidermis of aging skin. Aging (Albany NY) 2016;8:751–768. doi: 10.18632/aging.100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chang YT, Tseng CN, Tannenberg P, Eriksson L, Yuan K, de Jesus Perez VA, et al. Perlecan heparan sulfate deficiency impairs pulmonary vascular development and attenuates hypoxic pulmonary hypertension. Cardiovasc Res. 2015;107:20–31. doi: 10.1093/cvr/cvv143. [DOI] [PubMed] [Google Scholar]
- 72.Mongiat M, Taylor K, Otto J, Aho S, Uitto J, Whitelock J, et al. The protein core of the proteoglycan perlecan binds specifically to fibroblast growth factor-7. J Biol Chem. 2000;275:7095–7100. doi: 10.1074/jbc.275.10.7095. [DOI] [PubMed] [Google Scholar]
- 73.Smith SML, West LA, Govindraj P, Zhang X, Ornitz DM, Hassell JR. Heparan and chondroitin sulfate on growth plate perlecan mediate binding and delivery of FGF-2 to FGF receptors. Matrix Biol. 2007;26:175–184. doi: 10.1016/j.matbio.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 74.Muthusamy A, Cooper CR, Gomes RR., Jr Soluble perlecan domain I enhances vascular endothelial growth factor-165 activity and receptor phosphorylation in human bone marrow endothelial cells. BMC Biochemistry. 2010;11:43. doi: 10.1186/1471-2091-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noonan DM, Hassell JR. Perlecan, the large low-density proteoglycan of basement membranes: structure and variant forms. Kidney Int. 1993;43:53–60. doi: 10.1038/ki.1993.10. [DOI] [PubMed] [Google Scholar]
- 76.Xu Y, Ashline D, Liu L, Tassa C, Shaw SY, Ravid K, et al. The glycosylation-dependent interaction of perlecan core protein with LDL: implications for atherosclerosis. J Lipid Res. 2015;56:266–276. doi: 10.1194/jlr.M053017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tran-Lundmark K, Tran PK, Paulsson-Berne G, Fridén V, Soinen R, Tryggvason K, et al. Heparan sulfate in perlecan promotes mouse atherosclerosis. Roles of lipid permeability, lipid retention, and smooth muscle cell proliferation. Circ Res. 2008;103:43–52. doi: 10.1161/CIRCRESAHA.108.172833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sasaki M, Kleinman HK, Huber H, Deutzmann R, Yamada Y. Laminin, a mulitdomain protein. J Biol Chem. 1988;263:16536–16544. [PubMed] [Google Scholar]
- 79.Haaparanta T, Uitto J, Ruoslahti E, Engvall E. Molecular cloning of the cDNA encoding human laminin A chain. Matrix. 1991;11:151–160. doi: 10.1016/s0934-8832(11)80153-8. [DOI] [PubMed] [Google Scholar]
- 80.Noonan DM, Horigan EA, Ledbetter SR, Vogeli G, Sasaki M, Yamada Y, et al. Identification of cDNA clones encoding different domains of the basement membrane heparan sulfate proteoglycan. J Biol Chem. 1988;263:16379–16387. [PubMed] [Google Scholar]
- 81.Iwata S, Ito M, Nakata T, Noguchi Y, Okuno T, Ohkawara B, et al. A missense mutation in domain III in HSPG2 in Schwartz-Jampel syndrome compromises secretion of perlecan into the extracellular space. Neuromuscul Disord. 2015;25:667–671. doi: 10.1016/j.nmd.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 82.Nicole S, Davoine CS, Topaloglu H, Cattolico L, Barral D, Beighton P, et al. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz-Jampel syndrome (chondrodystrophic myotonia) Nat Genet. 2000;26:480–483. doi: 10.1038/82638. [DOI] [PubMed] [Google Scholar]
- 83.Chakravarti S, Horchar T, Jefferson B, Laurie GW, Hassell JR. Recombinant domain III of perlecan promotes cell attachment through its RGDS sequence. J Biol Chem. 1995;270:404–409. doi: 10.1074/jbc.270.1.404. [DOI] [PubMed] [Google Scholar]
- 84.Cunningham BA, Hemperly JJ, Murray BA, Prediger EA, Brackenbury R, Edelman GM. Neural cell adhesion molecule: structure, immunoglobulin-like domains, cell surface modulation, and alternative RNA splicing. Science. 1987;236:799–806. doi: 10.1126/science.3576199. [DOI] [PubMed] [Google Scholar]
- 85.Hopf M, Göhring W, Kohfeldt E, Yamada Y, Timpl R. Recombinant domain IV of perlecan binds to nidogens, laminin-nidogen complex, fibronectin, fibulin-2 and heparin. Eur J Biochem. 1999;259:917–925. doi: 10.1046/j.1432-1327.1999.00127.x. [DOI] [PubMed] [Google Scholar]
- 86.Grindel B, Li Q, Arnold R, Petros J, Zayzafoon M, Muldoon M, et al. Perlecan/HSPG2 and matrilysin/MMP-7 as indices of tissue invasion: tissue localization and circulating perlecan fragments in a cohort of 288 radical prostatectomy patients. Oncotarget. 2016;7:10433–10447. doi: 10.18632/oncotarget.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44–46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 88.Wells JM, Gaggar A, Blalock JE. MMP generated matrikines. Matrix Biol. 2015;44–46:122–129. doi: 10.1016/j.matbio.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015;44–46:94–112. doi: 10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015;44–46:113–121. doi: 10.1016/j.matbio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 91.Duarte S, Baber J, Fujii T, Coito AJ. Matrix metalloproteinases in liver injury, repair and fibrosis. Matrix Biol. 2015;44–46:147–156. doi: 10.1016/j.matbio.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eckhard U, Huesgen PF, Schilling O, Bellac CL, Butler GS, Cox JH, et al. Active site specificity profiling of the matrix metalloproteinase family: Proteomic identification of 4300 cleavage sites by nine MMPs explored with structural and synthetic peptide cleavage analyses. Matrix Biol. 2016;49:37–60. doi: 10.1016/j.matbio.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 93.Gonzalez EM, Reed CC, Bix G, Fu J, Zhang Y, Gopalakrishnan B, et al. BMP-1/Tolloid-like metalloproteases process endorepellin, the angiostatic C-terminal fragment of perlecan. J Biol Chem. 2005;280:7080–7087. doi: 10.1074/jbc.M409841200. [DOI] [PubMed] [Google Scholar]
- 94.Thadikkaran L, Crettaz D, Siegenthaler MA, Gallot D, Sapin V, Iozzo RV, et al. The role of proteomics in the assessment of premature rupture of fetal membranes. Clin Chim Acta. 2005;360:27–36. doi: 10.1016/j.cccn.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 95.Oda O, Shinzato T, Ohbayashi K, Takai I, Kunimatsu M, Maeda K, et al. Purification and characterization of perlecan fragment in urine of end-stage renal failure patients. Clin Chim Acta. 1996;255:119–132. doi: 10.1016/0009-8981(96)06395-4. [DOI] [PubMed] [Google Scholar]
- 96.O’Riordan E, Orlova TN, Mendelev N, Patschan D, Kemp R, Chander PN, et al. Urinary proteomic analysis of chronic renal allograft nephropathy. Proteomics Clin Appl. 2008;2:1025–1035. doi: 10.1002/prca.200780137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsangaris GT, Karamessinis P, Kolialexi A, Garbis SD, Antsaklis A, Mavrou A, et al. Proteomic analysis of amniotic fluid in pregnancies with Down syndrome. Proteomics. 2006;6:4410–4419. doi: 10.1002/pmic.200600085. [DOI] [PubMed] [Google Scholar]
- 98.Chang JW, Kang UB, Kim DH, Yi JK, Lee JW, Noh DY, et al. Identification of circulating endorepellin LG3 fragment: Potential use as a serological biomarker for breast cancer. Proteomics Clin Appl. 2008;2:23–32. doi: 10.1002/prca.200780049. [DOI] [PubMed] [Google Scholar]
- 99.Pilon EA, Dieude M, Qi S, Hamelin K, Pomerleau L, Beillevaire D, et al. The perlecan fragment LG3 regulates homing of mesenchymal stem cells and neointima formation during vascular rejection. Am J Transplant. 2015;15:1205–1218. doi: 10.1111/ajt.13119. [DOI] [PubMed] [Google Scholar]
- 100.Dieude M, Bell C, Turgeon J, Beillevaire D, Pomerleau L, Yang B, et al. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci Transl Med. 2015;7:318ra200. doi: 10.1126/scitranslmed.aac9816. [DOI] [PubMed] [Google Scholar]
- 101.SundarRaj N, Fite D, Ledbetter S, Chakravarti S, Hassell JR. Perlecan is a component of cartilage matrix and promotes chondrocyte attachment. J Cell Sci. 1995;108:2663–2672. doi: 10.1242/jcs.108.7.2663. [DOI] [PubMed] [Google Scholar]
- 102.Govindraj P, West L, Koob TJ, Neame P, Doege K, Hassell JR. Isolation and identification of the major heparan sulfate proteoglycans in the developing bovine rib growth plate. J Biol Chem. 2002;277:19461–19469. doi: 10.1074/jbc.M200786200. [DOI] [PubMed] [Google Scholar]
- 103.Govindraj P, West L, Smith S, Hassell JR. Modulation of FGF-2 binding to chondrocytes from the developing growth plate by perlecan. Matrix Biol. 2006;25:232–239. doi: 10.1016/j.matbio.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Merz DC, Alves G, Kawano T, Zheng H, Culotti JG. UNC-52/perlecan affects gonadal leader cell migrations in C. elegans hermaphrodites through alterations in growth factor signaling. Dev Biol. 2003;256:173–186. doi: 10.1016/s0012-1606(03)00014-9. [DOI] [PubMed] [Google Scholar]
- 105.Mullen GP, Rogalski TM, Bush JA, Gorji PR, Moerman DG. Complex patterns of alternative splicing mediate the spatial and temporal distribution of perlecan/UNC-52 in Caenorhabditis elegans. Mol Biol Cell. 1999;10:3205–3221. doi: 10.1091/mbc.10.10.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rogalski TM, Gilchrist EJ, Mullen GP, Moerman DG. Mutations in the unc-52 gene responsible for body wall muscle defects in adult Caenorhabditis elegans are located in alternatively spliced exons. Genetics. 1995;139:159–169. doi: 10.1093/genetics/139.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Voigt A, Pflanz R, Schafer U, Jackle H. Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev Dyn. 2002;224:403–412. doi: 10.1002/dvdy.10120. [DOI] [PubMed] [Google Scholar]
- 108.Lindner JR, Hillman PR, Barrett AL, Jackson MC, Perry TL, Park Y, et al. The Drosophila Perlecan gene trol regulates multiple signaling pathways in different developmental contexts. BMC Dev Biol. 2007;7:121. doi: 10.1186/1471-213X-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park Y, Rangel C, Reynolds MM, Caldwell MC, Johns M, Nayak M, et al. Drosophila perlecan modulates FGF and Hedgehog signals to activate neural stem cell division. Dev Biol. 2003;253:247–257. doi: 10.1016/s0012-1606(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 110.Grigorian M, Liu T, Banerjee U, Hartenstein V. The proteoglycan Trol controls the architecture of the extracellular matrix and balances proliferation and differentiation of blood progenitors in the Drosophila lymph gland. Dev Biol. 2013;384:301–312. doi: 10.1016/j.ydbio.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.San Antonio JD, Zoeller JJ, Habursky K, Turner K, Pimtong W, Burrows M, et al. A key role for the integrinα2β1 in experimental and developmental angiogenesis. Am J Pathol. 2009;175:1338–1347. doi: 10.2353/ajpath.2009.090234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Costell M, Gustafsson E, Aszódi A, Mörgelin M, Bloch W, Hunziker E, et al. Perlecan maintains the integrity of cartilage and some basement membranes. J Cell Biol. 1999;147:1109–1122. doi: 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Costell M, Carmona R, Gustafsson E, González-Iriarte M, Fässler R, Munoz-Chápuli R. Hyperplastic conotruncal endocardial cushions and transposition of great arteries in perlecan-null mice. Circ Res. 2002;91:158–164. doi: 10.1161/01.res.0000026056.81424.da. [DOI] [PubMed] [Google Scholar]
- 114.Arikawa-Hirasawa E, Watanabe E, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 115.Pan Y, Carbe C, Kupich S, Pickhinke U, Ohlig S, Frye M, et al. Heparan sulfate expression in the neural crest is essential for mouse cardiogenesis. Matrix Biol. 2014;35:253–265. doi: 10.1016/j.matbio.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rossi M, Morita H, Sormunen R, Airenne S, Kreivi M, Wang L, et al. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22:236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Inomata T, Ebihara N, Funaki T, Matsuda A, Wantanabe Y, Ning L, et al. Perlecan-deficient mutation impairs corneal epithelial structure. Invest Ophtalmol Vis Sci. 2012;53:1277–1284. doi: 10.1167/iovs.11-8742. [DOI] [PubMed] [Google Scholar]
- 118.Tran PK, Tran-Lundmark K, Soininen R, Tryggvason K, Thyberg J, Hedin U. Increased intimal hyperplasia and smooth muscle cell proliferation in transgenic mice with heparan sulfate-deficient perlecan. Circ Res. 2004;94:550–558. doi: 10.1161/01.RES.0000117772.86853.34. [DOI] [PubMed] [Google Scholar]
- 119.Zhou Z, Wang J, Cao R, Morita H, Soininen R, Chan KM, et al. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004;64:4699–4702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]
- 120.Nonaka R, Iesaki T, de VS, Daida H, Okada T, Sasaki T, et al. Perlecan deficiency causes endothelial dysfunction by reducing the expression of endothelial nitric oxide synthase. Physiol Rep. 2015;3:e12272. doi: 10.14814/phy2.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Arikawa-Hirasawa E, Wilcox WR, Le AH, Silverman N, Govindraj P, Hassell JR, et al. Dyssegmental dysplasia, Silverman-Handmaker type, is caused by functional null mutations of the perlecan gene. Nat Genet. 2001;27:431–434. doi: 10.1038/86941. [DOI] [PubMed] [Google Scholar]
- 122.Arikawa-Hirasawa E, Le AH, Nishino I, Nonaka I, Ho NC, Francomano CA, et al. Structural and functional mutations of the perlecan gene cause Schwartz-Jampel syndrome, with myotonic myopathy and chondrodysplasia. Am J Hum Genet. 2002;70:1368–1375. doi: 10.1086/340390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arikawa-Hirasawa E, Wilcox WR, Yamada Y. Dyssegmental dysplasia, Silverman-Handmaker type: unexpected role of perlecan in cartilage development. Am J Med Genet. 2001;106:254–257. doi: 10.1002/ajmg.10229. [DOI] [PubMed] [Google Scholar]
- 124.Cartaud A, Strochlic L, Guerra M, Blanchard B, Lambergeon M, Krejci E, et al. MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction. J Cell Biol. 2004;165:505–515. doi: 10.1083/jcb.200307164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peng HB, Xie H, Rossi SG, Rotundo RL. Acetylcholinesterase clustering at the neuromuscular junction involves perlecan and dystroglycan. J Cell Biol. 1999;145:911–921. doi: 10.1083/jcb.145.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Neill T, Schaefer L, Iozzo RV. Decoding the Matrix: Instructive Roles of Proteoglycan Receptors. Biochemistry. 2015;54:4583–4598. doi: 10.1021/acs.biochem.5b00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Arikawa-Hirasawa E, Rossi SG, Rotundo RL, Yamada Y. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat Neurosci. 2002;5:119–123. doi: 10.1038/nn801. [DOI] [PubMed] [Google Scholar]
- 128.Willis CD, Schaefer L, Iozzo RV. The biology of perlecan and its bioactive modules. In: Karamanos NK, editor. Extracellular Matrix: Pathobiology and Signaling. Walter de Gruyter GmbH & Co. KG; Berlin: 2012. pp. 171–184. [Google Scholar]
- 129.Woodall BP, Nyström A, Iozzo RA, Eble JA, Niland S, Krieg T, et al. Integrin α2β1 is the required receptor for endorepellin angiostatic activity. J Biol Chem. 2008;283:2335–2343. doi: 10.1074/jbc.M708364200. [DOI] [PubMed] [Google Scholar]
- 130.Aviezer D, Hecht D, Safran M, Eisinger M, David G, Yayon A. Perlecan, basal lamina proteoglycan, promotes basic fibroblast growth factor-receptor binding, mitogenesis, and angiogenesis. Cell. 1994;79:1005–1013. doi: 10.1016/0092-8674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 131.Zoeller JJ, Whitelock J, Iozzo RV. Perlecan regulates developmental angiogenesis by modulating the VEGF-VEGFR2 axis. Matrix Biol. 2009;28:284–291. doi: 10.1016/j.matbio.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kaji T, Yamamoto C, Oh-i M, Fujiwara Y, Yamazaki Y, Morita T, et al. The vascular endothelial growth factor VEGF165 induces perlecan synthesis via VEGF receptor-2 in cultured human brain microvascular endothelial cells. Biochem Biophys Acta. 2006;1760:1465–1474. doi: 10.1016/j.bbagen.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 133.Chuang CY, Lord MS, Melrose J, Rees MD, Knox SM, Freeman C, et al. Heparan sulfate-dependent signaling of fibroblast growth growth factor 18 by chondrocyte-derived perlecan. Biochemistry. 2010;49:5524–5532. doi: 10.1021/bi1005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bix G, Iozzo RV. Matrix revolutions: “tails” of basement-membrane components with angiostatic functions. Trends Cell Biol. 2005;15:52–60. doi: 10.1016/j.tcb.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 135.Bix G, Iozzo RV. Novel interactions of perlecan: Unraveling perlecan’s role in angiogenesis. Microsc Res. 2008;71:339–348. doi: 10.1002/jemt.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Goyal A, Pal N, Concannon M, Paulk M, Doran M, Poluzzi C, et al. Endorepellin, the angiostatic module of perlecan, interacts with both the α2β1 integrin and vascular endothelial growth factor receptor 2 (VEGFR2) J Biol Chem. 2011;286:25947–25962. doi: 10.1074/jbc.M111.243626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bix G, Fu J, Gonzalez E, Macro L, Barker A, Campbell S, et al. Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through the α2β1 integrin. J Cell Biol. 2004;166:97–109. doi: 10.1083/jcb.200401150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Willis CD, Poluzzi C, Mongiat M, Iozzo RV. Endorepellin laminin-like globular repeat 1/2 domains bind Ig3–5 of vascular endothelial growth factor(VEGF) receptor 2 and block pro-angiogenic signaling by VEGFA in endothelial cells. FEBS J. 2013;280:2271–2294. doi: 10.1111/febs.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bix G, Iozzo RA, Woodall B, Burrows M, McQuillan A, Campbell S, et al. Endorepellin, the C-terminal angiostatic module of perlecan, enhances collagen-platelet responses via the α2β1 integrin receptor. Blood. 2007;109:3745–3748. doi: 10.1182/blood-2006-08-039925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nyström A, Shaik ZP, Gullberg D, Krieg T, Eckes B, Zent R, et al. Role of tyrosine phosphatase SHP-1 in the mechanism of endorepellin angiostatic activity. Blood. 2009;114:4897–4906. doi: 10.1182/blood-2009-02-207134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Goyal A, Poluzzi C, Willis AC, Smythies J, Shellard A, Neill T, et al. Endorepellin affects angiogenesis by antagonizing diverse VEGFR2- evoked signaling pathways: transcriptional repression of HIF-1α and VEGFA and concurrent inhibition of NFAT1 activation. J Biol Chem. 2012;287:43543–43556. doi: 10.1074/jbc.M112.401786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bhattacharya R, Kwon J, Wang E, Mukherjee P, Mukhopadhyay D. Src homology 2 (SH2) domain containing protein tyrosine phosphatase-1 (SHP-1) dephosphorylates VEGF receptor-2 and attenuates endothelial DNA synthesis, but not migration. J Mol Signal. 2008;3:8. doi: 10.1186/1750-2187-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Choi AMK, Ryter SW, Levine B. Autophagy in human health and disease. New Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 144.Goyal A, Gubbiotti MA, Chery DR, Han L, Iozzo RV. Endorepellin-evoked autophagy contributes to angiostasis. J Biol Chem. 2016 doi: 10.1074/jbc.M116.740266. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thoreen CC, Kang SA, Chang JW, Liu Q, Zhang J, Gao Y, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vigetti D, Viola M, Karousou E, De LG, Passi A. Metabolic control of hyaluronan synthases. Matrix Biol. 2014;35:8–13. doi: 10.1016/j.matbio.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 147.Hascall VC, Wang A, Tammi M, Oikari S, Tammi R, Passi A, et al. The dynamic metabolism of hyaluronan regulates the cytosolic concentration of UDP-GlcNAc. Matrix Biol. 2014;35:14–17. doi: 10.1016/j.matbio.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang A, Sankaranarayanan NV, Yanagishita M, Templeton DM, Desai UR, Sugahara K, et al. Heparin interaction with a receptor on hyperglycemic dividing cells prevents intracellular hyaluronan synthesis and autophagy responses in models of type 1 diabetes. Matrix Biol. 2015;48:36–41. doi: 10.1016/j.matbio.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vigetti D, Clerici M, Deleonibus S, Karousou E, Viola M, Moretto P, et al. Hyaluronan synthesis is inhibited by adenosine monophosphate-activated protein kinase through the regulation of HAS2 activity in human aortic smooth muscle cells. J Biol Chem. 2011;286:7917–7924. doi: 10.1074/jbc.M110.193656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bix G, Castello R, Burrows M, Zoeller JJ, Weech M, Iozzo RA, et al. Endorepellin in vivo: targeting the tumor vasculature and retarding cancer growth and metabolism. J Natl Cancer Inst. 2006;98:1634–1646. doi: 10.1093/jnci/djj441. [DOI] [PubMed] [Google Scholar]
- 151.Nguyen TMB, Subramanian IV, Xiao X, Ghosh G, Nguyen P, Kelekar A, et al. Endostatin induces autophagy in endothelial cells by modulating Beclin 1 and β-catenin levels. J Cell Mol Med. 2009;13:3687–3698. doi: 10.1111/j.1582-4934.2009.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Järveläinen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biology. 2015;43:15–26. doi: 10.1016/j.matbio.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Buraschi S, Neill T, Goyal A, Poluzzi C, Smythies J, Owens RT, et al. Decorin causes autophagy in endothelial cells via Peg3. Proc Natl Acad Sci USA. 2013;110:E2582–E2591. doi: 10.1073/pnas.1305732110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Neill T, Torres AT, Buraschi S, Iozzo RV. Decorin has an appetite for endothelial cell autophagy. Autophagy. 2013;9:1626–1628. doi: 10.4161/auto.25881. [DOI] [PubMed] [Google Scholar]
- 155.Neill T, Schaefer L, Iozzo RV. Decorin, a guardian from the matrix. Am J Pathol. 2012;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Neill T, Torres A, Buraschi S, Owens RT, Hoek J, Baffa R, et al. Decorin induces mitophagy in breast carcinoma cells via peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) and mitostatin. J Biol Chem. 2014;289:4952–4968. doi: 10.1074/jbc.M113.512566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gubbiotti MA, Neill T, Frey H, Schaefer L, Iozzo RV. Decorin is an autophagy-inducible proteoglycan and is required for proper in vivo autophagy. Matrix Biol. 2015;48:14–25. doi: 10.1016/j.matbio.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gubbiotti MA, Iozzo RV. Proteoglycans regulate autophagy via outside-in signaling: An emerging new concept. Matrix Biol. 2015;48:6–13. doi: 10.1016/j.matbio.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Myren M, Kirby DJ, Noonan ML, Maeda A, Owens RT, Ricard-Blum S, et al. Biglycan potentially regulates angiogenesis during fracture repair by altering expression and function of endostatin. Matrix Biol. 2016;52–54:141–150. doi: 10.1016/j.matbio.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Carmignac V, Svensson M, Körner Z, Elowsson L, Matsumura C, Gawlik KI, et al. Autophagy is increased in laminin α2 chain-deficient muscle and its inhibition improves muscle morphology in a mouse model of MDC1A. Human Mol Gen. 2011;20:4891–4902. doi: 10.1093/hmg/ddr427. [DOI] [PubMed] [Google Scholar]