Abstract
The synapse between motor neurons and skeletal muscle is known as the neuromuscular junction (NMJ). Proper alignment of presynaptic and post-synaptic structures of motor neurons and muscle fibers, respectively, is essential for efficient motor control of skeletal muscles. The synaptic cleft between these two cells is filled with basal lamina. Laminins are heterotrimer extracellular matrix molecules that are key members of the basal lamina. Laminin α4, α5, and β2 chains specifically localize to NMJs, and these laminin isoforms play a critical role in maintenance of NMJs and organization of synaptic vesicle release sites known as active zones. These individual laminin chains exert their role in organizing NMJs by binding to their receptors including integrins, dystroglycan, and voltage-gated calcium channels (VGCCs). Disruption of these laminins or the laminin-receptor interaction occurs in neuromuscular diseases including Pierson syndrome and Lambert-Eaton myasthenic syndrome (LEMS). Interventions to maintain proper level of laminins and their receptor interactions may be insightful in treating neuromuscular diseases and aging related degeneration of NMJs.
Keywords: aged, Bassoon, neuromuscular junction, laminin, voltage-gated calcium channels
Introduction
Neuromuscular junctions (NMJs) are chemical synapses located between nerve terminals and specialized sites on the post-synaptic skeletal muscle fiber plasma membrane (i.e., motor endplates). Innervation of muscle fibers by motor neurons establishes proper control of skeletal muscle contraction by the nervous system, and this innervation can become disrupted during disease and aging. Depolarization of motor neurons results in subsequent depolarization of the skeletal muscle fiber plasma membrane or sarcolemma. On the presynaptic side, depolarization of motor neurons results in synaptic vesicle fusion with the plasma membrane and exocytosis of a chemical neurotransmitter, acetylcholine (ACh), into the synaptic cleft. Synaptic vesicle release sites of the nerve terminal are well-organized structures known as active zones (see Box 1 for expanded description of active zones) [1–5]. At the endplate, the sarcolemma uniquely folds to form junctional folds, and acetylcholine receptors (AChRs) accumulate at the crest of these junctional folds to rapidly and efficiently receive ACh released from motor neurons [6–10].
BOX #1. Active zone organization and active zone proteins.
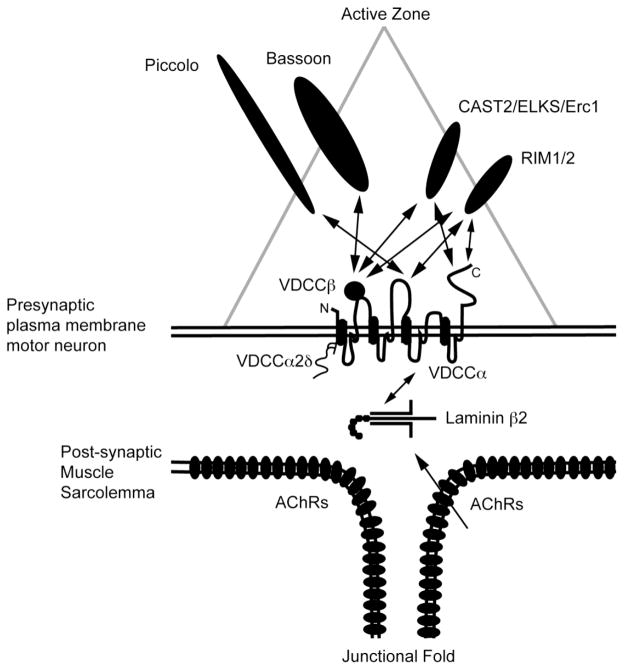
Active zones are a multiprotein complex accumulated at the presynaptic plasma membrane where synaptic vesicles accumulate, fuse with the plasma membrane, and release neurotransmitters into the synaptic cleft [1–5, 162, 204–208]. Using freeze-fracture electron microscopy, active zones were identified as two parallel arrays of 10–12 nm intramembranous particles arranged in two to four rows with each active zone containing 20 of these intramembranous particles [93, 96, 209, 210]. The density of active zones at the presynaptic membrane of NMJ is 2.4–2.7 active zones/μm2 in adult humans and mice [4, 93, 96, 97], and this density is maintained during postnatal maturation periods when NMJs enlarge [175]. A collection of proteins make up the active zones (also known as the cytoskeletal matrix of the active zone (CAZ)) including Bassoon, CAST/ELKS2/Erc2, CAST2/ ELKS/Erc1, Munc13, Piccolo, Rab3 interacting protein-1/2 (RIM1/2), as well as Brunchpilot inDrosophila that is a homolog of CAST/ELKS/Erc (Figure 3) [211–223]. These active zone proteins are involved in accumulation of synaptic vesicles to the plasma membrane and neurotransmitter release upon stimulation by calcium influx through VGCCs. It is important to note that active zone density is independent of nerve transmission. In acetylcholine transferase knockout mice, ACh cannot be properly synthesized therefore synaptic transmission is absent. However, active zone density is normal in these mice [224]. Thus, proper nerve transmission is not necessary for proper active zone organization and formation.
The active zone proteins Bassoon and Piccolo were discovered in screenings to determine structural proteins in rat brain synaptic junctions, and these two proteins share many structural similarities [212, 215, 219, 225]. In NMJs of rodents, Bassoon and Piccolo display a punctate staining pattern by fluorescent immunohistochemistry (Figure 4), with these puncta localizing to presynaptic active zones [63, 175, 189]. Mice without functional Bassoon have normal synapse formation, but impaired synaptic transmission, abnormal dendritic branches, ectopic formation of active zones, and lack proper anchoring of photoreceptor ribbon synapses to active zones with otherwise normal retinal anatomy [226, 227]. Piccolo has been shown to aid in synaptic vesicle exocytosis, and when absent, synaptic vesicle trafficking is disrupted [228]. Importantly, Bassoon helps to position VGCCs near the synaptic vesicle release sites [223]. Although disruption of Bassoon reduces synaptic functionality in some regions of the central nervous system, the disruption of active zone proteins may or may not result in aberrant NMJ formation and needs to be further tested.
The role of other active zone specific proteins in the formation of NMJ active zones is not as well established. CAST family of scaffolding protein CAST2/ELKS/Erc1 along with Piccolo, but not CAST/ELKS2/Erc2, are detected at NMJs [229]. In addition, CAST/ELKS2/Erc2, similar to Bassoon, has been linked to VGCC functionality [230]. The role of CAST/ELKS2/Erc2 in active zone organization was confirmed in photoreceptor synapses and inhibitory synapses due to the loss of CAST/ELSK2α [231, 232]. Munc13 has three homologous family members (Munc13-1/2/3) and has an essential role in neurotransmitter release and synaptic vesicle priming in synapses of the central nervous system [217, 233, 234]. Munc13-1/2 double knockout mice totally lack spontaneous and evoked synaptic transmission in excitatory and inhibitory synapses of hippocampal neurons [235], but exhibited residual amount of synaptic transmission at NMJs [236]. At the NMJ, these mice exhibit normal apposition between motor neuron terminals with endplates, and active zones with docked vesicles were detected [236, 237]. Therefore, Munc family of proteins may not be essential for the structural assembly of active zones. RIMs are multi-domain proteins consisting of three isoforms (α, β, γ) that also play essential roles in connecting active zone specific proteins to active zone structures and synaptic vesicles [238–244]. For example, direct interactions of PDZ domains in RIM and N- and P/Q-type VGCCs tether these VGCCs to presynaptic active zones [244]. RIM1 interacts directly with VGCC β subunit and suppresses voltage-dependent inactivation of neuronal VGCCs [242, 243]. RIM1/2 knockout mice have a reduced number of docked vesicles at active zones with reduced density of VGCCs in the calyx of Held synapse without loss of active zone density [245]. Further work is needed to elucidate the molecular mechanisms that underlie changes in active zone protein content during aging and other neuromuscular diseases.
During development, motor neurons must seek out and find nascent endplates making up only approximately 0.1% of the sarcolemmal surface area [11]. Individual components of the skeletal muscle fiber basement membrane help to guide the process of innervation by motor neurons, and also proper organization of pre- and post-synaptic NMJ morphology. Laminins play a large role in this process and individual laminin subunits are responsible for organizing different components of the NMJ structure [11, 12]. Laminin receptors, including basal cell adhesion molecule/Lutheran blood group antigen (Bcam), dystroglycan, integrins, and voltage-gated calcium channels (VGCCs), assume different roles influenced by each laminin chain (see Box 2 for expanded description of VGCCs at NMJs) [10–20]. These interactions are disturbed during some diseases and aging, thus influencing the morphology and function of pre- and post-synaptic NMJ structures. In this review, we will highlight the relationship of laminins and laminin receptors in the organization of the pre- and post-synaptic components of NMJs and how these molecular mechanisms relate to some disease processes.
BOX #2. Voltage-gated calcium channels.
VGCCs localize at presynaptic active zones and are responsible for calcium influx upon sensing depolarization of the presynaptic plasma membrane. The rise of calcium concentration in nerve terminals results in fusion of synaptic vesicles and exocytosis of neurotransmitters into the synaptic cleft. This process is essential for communication between neurons as well as between motor neurons and skeletal muscle fibers. A number of VGCC types exist, including P/Q-, N-, L-, and R-type VGCCs (Cav2.1, Cav2.2, Cav2.3, and Cav1.2, respectively), but the P/Q- and N-type VGCCs are enriched specifically at motor nerve terminals [64, 246–248]. Synaptic transmission in NMJs at perinatal stages depends on both N-and P/Q-type VGCCs; however, the electrophysiological dependence shifts towards P/Q-type VGCCs for synaptic transmission in adulthood [64]. These calcium channels have two transmembrane subunits (α1 and α2δ) and a cytosolic β subunit [246]. Interestingly, N- and P/Q-type VGCCs are receptors for laminins containing the β2 chain. A leucine-arginine-glutamic acid (LRE) segment in laminin β2 binds directly to the 11th extracellular loop of the α subunit of P/Q- and N-type VGCCs [63]. The 11th extracellular loop of the P/Q-type VGCC has structural homology with the L5III loop of L-type VGCC (Cav1.1), whose 3-dimensional structure has been elucidated recently using single-particle cryo-electron microscopy [249]. The L5III domain formed one of the most exposed domains of the α subunit. The interaction between laminin β2 chain and P/Q- and N-type VGCCs is essential for NMJ function as well as presynaptic active zone formation and organization [63, 90].
P/Q-type VGCCs localize at motor terminal active zones [89, 189]. These VGCCs directly interact with synaptic vesicle related proteins syntaxin, synaptosomal associated protein of 25 kDa (SNAP-25), and synaptotagmin, and in turn SNAP-25 and syntaxin modify VGCC function [250–255]. In addition, a number of active zone proteins interact with different subunits of the VGCCs (Figure 3)[63, 90, 223, 242–244, 256–258]. Bassoon and CAST/ELKS2/Erc2 interact with the P/Q-type VGCC β subunits [90, 258], Bassoon interacts indirectly with P/Q-type VGCCs [223], and RIM1/2 interacts with P/Q- and N-type VGCC α subunits and β subunit [242–244, 256]. Bassoon and RIM1 have also been shown to inhibit the inactivation properties of P/Q-type VGCCs, prolonging the opening of these calcium channels localized with Bassoon and RIM1 compared to VGCCs without interacting with these active zone proteins [189, 242, 243]. Piccolo has been shown in pancreatic beta cells to interact with L-type VGCC α subunits aiding in vesicle secretion [257]. Thus, binding of active zone proteins to the VGCCs appears to be important for properly positioning these channels within active zones and regulating calcium influx. Alternatively, evidence supports that laminin β2 in the synaptic cleft binds to and organizes the locations of VGCCs, and it is this interaction that results in proper positioning of the active zones in front of junctional folds [63, 65]. Disruption of the VGCC-active zone protein relationship may reduce calcium influx during repeated depolarization and aged NMJs.
The basal lamina of NMJs
Skeletal muscle fibers are surrounded by a layer of connective tissue, the basement membrane, which can be further subdivided into an internal basal lamina layer connecting to the sarcolemma and an external reticular lamina layer [11, 12]. The basal lamina envelops the entire muscle fiber including the synaptic cleft between the nerve terminal and endplate, but the reticular lamina is excluded from the synaptic cleft of NMJs [12]. The basal lamina is made up largely of collagen IV molecules, laminins, and other non-collagenous proteins including entactin/nidogen, perlecan, and fibronectin [11, 12, 21–23]. The network of laminins and collagen are connected to one another by the glycoprotein entactin/nidogen, but also anchored to the sarcolemma and intracellular cytoskeleton by binding to laminin receptors integrin and dystroglycan [14, 17, 24, 25]. The basal lamina provides strength and structure to the skeletal muscle fibers, but rather than being a static structure, the basal lamina plays a direct role in key biological functions.
Components of the basal lamina have important regulatory roles in myogenesis and synaptogenesis, as these molecules help guide cell growth, cell migration, and adhesion of motor neuron axons [12, 26–30]. Localization of specific collagen and laminin chains to regions of the endplate basal lamina plays a role in organizing the pre- and post-synaptic structures of the NMJ. In mammals, collagen IV α2, α3, and α6 chains, as well as collagen XIII, are concentrated at mouse NMJs [12, 31–34]. Collagen molecules are important for development of NMJs. For instance, collagen IV α2 chains are necessary for formation of NMJs and differentiation of motor neuron terminals during embryonic development, and collagens IV α3 and α6 are required for maintenance of mature NMJs [33]. As will be described below, both laminin α and β chains play a prominent role in the development of pre- and post-synaptic structures of NMJs, with each laminin chain playing a specific role. Disruption of individual components of the basal lamina or the connections of the basal lamina to the muscle fiber itself results in muscular dystrophy and degeneration of muscle fibers and NMJs [35–38].
The role of laminins in NMJs and active zone organization
Laminins are important components of the basal lamina. These large, multi-armed glycoproteins (~400–900 kDa) assemble into heterotrimers composed of three different chains: α, β, and γ [11, 12, 21, 39–43]. In mice and humans, five different α chains, three different β chains, and three different γ chains have been identified. To date, 19 laminin trimers have been described (laminin 1–15, as well as laminin 5B) with laminin-111 (α1β1γ1, formerly laminin-1) being the first characterized in Engelbreth-Holm-Swarm (EHS) tumors. The different laminin chains assemble into cross or T-shaped molecules. C-terminal ends of laminin α chains wrap into a coiled structure containing laminin globular (LG) domains. N-terminal ends, known as “short arms,” are free and contain N-terminal globular (LN) domains [11, 12, 21, 39–43].
Laminins have been shown to play an essential role in myogenesis and synaptogenesis as these molecules guide cell differentiation, migration, and adhesion [12, 26–29, 44, 45]. Specific heterotrimers of laminin chains assemble in specific regions of different tissues. In the case of skeletal muscle, laminin-211 (α2β1γ1, laminin-2) predominates throughout the extrasynaptic basal lamina, but laminin-421 (α4β2γ1, laminin-9), laminin-521 (α5β2γ1, laminin-11), and laminin-221 (α2β2γ1, laminin-4) are expressed specifically at the synaptic cleft of NMJs (Figure 1A) [46]. Laminin γ1 chains are found throughout the sarcolemma and are essential for formation of basement membranes as knockout of laminin γ1 is lethal [45]. Both laminin α and β chains have been described to play specific roles in different aspects of development and maintenance of NMJs, as well as organization of presynaptic active zones.
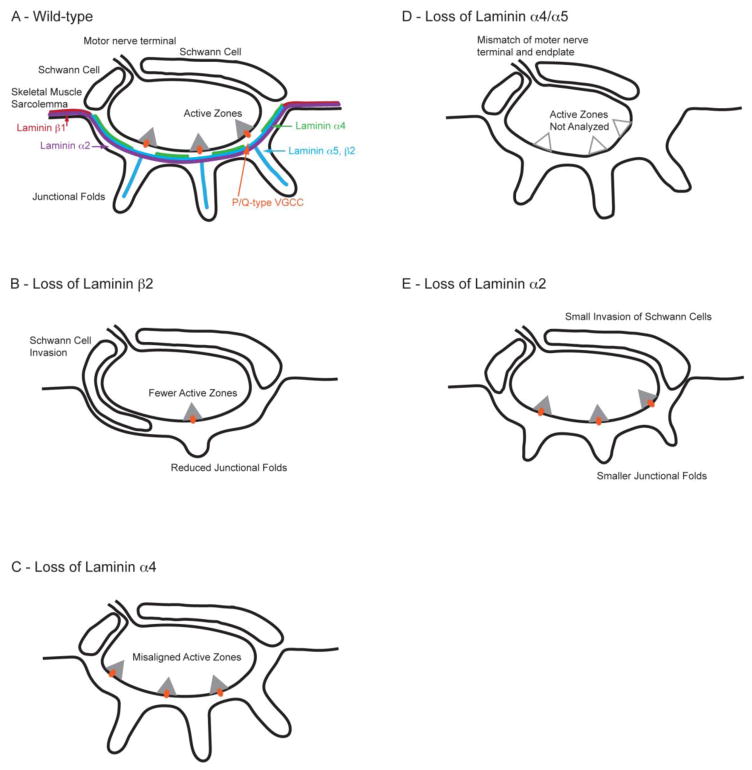
Figure 1.
The role of laminins at the NMJ. Laminin chains have specific roles in organizing the presynaptic active zones and endplate morphology. (A) Represents an NMJ of adult wild-type mouse. (B) Represents an NMJ of laminin β2 knockout mouse. There is a loss of active zones, reduced number of synaptic vesicles and junctional folds, and infiltration of the synaptic cleft by Schwann cells. (C) Represents an NMJ of laminin α4 knockout mouse. There is a misalignment between active zones and junctional folds. (D) Represents an NMJ of laminin α4 and α5 double knockout mouse where the nerve terminal size is small. (E) Represents an NMJ laminin α2 knockout mouse where there is a reduced number of junctional folds and moderate infiltration of synaptic cleft by Schwann cells.
Laminin β chains
Laminin β1 and β2 chains are homologous with nearly identical structures [31, 47]; however, localization patterns of these laminin chains vary even between different locations on the same cellular basal lamina. Laminin β2, formerly known as s-laminin due to its synaptic localization, is located at NMJs (Figure 1A and Figure 2), and also in developing brain, spinal cord, retina, and lens, capillaries, and kidney glomerulus [47–53]. In skeletal muscles, laminin β2 chains assemble with γ1 chains and either α2, α4, or α5 chains to form specific laminin molecules that are secreted from muscle and incorporated into the synaptic basal lamina [46, 47, 49, 54, 55]. Importantly, laminin β2 is specifically enriched at the basal lamina of NMJs but is not found at the extrasynaptic basal lamina (Figure 1A) [46, 55]. On the other hand, laminin β1 is found throughout the extrasynaptic basal lamina but excluded from NMJs [46, 55].
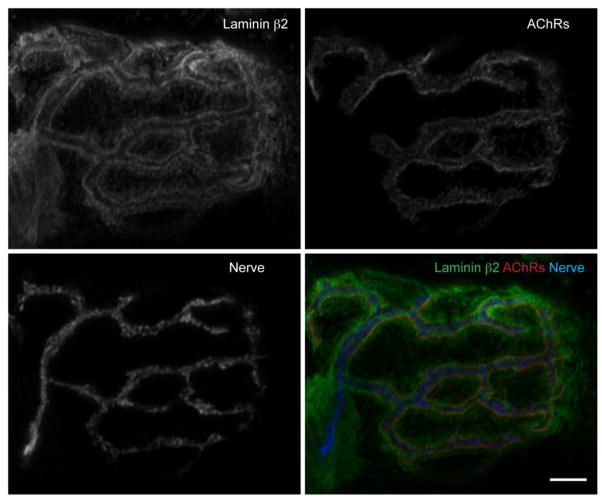
Figure 2.
Laminin β2 localization at an NMJ. Immunohistochemistry detection of laminin β2 in an NMJ of an adult (4 month old) wild-type C57Bl/6 mouse. Longitudinal sections of gastrocnemius muscle were stained with primary antibodies against laminin β2 (green), synaptic vesicle protein-2 (SV-2) and neurofilament to visualize the nerve terminal (Nerve, blue), and Alexa Fluor 594-labeled α-bungarotoxin to visualize acetylcholine receptors (AChRs, red). An NMJ was imaged on a confocal microscope and deconvolved. Scale bar represents 5 μm.
Early on, laminin β2 was shown in vitro to guide neurite outgrowth from motor neurons and promote differentiation into mature nerve terminals [30, 46, 56, 57]. The generation of a mouse model lacking laminin β2 proved insightful in the precise role of laminin β2 in synaptic development at the NMJ. Laminin β2 knockout mice die between days P15 and P30 due to failure of the renal glomerular filtration barrier [58, 59], and display malformed NMJs [58, 60, 61]. Despite being innervated initially, NMJs of laminin β2 knockout mice lack appropriate formation of pre- and post-terminal structures including a lack of junctional folds, reduced number of active zones, and Schwann cell infiltration of the synaptic cleft (Figure 1B) [58, 62, 63]. Different molecules have been shown to sequentially play a role in the development of NMJs from early embryonic stages to maintenance of adult NMJs [33]. Laminin β2 is important for maintenance and maturation of NMJs during the first few weeks after birth [33, 63]. On a functional level, NMJs of laminin β2 knockout mice have lower miniature endplate potential (mEPP) frequency and a higher likelihood of failure of synaptic transmission compared to wild-type mice [58, 60]. The reduction in neurotransmitter release in these mice relates to a reduced number of active zones and synaptic vesicles near the presynaptic membrane, as well as reduced protein levels of the active zone specific protein Bassoon and synaptic vesicle related proteins [58, 60, 61, 63]. Furthermore, laminin β2 knockout mice also have a failure in maturation of VGCC types that are present at presynaptic terminals of NMJs [61]. Laminin β2 knockout mice retained N-type VGCCs at presynaptic terminals, and fail to transition from expressing N- and P/Q-type VGCCs at presynaptic terminals shortly after birth to expressing predominately P/Q-type VGCCs during the first 2–3 weeks following birth that typically occurs in wild-type mice [61, 64]. Due to the loss of laminin β2, these knockout mice also have a loss of laminin-421 (laminin-9) and laminin-521 (laminin-11) at NMJs, which leads to investigation of the role of laminin α chains in organization of NMJs [58]. In summary, the loss of laminin β2 influences NMJ morphology and functionality partly by disrupting active zone formation and organization, and changing the composition of VGCC types at NMJs.
Laminin α chains
Laminin α2, α4, and α5 chains are essential for proper muscle development, as well as specifically establishing and maintaining the structure of NMJs and alignment of presynaptic active zones [20, 65–67]. Each of these laminin α chains plays a specific role in these processes. Laminins containing the laminin α2 chain are collectively known as merosin. Laminin α2 subunits assemble into laminin-211 (laminin-2) throughout the extrasynaptic skeletal muscle basal lamina [67–69], and assemble into laminin-221 (laminin-4) that is specific to NMJs. Laminin-211 promotes axon growth, migration of muscle cells, and Schwann cell functionality [56, 70, 71]. Mice harboring mutations of the laminin α2 gene display a muscular dystrophy-like syndrome [67], which is also observed in humans harboring genetic mutations in the gene encoding laminin α2 (lama2) [37, 72–75]. Laminin α2 mutant mice with a spontaneous hypomorphic allele (i.e., dy and dy2J mice) and other laminin α2 mutant models (dyW and dy3K mice) do not develop post-synaptic junctional folds at motor endplates, display partial detachment of motor neuron terminals from the endplate, have demyelination of motor axons, and minor Schwann cell infiltration into synaptic cleft (Figure 1E) [35, 36, 76–86]. However, these mutant mice do assemble active zones, which properly oppose AChRs that accumulate at endplates [11, 77]. Thus, laminin α2 is essential for skeletal muscle maintenance and formation of structures at muscle endplates, but may not be required for formation of presynaptic structures of NMJs.
Laminin α4 and α5 chains appear to be directly responsible for organizing presynaptic active zones and endplate structures in NMJs, with each of these laminin chains playing an individual role. Laminin α4 chains are part of laminin-421 (laminin-9) molecules and concentrate to the synaptic cleft of NMJs [46]. Laminin α4 localizes within the basal lamina where active zones are absent, and does not localize at the crest of junctional folds (Figure 1A) [65]. This locational specificity may serve as a mechanism by which laminin α4 aligns presynaptic active zones and post-synaptic endplate structures. Consistent with this hypothesis, laminin α4 knockout mice have a normal number of active zones and junctional folds; however, the alignment of these pre- and post-synaptic structures is disrupted (Figure 1C) [65]. In wild-type mice, 78% of active zones align with the junctional folds; however, this was reduced to 23% in laminin α4 knockout mice [65]. Furthermore, laminin α4 knockout mice suffer a neuromuscular defect and display a phenotype that resembles premature aging of NMJs; however, these mice do not display signs of myopathy or muscular dystrophy [65, 87].
Laminin α5 is expressed throughout the basement membrane of developing skeletal muscle during embryonic stage, but becomes concentrated at the synaptic cleft shortly after birth [46]. In postnatal stages, laminin α5 chains follow an expression pattern paralleling laminin β2 chain location, but the role of laminin α5 is different from laminin β2. Complete loss of laminin α5 is embryonically lethal due to multiple developmental defects [88]. However, mice with a muscle-specific laminin α5 knockout display arrested postsynaptic maturation of NMJs to the early postnatal stage [20]. To further investigate the role of laminin α4 and α5 in skeletal muscle, laminin α4 knockout mice were crossed with muscle-specific laminin α5 knockout mice to generate double knockout mice of laminin α4/α5 [20]. These double mutants are smaller and weaker than either single mutant and survived to three months of age [20]. In these double knockout mice, synapse elimination occurs normally. However, the remaining axon does not take over the AChR territory that was previously innervated by the losing axons (Figure 1D) [20]. In wild-type mice, the vacated area of an endplate will be occupied by the remaining winning axon. These results suggest a role for laminin α4 and α5 in presynaptic maturation. Furthermore, maturation of AChR clusters is arrested in laminin α4/α5 double knockout mice, and levels of the laminin receptor dystroglycan are reduced at NMJs [20]. Dystroglycan conditional knockout mice also have arrested maturation of AChR clusters on the post-synaptic membrane [20]. In support of these in vivo observations, primary myotube cultures prepared from laminin α4/α5 double knockout mice or muscle-specific laminin α5 knockout mice fail to form mature AChR clusters appropriately; however, myotube cultures prepared from laminin α4 or laminin β2 knockout mice are still capable of forming mature AChR clusters in vitro [20]. These results implicate the laminin α5-dystroglycan interaction is important for the maturation of AChR clusters in postnatal stages.
The role of laminin receptors in NMJ organization
A number of laminin receptors, including Bcam, dystroglycan, integrins, and VGCCs are localized to NMJs and participate in organizing pre- and post-synaptic structures as described briefly for dystroglycan above [10–20].
Voltage-gated calcium channels
In adult NMJs, P/Q-type VGCCs localize at presynaptic terminals specifically at active zones. For a more detailed description of VGCCs, please refer to Box #2. P/Q-type and N-type VGCCs have been identified as receptors for laminin β2, and the interaction of these two molecules is important for organizing presynaptic active zones as cytosolic domains of VGCCs also bind with active zone proteins (see Box #2, Figure 3, and [63]). A leucine-arginine-glutamic acid (LRE) segment in laminin β2 binds to the 11th extracellular loop of the α subunit of P/Q- and N-type VGCCs, but does not bind to other VGCC types (R- and L-type VGCCs) expressed in motor neurons [63]. The LRE sequence mediates cell adhesion and neurite outgrowth of ciliary ganglion neurons (muscle innervating neuron) induced by laminin β2 [28, 30]. Laminin β1 does not bind to P/Q-, N-, R-, or L-type VGCCs [63]. Disruption of the interaction between VGCCs and laminin β2 results in a loss of active zone proteins without influencing NMJ area or morphology [63].
Figure 3.
Schematic of active zone organization in mammalian neuromuscular junctions. Laminin β2 is a muscle derived active zone organizer that binds with presynaptic voltage-gated calcium channels (VGCC). Active zone proteins, Bassoon, CAST2/ELKS/Erc1, Piccolo, and Rim1/2, bind with VGCCs at the cytosolic domains and subunits. This link of laminin β2 of the basal lamina with active zone proteins organizes the active zones.
Direct evidence for the role of VGCCs in organizing NMJ active zones comes from VGCC knockout mice. P/Q-type VGCC knockout mice show decreased active zone formation and reduced protein levels of Bassoon in NMJs [63]. These knockout mice display a compensatory increase in NMJ localization of other VGCC isoforms (i.e., N- or R-type VGCCs) [63, 89]. This is problematic as typically during postnatal maturation there is a decrease in the dependence on N-type VGCCs and a shift towards dependence on P/Q-type VGCCs for synaptic transmission at NMJs [64]. Thus, NMJs from these mice display delayed maturation of NMJs as they fail to properly switch from N-type to P/Q-type VGCCs with compensatory upregulation of N-type VGCCs [61] that also bind laminin β2 chains confounding the interpretation of these results. To overcome the confluence of this compensation, P/Q- and N-type VGCC double knockout mice were generated [90]. These double knockout mice display proper NMJ innervation, but a loss of synaptic transmission and reduced levels of active zone specific proteins Bassoon, CAST2/ELKS/Erc1, and Piccolo [90]. Furthermore, VGCC α2δ subunit in Drosophila plays a role in synapse formation and loss of the α2δ-3 subunit results in disorganized active zones [91]. These findings support the role of VGCCs in active zone organization in NMJs.
Disruption of the interaction between laminin β2 and P/Q-type VGCCs also results in loss of active zone structures. Soluble recombinant proteins generated from P/Q-type VGCCs containing only the 11th extracellular loop block the binding of P/Q-type VGCCs with laminin-421 (laminin-9) or a recombinant protein of LRE segment [63]. This indicates that the binding site for laminin in P/Q-type VGCCs is contained in the 11th extracellular loop of the α subunit. In contrast, recombinant protein containing the 11th extracellular loop of L-type VGCCs did not block this binding [63]. Consistently, injecting mice with the recombinant protein of P/Q-type VGCC’s 11th extracellular loop to block the binding of laminin β2 with P/Q-type VGCCs results in a greater than 50% decrease in active zone number without altering postsynaptic AChR clustering [63]. Similarly, LEMS patients develop autoantibodies against P/Q-type VGCCs and cause internalization of P/Q-type VGCCs into nerve terminals [92–94]. Interestingly, some LEMS patients develop autoantibodies specifically against the laminin β2-binding domain on P/Q-type VGCCs, which may disrupt the binding of these molecules [95]. This loss of interaction between laminin β2 and VGCCs may lead to a reduced number of presynaptic active zones and muscle weakness [63, 96]. Consistently, mice injected with LEMS patient IgGs display reduced active zone number similar to patients [97]. Thus, VGCCs and their interaction with laminin β2 play an essential role in organizing the presynaptic active zones.
Dystroglycan
Dystroglycan is a laminin receptor that anchors the basement membrane laminin chains to the intracellular cytoskeleton of skeletal muscles by binding with dystrophin/utrophin/syntrophins/dystrobrevin [18, 98–104]. Dystroglycan is a component of the dystrophin-glycoprotein complex and is composed of α- and β-dystroglycan subunits encoded by a single gene [18]. α-dystroglycan functions as an extracellular receptor that binds to laminins but it also binds to perlecan, agrin, and neurexin. β-dystroglycan subunit is a transmembrane protein that links the extracellular α-dystroglycan to the cytoskeletal protein dystrophin [18]. Although dystroglycan is expressed throughout the sarcolemma, this receptor has higher concentration at NMJs [105]. Laminins (α4 and α5 chains) appear to be responsible for accumulating dystroglycan receptors to the post-synaptic membrane and this relationship is important for the maturation of AChR clusters [20, 106, 107]. Loss of dystroglycan in mice is embryonically lethal [108], and embryonic myotubes from dystroglycan null mice have poor AChR clustering and NMJ formation [109–111]. Arrested maturation of AChR cluster was confirmed in postnatal skeletal muscles of muscle-specific dystroglycan knockout mice [20]. As described earlier, mice lacking both laminin α4 and a muscle-specific knockout of laminin α5 (laminin α4/α5 knockout mice mentioned previously) also have arrested maturation of AChR clustering and reduced dystroglycan content at NMJs [20]. Thus, the relationship between laminin α4/α5 and dystroglycan is important for appropriate endplate development.
Integrins
Integrins are a well-characterized family of laminin receptors. Cooperation between laminin and integrins help to link the interior cytoskeleton of muscle cells to the extracellular laminin molecules of the basal lamina [14]. These transmembrane proteins mediate cell-to-cell and cell-to-matrix interactions, and these receptors also possess the ability to activate intracellular signaling pathways [14]. Integrins form heterodimers consisting of integrin α and β chains, each consisting of a number of different variants. Six members of the β1 family of integrins (α1β1, α2β1, α3β1, α6β1, α7β1, and α9β1) and three αv family members (αvβ3, αvβ5, αvβ8), as well as α6β4, have been shown to interact with different laminin chains [14, 39, 112–117]. Laminin α1, α2, α3, and α5 chains have integrin binding sites located mainly in the C-terminal LG domain, although binding sites in the “short arm” N-terminus have been shown for integrin α1β1 and α2β1 [116–119].
Integrin α7 is enriched at NMJs and remain present at NMJs following denervation [13]. Through alternative splicing, integrin α7 is cleaved into three cytoplasmic domain variants (α7A, α7B, and α7C) [120–122]. Variants of integrin α7 are expressed differentially during development with the integrin α7A variant appearing immediately postnatally at NMJs, while the integrin α7B variant does not concentrate at NMJs until 2 weeks postnatally [13]. The integrin α7C variant is expressed both at NMJs and extrasynaptically [13]. In humans, genetic mutations of the gene encoding integrin α7 result in myopathy [123]. In mice, knockout of integrin α7 result in a loss of postsynaptic junctional folds, detachment of the myotendinous junction through loss of binding with laminin-211 (laminin-4), and myopathies similar to muscular dystrophy [124–126]. Dimers of integrin α7 and integrin β1 are predominately expressed in skeletal muscle [121, 127]. Both integrin α7β1 and dystroglycan are required for polymerization of laminins on the cell surface of cultured myotubes and these two receptors link actin filaments of the skeletal muscle fiber cytoskeleton to extracellular laminins [14, 71, 128]. Dystroglycan links to actin by binding to a complex of dystrophin/utrophin/syntrophins while integrin α7β1 links to actin by binding to a complex of talin and vinculin/metavinculin, and these two structures are linked to one another extracellularly by laminin [14]. Thus, cooperation between integrin α7β1 and dystroglycan link the cytoskeleton to laminins in the basal lamina in skeletal muscle, anchoring interior components of the muscle fiber with the basement membrane.
Other integrin subunits are important for muscle and active zone formation. Integrin β1 regulates skeletal muscle fiber development and is essential for myotube fusion and assembly of sarcomeres [129]. Furthermore, integrin β1 in skeletal muscle, but not in motor neurons, is required for innervation of NMJs [130]. Similar to integrin α7, integrin β1 is spliced and the integrin β1D variant appears to be the dominant integrin variant expressed in adult skeletal muscle [131, 132]. Integrin α3β1 has also emerged as important laminin receptor for active zone organization aiding laminin α4 in this process. Both integrins α3 and β1 are located at NMJs and expressed in motor neurons [13]. Integrin α3 is found at nerve terminal of Torpedo californica electric organs and concentrates near active zones of nerve terminals in Xenopus laevis [133–135]. Laminin molecules containing laminin α4 chains bind with integrin α3, α6, and α7 chains, which complex with integrin β1 chains [136–138]. Integrin α3β1 dimers have been reported to complex with laminin 421 (laminin-9), active zone proteins Bassoon and Piccolo, and VGCCs in Torpedo californica electric organ synapses, which is a modified neuromuscular synapse [135, 139]. Thus, it has been hypothesized that this interaction between laminin α4 and integrin α3β1 is important for laminin α4’s role as an active zone organizer in Torpedo electric organ synapses [135]. In summary, interactions between laminin receptors such as VGCCs, dystroglycan, and integrins with specific laminin chains are important for multiple aspects of NMJ development and maturation, especially development of presynaptic active zones.
Diseases associated with the loss of laminins or laminin-receptor interactions
Disorders that alter the NMJ organization and muscle function have been associated with the loss of specific laminin chains or disruption of the interaction between laminins and laminin receptors. In humans, laminin β2 gene (lamb2) has been localized on chromosome 3p21 and consists of 32 exons spanning 12 kb of genomic DNA making a protein consisting of 1,798 amino acids [140]. Recently, 49 different mutations in lamb2 gene have been described resulting in recessive genetic disorders of varying degrees of severity including Pierson syndrome [140]. Pierson syndrome gives rise to a disruption of NMJs, ocular anomalies (i.e., microcoria, abnormal lens shape, and retinal abnormalities), and severe congenital nephrotic syndrome [140–144]. Pierson Syndrome patients usually pass away shortly after birth due to kidney disease. Individuals that do survive for longer can display developmental and neurological defects, including disrupted function and organization of NMJs [140, 142, 143, 145]. A patient suffering from a congenital myasthenic syndrome with a mutation of lamb2 showed reduced mEPP amplitude, decay, and frequency, reduced quantal content, reduced endplate area, reduced number of active zones, reduced number of synaptic vesicles, and Schwann cell infiltration of NMJs [144]. The symptoms of Pierson syndrome patients are consistent with what is observed in laminin β2 knockout mice.
Loss of laminin α2 results in a muscular dystrophy-like syndrome. In humans, recessive familial disruption of the gene encoding laminin α2 protein (lama2), located on chromosome 6q22-q23, results in merosin-deficient congenital muscular dystrophy type 1A (MDC1A) [66, 146]. At birth or shortly thereafter, patients are hypotonic becoming non-ambulatory, require respiratory assistance, and pass away in the first decade of life or soon after in severe cases. Cerebral white matter abnormalities have been found by magnetic resonance imaging (MRI) in many cases, and seizures and mental retardation have been reported in a small portion of cases [66]. A number of different mutations have been observed resulting in a complete or partial loss of laminin α2 protein [37, 66, 72–75, 146–150]. Some patients have only a partial decrease in laminin α2 protein levels, and these patients have better prognosis becoming ambulatory with longer life expectancy although a progressive muscle weakness may persist [72, 149]. The human form of MDC1A mimics animal models where reduced levels of laminin α2 protein results in muscular dystrophy. In these animal models, severity of reductions in laminin α2 protein levels correlates with the severity of phenotype [66, 67]. As mentioned previously, these mice have reduced junctional folds, detachment of motor neuron from the endplate, demyelination, and infiltration of Schwann cells into the synaptic cleft.
LEMS is a form of myasthenia resulting from autoimmune attack of VGCCs leading to neuromuscular failure [92–97, 151]. These patients have muscle weakness and fatigue, reduced synaptic transmission, and reduced active zone number. Over half of the patients have small cell lung carcinoma and the treatment of cancer reduces auto-antibodies [152–155], which can be the source of the VGCC antigens [156, 157]. The auto-antibodies produced against P/Q-type VGCCs result in internalization of VGCCs into nerve terminals, and potentially disrupt binding between laminin β2 and VGCCs, both of which leads to a loss of presynaptic active zones and muscle weakness [96]. The most common treatment for LEMS patients is the use of 3,4-diaminopyridine (3,4-DAP), a potassium channel blocker that prolongs the length of action potentials, leading to modest improvements in symptomology [158]. Recently, combining 3,4-DAP with a novel calcium channel agonist (GV-58) has led to a reversal of symptomology in a mouse model of LEMS [159, 160]. GV-58 acts specifically towards N- and P/Q-type VGCCs, not L-type VGCCs, increasing calcium influx during depolarization [159]. Use of this pharmacological combination restores neurotransmitter release in a LEMS mouse model [160]. These findings highlight the importance of VGCCs in the organization of presynaptic active zones, but also introduce this interaction as a favorable treatment target in some neuromuscular disorders.
Laminins, aging, and exercise
Aging skeletal muscle undergoes a progressive loss of muscle mass (sarcopenia) and strength leading to decreased quality of life and increased risk of falling, fractures, and mortality [161–163]. During aging, the pre- and post-synaptic NMJ morphology becomes disrupted. On the presynaptic side, motor neuron terminals atrophy during aging compared to young humans and rodents although the number of nerve terminal branches increases [164–170]. In addition, there is altered synaptic transmission, increased quantal release, decreased synaptic vesicle number, and a decrease in the number of active zones and content of active zone proteins [164, 171–175]. On the post-synaptic side, the endplate area expands despite AChR cluster area becoming fragmented ultimately leading to a progressive denervation of the NMJ [164, 166, 170, 172, 176–185]. These findings have been complicated by the examination of different muscle fiber types as fast and slow muscle fibers display differences in the influence of aging on NMJ morphology. Fast muscle fibers (i.e., extensor digitorum longus, EDL, muscle) typically show age-related changes in morphology prior to slow muscle fibers (i.e., soleus, diaphragm muscles) [179, 181, 186, 187]. Denervation during aging may be different between muscle fiber types as one report showed that denervation was greater in 29 month old mice in fast-twitch muscle fibers (i.e., EDL muscle) compared to slow twitch muscle fibers (i.e., soleus) [181]; however, another report shows that denervation differences between muscle fiber types was not different in two year old mice [188]. Thus advanced aged beyond two years may be necessary to compare denervation patterns between muscle types.
The loss of NMJ innervation precedes the degradation of spinal motor neurons [180, 181], which suggest a dying back neuropathy or distal axonopathy. In mice, partial denervation within individual NMJs begins to occur at approximately 18 months of age, and becomes more pronounced by 24 months of age and older [180]. In addition, we have observed that aging results in a reduced number of presynaptic active zones and a reduced level of Bassoon protein at presynaptic terminals [175, 189]. The loss of Bassoon will impair VGCC functionality and depress nerve transmission potentially leading to a loss of muscle strength or denervation [189]. One study has shown that protein levels of laminin α4, α5, and β2 chains are not reduced in NMJs during aging; however, the pattern of laminin localization in NMJs changes in parallel to changes of NMJ morphology [87]. This opens the possibility of changes in localization of specific laminin chains are partly causative of NMJ degeneration during aging.
Exercise has been shown to positively influence NMJs in aging muscle [168, 180, 183–185, 190–193]. Voluntary wheel running and treadmill running in aged mice and rats has been shown to reduce aging induced post-synaptic AChR fragmentation and help maintain NMJ innervation [180, 184, 193]. Even just one month of voluntary wheel running in 22-month-old mice reduces denervation of aged NMJs [180]. In rodents, endplate area expands and AChR clusters become fragmented during aging. However, forced treadmill running reduces post-synaptic endplate area and decreases fragmentation of AChR clusters in aged animals [168, 183, 191, 194]. Importantly, resistance training has also been shown to positively influence NMJ morphology and reduce AChR fragmentation in both adult and aged humans and rodents [185, 195].
Exercise has also been shown to modulate presynaptic active zone protein content. Active zone protein levels in NMJs are reduced during aging, but resistance training of 24-month old rats restores Bassoon to young adult levels [189]. No other reports, to our knowledge, have investigated active zone proteins at the NMJ with exercise training. More work is needed to elucidate the molecular mechanisms by which exercise modulates active zones and active zone proteins during aging. It is possible that exercise stimulates expression of activity dependent genes, synaptic laminin expression, and laminin-receptor interactions that in turn positively influence pre- and post-synaptic gene expression level, NMJ morphology, and innervation. Exercise activates AMPK by phosphorylating AMPKα (Thr172) in muscles [196–199] and directly phosphorylates and activates peroxisome-proliferator-activated receptor gamma co-activator 1α (PGC-1α) [200]. PGC-1α enhances transcription of NMJ genes including laminin β2 in C2C12 muscle cells [201]. However, laminin β2 mRNA expression did not increase in vivo in gastrocnemius muscle from transgenic mice overexpressing PGC-1α [201]. In a single human study, four hours after a single bout of resistance exercise laminin β2 gene expression was increased in subjects that previously performed 12 weeks of resistance training [202]. Elsewhere, Ogasawara et al. showed that resistance exercise increases integrin β1 levels without changing laminin α2 or integrin α7, however other laminin chains were not examined [203]. Thus, upstream regulators that govern the response to exercise may have a direct influence over synaptic laminins and NMJ organizers.
Conclusion
Synapse specific laminin chains and their interaction with laminin receptors are essential for NMJ formation and organization of pre- and post-synaptic structures. Loss of specific laminin chains or laminin receptors, as well as blocking the interaction between laminins and laminin receptors results in changes in NMJ innervation, morphology, and organization of pre- and post-synaptic structures including the active zones and junctional folds. Increasing levels of laminins, especially laminin β2, or increasing the signaling pathways stimulated by synaptic laminins are a viable molecular target to improve neuromuscular disease outcomes.
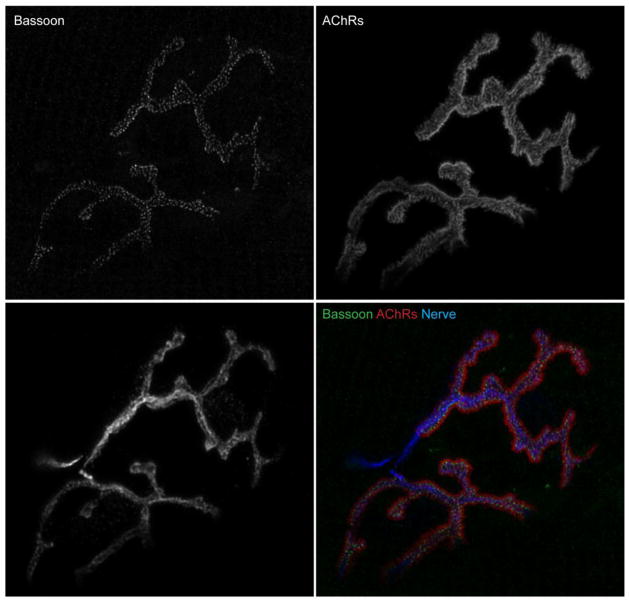
Figure 4.
Bassoon localization at an NMJ. Immunohistochemistry detection of active zone specific protein Bassoon in an NMJ of an adult (4 month old) wild-type C57Bl/6 mouse. Longitudinal sections of gastrocnemius muscle were stained with primary antibodies against Bassoon (green), SV-2 and neurofilament to visualize nerve terminal (Nerve, blue), and Alexa Fluor 594-labeled α-bungarotoxin to visualize acetylcholine receptors (AChRs, red). An NMJ was imaged on a confocal microscope and deconvolved. Scale bar represents 5 μm.
Acknowledgments
We thank Dr. Ambra Pozzi for the invitation for this manuscript. Funding: This work was supported by the National Institutes of Health 1R01NS078214 and 1R01AG051470 (H.N.), from National Institutes of Health K-INBRE postdoctoral award P20 GM103418 (R.S.R.).
Abbreviations
- 3,4-DAP
3,4-diaminopyridine
- ACh
acetylcholine
- AChR
acetylcholine receptor
- AMPK
adenosine monophosphate-activated protein kinase
- Bcam
basal cell adhesion molecule/Lutheran blood group antigen
- CAZ
cytomatrix of the active zone
- CAST
CAZ-associated structural protein
- EHS
Engelbreth-Holm-Swarm
- EDL
extensor digitorum longus
- LEMS
Lambert-Eaton myasthenic syndrome
- LRE
leucine-arginine-glutamic acid
- MDC1A
segment, merosin deficient congenital muscular dystrophy type 1A
- mEPP
miniature end plate potential
- NMJ
neuromuscular junction
- RIM
Rab3-interacting molecule
- SNAP-25
synaptosomal associated protein of 25 kDa
- VGCC
voltage-gated calcium channel
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Author contributions: RSR and HN wrote the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Couteaux R, Pecot-Dechavassine M. Synaptic vesicles and pouches at the level of “active zones” of the neuromuscular junction. C R Acad Sci Hebd Seances Acad Sci D. 1970;271(25):2346–9. [PubMed] [Google Scholar]
- 2.Heuser JE, Reese TS, Dennis MJ, Jan Y, Jan L, Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirokawa N, Heuser JE. Internal and external differentiations of the postsynaptic membrane at the neuromuscular junction. J Neurocytol. 1982;11(3):487–510. doi: 10.1007/BF01257990. [DOI] [PubMed] [Google Scholar]
- 4.Clarke GL, Chen J, Nishimune H. Presynaptic Active Zone Density during Development and Synaptic Plasticity. Front Mol Neurosci. 2012;5:12. doi: 10.3389/fnmol.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudhof TC. The presynaptic active zone. Neuron. 2012;75(1):11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fertuck HC, Salpeter MM. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-alpha-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976;69(1):144–58. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson MJ, Cohen MW. Nerve-induced and spontaneous redistribution of acetylcholine receptors on cultured muscle cells. J Physiol. 1977;268(3):757–73. doi: 10.1113/jphysiol.1977.sp011880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank E, Fischbach GD. Early events in neuromuscular junction formation in vitro: induction of acetylcholine receptor clusters in the postsynaptic membrane and morphology of newly formed synapses. J Cell Biol. 1979;83(1):143–58. doi: 10.1083/jcb.83.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanes JR, Lichtman JW. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat Rev Neurosci. 2001;2(11):791–805. doi: 10.1038/35097557. [DOI] [PubMed] [Google Scholar]
- 10.Darabid H, Perez-Gonzalez AP, Robitaille R. Neuromuscular synaptogenesis: coordinating partners with multiple functions. Nat Rev Neurosci. 2014;15(11):703–18. [PubMed] [Google Scholar]
- 11.Patton BL. Basal lamina and the organization of neuromuscular synapses. J Neurocytol. 2003;32(5–8):883–903. doi: 10.1023/B:NEUR.0000020630.74955.19. [DOI] [PubMed] [Google Scholar]
- 12.Sanes JR. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 2003;278(15):12601–4. doi: 10.1074/jbc.R200027200. [DOI] [PubMed] [Google Scholar]
- 13.Martin PT, Kaufman SJ, Kramer RH, Sanes JR. Synaptic integrins in developing, adult, and mutant muscle: selective association of alpha1, alpha7A, and alpha7B integrins with the neuromuscular junction. Dev Biol. 1996;174(1):125–39. doi: 10.1006/dbio.1996.0057. [DOI] [PubMed] [Google Scholar]
- 14.Belkin AM, Stepp MA. Integrins as receptors for laminins. Microsc Res Tech. 2000;51(3):280–301. doi: 10.1002/1097-0029(20001101)51:3<280::AID-JEMT7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 15.Timpl R, Tisi D, Talts JF, Andac Z, Sasaki T, Hohenester E. Structure and function of laminin LG modules. Matrix Biol. 2000;19(4):309–17. doi: 10.1016/s0945-053x(00)00072-x. [DOI] [PubMed] [Google Scholar]
- 16.Kikkawa Y, Miner JH. Review: Lutheran/B-CAM: a laminin receptor on red blood cells and in various tissues. Connect Tissue Res. 2005;46(4–5):193–9. doi: 10.1080/03008200500344074. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki N, Yokoyama F, Nomizu M. Functional sites in the laminin alpha chains. Connect Tissue Res. 2005;46(3):142–52. doi: 10.1080/03008200591008527. [DOI] [PubMed] [Google Scholar]
- 18.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119(Pt 2):199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- 19.Nishiuchi R, Takagi J, Hayashi M, Ido H, Yagi Y, Sanzen N, Tsuji T, Yamada M, Sekiguchi K. Ligand-binding specificities of laminin-binding integrins: a comprehensive survey of laminin-integrin interactions using recombinant alpha3beta1, alpha6beta1, alpha7beta1 and alpha6beta4 integrins. Matrix Biol. 2006;25(3):189–97. doi: 10.1016/j.matbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Nishimune H, Valdez G, Jarad G, Moulson CL, Muller U, Miner JH, Sanes JR. Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J Cell Biol. 2008;182(6):1201–15. doi: 10.1083/jcb.200805095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin--a glycoprotein from basement membranes. J Biol Chem. 1979;254(19):9933–7. [PubMed] [Google Scholar]
- 22.Timpl R. Processed and non-processed forms of procollagens. Biochem Soc Trans. 1984;12(6):924–7. doi: 10.1042/bst0120924. [DOI] [PubMed] [Google Scholar]
- 23.Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18(2):123–32. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- 24.Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem. 2003;278(17):14587–90. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- 25.Michele DE, Campbell KP. Dystrophin-glycoprotein complex: post-translational processing and dystroglycan function. J Biol Chem. 2003;278(18):15457–60. doi: 10.1074/jbc.R200031200. [DOI] [PubMed] [Google Scholar]
- 26.Hauschka SD, Konigsberg IR. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966;55(1):119–26. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foster RF, Thompson JM, Kaufman SJ. A laminin substrate promotes myogenesis in rat skeletal muscle cultures: analysis of replication and development using antidesmin and anti-BrdUrd monoclonal antibodies. Dev Biol. 1987;122(1):11–20. doi: 10.1016/0012-1606(87)90327-7. [DOI] [PubMed] [Google Scholar]
- 28.Hunter DD, Porter BE, Bulock JW, Adams SP, Merlie JP, Sanes JR. Primary sequence of a motor neuron-selective adhesive site in the synaptic basal lamina protein S-laminin. Cell. 1989;59(5):905–13. doi: 10.1016/0092-8674(89)90613-2. [DOI] [PubMed] [Google Scholar]
- 29.Hunter DD, Cashman N, Morris-Valero R, Bulock JW, Adams SP, Sanes JR. An LRE (leucine-arginine-glutamate)-dependent mechanism for adhesion of neurons to S-laminin. J Neurosci. 1991;11(12):3960–71. doi: 10.1523/JNEUROSCI.11-12-03960.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter BE, Weis J, Sanes JR. A motoneuron-selective stop signal in the synaptic protein S-laminin. Neuron. 1995;14(3):549–59. doi: 10.1016/0896-6273(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 31.Sanes JR, Engvall E, Butkowski R, Hunter DD. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990;111(4):1685–99. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miner JH, Sanes JR. Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. J Cell Biol. 1994;127(3):879–91. doi: 10.1083/jcb.127.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fox MA, Sanes JR, Borza DB, Eswarakumar VP, Fassler R, Hudson BG, John SW, Ninomiya Y, Pedchenko V, Pfaff SL, Rheault MN, Sado Y, Segal Y, Werle MJ, Umemori H. Distinct target-derived signals organize formation, maturation, and maintenance of motor nerve terminals. Cell. 2007;129(1):179–93. doi: 10.1016/j.cell.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 34.Latvanlehto A, Fox MA, Sormunen R, Tu H, Oikarainen T, Koski A, Naumenko N, Shakirzyanova A, Kallio M, Ilves M, Giniatullin R, Sanes JR, Pihlajaniemi T. Muscle-derived collagen XIII regulates maturation of the skeletal neuromuscular junction. J Neurosci. 2010;30(37):12230–41. doi: 10.1523/JNEUROSCI.5518-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Christmas P, Wu XR, Wewer UM, Engvall E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc Natl Acad Sci U S A. 1994;91(12):5572–6. doi: 10.1073/pnas.91.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H, Wu XR, Wewer UM, Engvall E. Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nat Genet. 1994;8(3):297–302. doi: 10.1038/ng1194-297. [DOI] [PubMed] [Google Scholar]
- 37.Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tome FM, Schwartz K, Fardeau M, Tryggvason K, et al. Mutations in the laminin alpha 2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet. 1995;11(2):216–8. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- 38.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82(2):291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- 39.Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn. 2000;218(2):213–34. doi: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 40.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–84. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 41.Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JC, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, von der Mark K, Wewer UM, Yamada Y, Yurchenco PD. A simplified laminin nomenclature. Matrix Biol. 2005;24(5):326–32. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 42.Tzu J, Marinkovich MP. Bridging structure with function: structural, regulatory, and developmental role of laminins. Int J Biochem Cell Biol. 2008;40(2):199–214. doi: 10.1016/j.biocel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durbeej M. Laminins. Cell Tissue Res. 2010;339(1):259–68. doi: 10.1007/s00441-009-0838-2. [DOI] [PubMed] [Google Scholar]
- 44.Vachon PH, Loechel F, Xu H, Wewer UM, Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J Cell Biol. 1996;134(6):1483–97. doi: 10.1083/jcb.134.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol. 1999;144(1):151–60. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patton BL, Miner JH, Chiu AY, Sanes JR. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J Cell Biol. 1997;139(6):1507–21. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989;338(6212):229–34. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- 48.Sanes JR, Hall ZW. Antibodies that bind specifically to synaptic sites on muscle fiber basal lamina. J Cell Biol. 1979;83(2 Pt 1):357–70. doi: 10.1083/jcb.83.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chiu AY, Sanes JR. Development of basal lamina in synaptic and extrasynaptic portions of embryonic rat muscle. Dev Biol. 1984;103(2):456–67. doi: 10.1016/0012-1606(84)90333-6. [DOI] [PubMed] [Google Scholar]
- 50.Hunter DD, Murphy MD, Olsson CV, Brunken WJ. S-laminin expression in adult and developing retinae: a potential cue for photoreceptor morphogenesis. Neuron. 1992;8(3):399–413. doi: 10.1016/0896-6273(92)90269-j. [DOI] [PubMed] [Google Scholar]
- 51.Hunter DD, Llinas R, Ard M, Merlie JP, Sanes JR. Expression of s-laminin and laminin in the developing rat central nervous system. J Comp Neurol. 1992;323(2):238–51. doi: 10.1002/cne.903230208. [DOI] [PubMed] [Google Scholar]
- 52.Libby RT, Hunter DD, Brunken WJ. Developmental expression of laminin beta 2 in rat retina. Further support for a role in rod morphogenesis. Invest Ophthalmol Vis Sci. 1996;37(8):1651–61. [PubMed] [Google Scholar]
- 53.Libby RT, Xu Y, Selfors LM, Brunken WJ, Hunter DD. Identification of the cellular source of laminin beta2 in adult and developing vertebrate retinae. J Comp Neurol. 1997;389(4):655–67. doi: 10.1002/(sici)1096-9861(19971229)389:4<655::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 54.Green TL, Hunter DD, Chan W, Merlie JP, Sanes JR. Synthesis and assembly of the synaptic cleft protein S-laminin by cultured cells. J Biol Chem. 1992;267(3):2014–22. [PubMed] [Google Scholar]
- 55.Martin PT, Ettinger AJ, Sanes JR. A synaptic localization domain in the synaptic cleft protein laminin beta 2 (s-laminin) Science. 1995;269(5222):413–6. doi: 10.1126/science.7618109. [DOI] [PubMed] [Google Scholar]
- 56.Cho SI, Ko J, Patton BL, Sanes JR, Chiu AY. Motor neurons and Schwann cells distinguish between synaptic and extrasynaptic isoforms of laminin. J Neurobiol. 1998;37(3):339–58. [PubMed] [Google Scholar]
- 57.Son YJ, Patton BL, Sanes JR. Induction of presynaptic differentiation in cultured neurons by extracellular matrix components. Eur J Neurosci. 1999;11(10):3457–67. doi: 10.1046/j.1460-9568.1999.00766.x. [DOI] [PubMed] [Google Scholar]
- 58.Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature. 1995;374(6519):258–62. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- 59.Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat Genet. 1995;10(4):400–6. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- 60.Knight D, Tolley LK, Kim DK, Lavidis NA, Noakes PG. Functional analysis of neurotransmission at beta2-laminin deficient terminals. J Physiol. 2003;546(Pt 3):789–800. doi: 10.1113/jphysiol.2002.030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chand KK, Lee KM, Schenning MP, Lavidis NA, Noakes PG. Loss of beta2-laminin alters calcium sensitivity and voltage-gated calcium channel maturation of neurotransmission at the neuromuscular junction. J Physiol. 2015;593(1):245–65. doi: 10.1113/jphysiol.2014.284133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patton BL, Chiu AY, Sanes JR. Synaptic laminin prevents glial entry into the synaptic cleft. Nature. 1998;393(6686):698–701. doi: 10.1038/31502. [DOI] [PubMed] [Google Scholar]
- 63.Nishimune H, Sanes JR, Carlson SS. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature. 2004;432(7017):580–7. doi: 10.1038/nature03112. [DOI] [PubMed] [Google Scholar]
- 64.Rosato Siri MD, Uchitel OD. Calcium channels coupled to neurotransmitter release at neonatal rat neuromuscular junctions. J Physiol. 1999;514( Pt 2):533–40. doi: 10.1111/j.1469-7793.1999.533ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patton BL, Cunningham JM, Thyboll J, Kortesmaa J, Westerblad H, Edstrom L, Tryggvason K, Sanes JR. Properly formed but improperly localized synaptic specializations in the absence of laminin alpha4. Nat Neurosci. 2001;4(6):597–604. doi: 10.1038/88414. [DOI] [PubMed] [Google Scholar]
- 66.Mendell JR, Boue DR, Martin PT. The congenital muscular dystrophies: recent advances and molecular insights. Pediatr Dev Pathol. 2006;9(6):427–43. doi: 10.2350/06-07-0127.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Holmberg J, Durbeej M. Laminin-211 in skeletal muscle function. Cell Adh Migr. 2013;7(1):111–21. doi: 10.4161/cam.22618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leivo I, Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci U S A. 1988;85(5):1544–8. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ehrig K, Leivo I, Argraves WS, Ruoslahti E, Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci U S A. 1990;87(9):3264–8. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao CC, Ziober BL, Sutherland AE, Mendrick DL, Kramer RH. Laminins promote the locomotion of skeletal myoblasts via the alpha 7 integrin receptor. J Cell Sci. 1996;109( Pt 13):3139–50. doi: 10.1242/jcs.109.13.3139. [DOI] [PubMed] [Google Scholar]
- 71.Montanaro F, Lindenbaum M, Carbonetto S. alpha-Dystroglycan is a laminin receptor involved in extracellular matrix assembly on myotubes and muscle cell viability. J Cell Biol. 1999;145(6):1325–40. doi: 10.1083/jcb.145.6.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herrmann R, Straub V, Meyer K, Kahn T, Wagner M, Voit T. Congenital muscular dystrophy with laminin alpha 2 chain deficiency: identification of a new intermediate phenotype and correlation of clinical findings to muscle immunohistochemistry. Eur J Pediatr. 1996;155(11):968–76. doi: 10.1007/BF02282889. [DOI] [PubMed] [Google Scholar]
- 73.Nissinen M, Helbling-Leclerc A, Zhang X, Evangelista T, Topaloglu H, Cruaud C, Weissenbach J, Fardeau M, Tome FM, Schwartz K, Tryggvason K, Guicheney P. Substitution of a conserved cysteine-996 in a cysteine-rich motif of the laminin alpha2-chain in congenital muscular dystrophy with partial deficiency of the protein. Am J Hum Genet. 1996;58(6):1177–84. [PMC free article] [PubMed] [Google Scholar]
- 74.Pegoraro E, Marks H, Garcia CA, Crawford T, Mancias P, Connolly AM, Fanin M, Martinello F, Trevisan CP, Angelini C, Stella A, Scavina M, Munk RL, Servidei S, Bonnemann CC, Bertorini T, Acsadi G, Thompson CE, Gagnon D, Hoganson G, Carver V, Zimmerman RA, Hoffman EP. Laminin alpha2 muscular dystrophy: genotype/phenotype studies of 22 patients. Neurology. 1998;51(1):101–10. doi: 10.1212/wnl.51.1.101. [DOI] [PubMed] [Google Scholar]
- 75.Coral-Vazquez RM, Rosas-Vargas H, Meza-Espinosa P, Mendoza I, Huicochea JC, Ramon G, Salamanca F. Severe congenital muscular dystrophy in a Mexican family with a new nonsense mutation (R2578X) in the laminin alpha-2 gene. J Hum Genet. 2003;48(2):91–5. doi: 10.1007/s100380300013. [DOI] [PubMed] [Google Scholar]
- 76.Meier H, Southard JL. Muscular dystrophy in the mouse caused by an allele at the dy-locus. Life Sci. 1970;9(3):137–44. doi: 10.1016/0024-3205(70)90306-1. [DOI] [PubMed] [Google Scholar]
- 77.Gilbert JJ, Steinberg MC, Banker BQ. Ultrastructural alterations of the motor end plate in myotonic dystrophy of the mouse (dy2J dy2J) J Neuropathol Exp Neurol. 1973;32(3):345–64. doi: 10.1097/00005072-197307000-00001. [DOI] [PubMed] [Google Scholar]
- 78.Banker BQ, Hirst NS, Chester CS, Fok RY. Histometric and electron cytochemical study of muscle in the dystrophic mouse. Ann N Y Acad Sci. 1979;317:115–31. [PubMed] [Google Scholar]
- 79.Law PK, Saito A, Fleischer S. Ultrastructural changes in muscle and motor end-plate of the dystrophic mouse. Exp Neurol. 1983;80(2):361–82. doi: 10.1016/0014-4886(83)90289-3. [DOI] [PubMed] [Google Scholar]
- 80.Sunada Y, Bernier SM, Kozak CA, Yamada Y, Campbell KP. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J Biol Chem. 1994;269(19):13729–32. [PubMed] [Google Scholar]
- 81.Desaki J, Matsuda S, Sakanaka M. Morphological changes of neuromuscular junctions in the dystrophic (dy) mouse: a scanning and transmission electron microscopic study. J Electron Microsc (Tokyo) 1995;44(2):59–65. [PubMed] [Google Scholar]
- 82.Sunada Y, Bernier SM, Utani A, Yamada Y, Campbell KP. Identification of a novel mutant transcript of laminin alpha 2 chain gene responsible for muscular dystrophy and dysmyelination in dy2J mice. Hum Mol Genet. 1995;4(6):1055–61. doi: 10.1093/hmg/4.6.1055. [DOI] [PubMed] [Google Scholar]
- 83.Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Nabeshima Y, Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415(1):33–9. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- 84.Kuang W, Xu H, Vachon PH, Engvall E. Disruption of the lama2 gene in embryonic stem cells: laminin alpha 2 is necessary for sustenance of mature muscle cells. Exp Cell Res. 1998;241(1):117–25. doi: 10.1006/excr.1998.4025. [DOI] [PubMed] [Google Scholar]
- 85.Kuang W, Xu H, Vachon PH, Liu L, Loechel F, Wewer UM, Engvall E. Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. J Clin Invest. 1998;102(4):844–52. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patton BL. Laminins of the neuromuscular system. Microsc Res Tech. 2000;51(3):247–61. doi: 10.1002/1097-0029(20001101)51:3<247::AID-JEMT5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 87.Samuel MA, Valdez G, Tapia JC, Lichtman JW, Sanes JR. Agrin and synaptic laminin are required to maintain adult neuromuscular junctions. PLoS One. 2012;7(10):e46663. doi: 10.1371/journal.pone.0046663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miner JH, Cunningham J, Sanes JR. Roles for laminin in embryogenesis: exencephaly, syndactyly, and placentopathy in mice lacking the laminin alpha5 chain. J Cell Biol. 1998;143(6):1713–23. doi: 10.1083/jcb.143.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Urbano FJ, Piedras-Renteria ES, Jun K, Shin HS, Uchitel OD, Tsien RW. Altered properties of quantal neurotransmitter release at endplates of mice lacking P/Q-type Ca2+ channels. Proc Natl Acad Sci U S A. 2003;100(6):3491–6. doi: 10.1073/pnas.0437991100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen J, Billings SE, Nishimune H. Calcium channels link the muscle-derived synapse organizer laminin beta2 to Bassoon and CAST/Erc2 to organize presynaptic active zones. J Neurosci. 2011;31(2):512–25. doi: 10.1523/JNEUROSCI.3771-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci. 2009;12(11):1415–23. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lambert EH, Elmqvist D. Quantal components of end-plate potentials in the myasthenic syndrome. Ann N Y Acad Sci. 1971;183:183–99. doi: 10.1111/j.1749-6632.1971.tb30750.x. [DOI] [PubMed] [Google Scholar]
- 93.Fukunaga H, Engel AG, Osame M, Lambert EH. Paucity and disorganization of presynaptic membrane active zones in the lambert-eaton myasthenic syndrome. Muscle & Nerve. 1982;5(9):686–697. [Google Scholar]
- 94.Flink MT, Atchison WD. Ca2+ channels as targets of neurological disease: Lambert-Eaton Syndrome and other Ca2+ channelopathies. J Bioenerg Biomembr. 2003;35(6):697–718. doi: 10.1023/b:jobb.0000008033.02320.10. [DOI] [PubMed] [Google Scholar]
- 95.Takamori M, Maruta T, Komai K. Lambert-Eaton myasthenic syndrome as an autoimmune calcium-channelopathy. Neurosci Res. 2000;36(3):183–91. doi: 10.1016/s0168-0102(99)00135-2. [DOI] [PubMed] [Google Scholar]
- 96.Fukuoka T, Engel AG, Lang B, Newsom-Davis J, Prior C, Wray DW. Lambert-Eaton myasthenic syndrome: I. Early morphological effects of IgG on the presynaptic membrane active zones. Ann Neurol. 1987;22(2):193–9. doi: 10.1002/ana.410220203. [DOI] [PubMed] [Google Scholar]
- 97.Fukunaga H, Engel AG, Lang B, Newsom-Davis J, Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc Natl Acad Sci U S A. 1983;80(24):7636–40. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, Campbell KP. Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature. 1992;355(6362):696–702. doi: 10.1038/355696a0. [DOI] [PubMed] [Google Scholar]
- 99.Gee SH, Blacher RW, Douville PJ, Provost PR, Yurchenco PD, Carbonetto S. Laminin-binding protein 120 from brain is closely related to the dystrophin-associated glycoprotein, dystroglycan, and binds with high affinity to the major heparin binding domain of laminin. J Biol Chem. 1993;268(20):14972–80. [PubMed] [Google Scholar]
- 100.Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90(4):729–38. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- 101.Grady RM, Grange RW, Lau KS, Maimone MM, Nichol MC, Stull JT, Sanes JR. Role for alpha-dystrobrevin in the pathogenesis of dystrophin-dependent muscular dystrophies. Nat Cell Biol. 1999;1(4):215–20. doi: 10.1038/12034. [DOI] [PubMed] [Google Scholar]
- 102.Talts JF, Andac Z, Gohring W, Brancaccio A, Timpl R. Binding of the G domains of laminin alpha1 and alpha2 chains and perlecan to heparin, sulfatides, alpha-dystroglycan and several extracellular matrix proteins. EMBO J. 1999;18(4):863–70. doi: 10.1093/emboj/18.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu H, Talts JF. Beta1 integrin and alpha-dystroglycan binding sites are localized to different laminin-G-domain-like (LG) modules within the laminin alpha5 chain G domain. Biochem J. 2003;371(Pt 2):289–99. doi: 10.1042/BJ20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ido H, Harada K, Futaki S, Hayashi Y, Nishiuchi R, Natsuka Y, Li S, Wada Y, Combs AC, Ervasti JM, Sekiguchi K. Molecular dissection of the alpha-dystroglycan- and integrin-binding sites within the globular domain of human laminin-10. J Biol Chem. 2004;279(12):10946–54. doi: 10.1074/jbc.M313626200. [DOI] [PubMed] [Google Scholar]
- 105.Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature. 1992;360(6404):588–91. doi: 10.1038/360588a0. [DOI] [PubMed] [Google Scholar]
- 106.Cohen MW, Jacobson C, Yurchenco PD, Morris GE, Carbonetto S. Laminin-induced clustering of dystroglycan on embryonic muscle cells: comparison with agrin-induced clustering. J Cell Biol. 1997;136(5):1047–58. doi: 10.1083/jcb.136.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Montanaro F, Gee SH, Jacobson C, Lindenbaum MH, Froehner SC, Carbonetto S. Laminin and alpha-dystroglycan mediate acetylcholine receptor aggregation via a MuSK-independent pathway. J Neurosci. 1998;18(4):1250–60. doi: 10.1523/JNEUROSCI.18-04-01250.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Williamson RA, Henry MD, Daniels KJ, Hrstka RF, Lee JC, Sunada Y, Ibraghimov-Beskrovnaya O, Campbell KP. Dystroglycan is essential for early embryonic development: disruption of Reichert’s membrane in Dag1-null mice. Hum Mol Genet. 1997;6(6):831–41. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- 109.Cote PD, Moukhles H, Lindenbaum M, Carbonetto S. Chimaeric mice deficient in dystroglycans develop muscular dystrophy and have disrupted myoneural synapses. Nat Genet. 1999;23(3):338–42. doi: 10.1038/15519. [DOI] [PubMed] [Google Scholar]
- 110.Grady RM, Zhou H, Cunningham JM, Henry MD, Campbell KP, Sanes JR. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin--glycoprotein complex. Neuron. 2000;25(2):279–93. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- 111.Jacobson C, Cote PD, Rossi SG, Rotundo RL, Carbonetto S. The dystroglycan complex is necessary for stabilization of acetylcholine receptor clusters at neuromuscular junctions and formation of the synaptic basement membrane. J Cell Biol. 2001;152(3):435–50. doi: 10.1083/jcb.152.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuhn K, Eble J. The structural bases of integrin-ligand interactions. Trends Cell Biol. 1994;4(7):256–61. doi: 10.1016/0962-8924(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 113.Mercurio AM. Laminin receptors: achieving specificity through cooperation. Trends Cell Biol. 1995;5(11):419–23. doi: 10.1016/s0962-8924(00)89100-x. [DOI] [PubMed] [Google Scholar]
- 114.Nomizu M, Kim WH, Yamamura K, Utani A, Song SY, Otaka A, Roller PP, Kleinman HK, Yamada Y. Identification of cell binding sites in the laminin alpha 1 chain carboxyl-terminal globular domain by systematic screening of synthetic peptides. J Biol Chem. 1995;270(35):20583–90. doi: 10.1074/jbc.270.35.20583. [DOI] [PubMed] [Google Scholar]
- 115.Nomizu M, Weeks BS, Weston CA, Kim WH, Kleinman HK, Yamada Y. Structure-activity study of a laminin alpha 1 chain active peptide segment Ile-Lys-Val-Ala-Val (IKVAV) FEBS Lett. 1995;365(2–3):227–31. doi: 10.1016/0014-5793(95)00475-o. [DOI] [PubMed] [Google Scholar]
- 116.Colognato H, MacCarrick M, O’Rear JJ, Yurchenco PD. The laminin alpha2-chain short arm mediates cell adhesion through both the alpha1beta1 and alpha2beta1 integrins. J Biol Chem. 1997;272(46):29330–6. doi: 10.1074/jbc.272.46.29330. [DOI] [PubMed] [Google Scholar]
- 117.Mizushima H, Takamura H, Miyagi Y, Kikkawa Y, Yamanaka N, Yasumitsu H, Misugi K, Miyazaki K. Identification of integrin-dependent and -independent cell adhesion domains in COOH-terminal globular region of laminin-5 alpha 3 chain. Cell Growth Differ. 1997;8(9):979–87. [PubMed] [Google Scholar]
- 118.Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J Biol Chem. 1998;273(25):15854–9. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- 119.Orian-Rousseau V, Aberdam D, Rousselle P, Messent A, Gavrilovic J, Meneguzzi G, Kedinger M, Simon-Assmann P. Human colonic cancer cells synthesize and adhere to laminin-5. Their adhesion to laminin-5 involves multiple receptors among which is integrin alpha2beta1. J Cell Sci. 1998;111( Pt 14):1993–2004. doi: 10.1242/jcs.111.14.1993. [DOI] [PubMed] [Google Scholar]
- 120.Collo G, Starr L, Quaranta V. A new isoform of the laminin receptor integrin alpha 7 beta 1 is developmentally regulated in skeletal muscle. J Biol Chem. 1993;268(25):19019–24. [PubMed] [Google Scholar]
- 121.Song WK, Wang W, Sato H, Bielser DA, Kaufman SJ. Expression of alpha 7 integrin cytoplasmic domains during skeletal muscle development: alternate forms, conformational change, and homologies with serine/threonine kinases and tyrosine phosphatases. J Cell Sci. 1993;106( Pt 4):1139–52. doi: 10.1242/jcs.106.4.1139. [DOI] [PubMed] [Google Scholar]
- 122.Ziober BL, Chen YQ, Ramos DM, Waleh N, Kramer RH. Expression of the alpha7beta1 laminin receptor suppresses melanoma growth and metastatic potential. Cell Growth Differ. 1999;10(7):479–90. [PubMed] [Google Scholar]
- 123.Hayashi YK, Chou FL, Engvall E, Ogawa M, Matsuda C, Hirabayashi S, Yokochi K, Ziober BL, Kramer RH, Kaufman SJ, Ozawa E, Goto Y, Nonaka I, Tsukahara T, Wang JZ, Hoffman EP, Arahata K. Mutations in the integrin alpha7 gene cause congenital myopathy. Nat Genet. 1998;19(1):94–7. doi: 10.1038/ng0598-94. [DOI] [PubMed] [Google Scholar]
- 124.Mayer U, Saher G, Fassler R, Bornemann A, Echtermeyer F, von der Mark H, Miosge N, Poschl E, von der Mark K. Absence of integrin alpha 7 causes a novel form of muscular dystrophy. Nat Genet. 1997;17(3):318–23. doi: 10.1038/ng1197-318. [DOI] [PubMed] [Google Scholar]
- 125.Miosge N, Klenczar C, Herken R, Willem M, Mayer U. Organization of the myotendinous junction is dependent on the presence of alpha7beta1 integrin. Lab Invest. 1999;79(12):1591–9. [PubMed] [Google Scholar]
- 126.Nawrotzki R, Willem M, Miosge N, Brinkmeier H, Mayer U. Defective integrin switch and matrix composition at alpha 7-deficient myotendinous junctions precede the onset of muscular dystrophy in mice. Hum Mol Genet. 2003;12(5):483–95. doi: 10.1093/hmg/ddg047. [DOI] [PubMed] [Google Scholar]
- 127.Bao ZZ, Lakonishok M, Kaufman S, Horwitz AF. Alpha 7 beta 1 integrin is a component of the myotendinous junction on skeletal muscle. J Cell Sci. 1993;106( Pt 2):579–89. doi: 10.1242/jcs.106.2.579. [DOI] [PubMed] [Google Scholar]
- 128.Colognato H, Winkelmann DA, Yurchenco PD. Laminin polymerization induces a receptor-cytoskeleton network. J Cell Biol. 1999;145(3):619–31. doi: 10.1083/jcb.145.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Schwander M, Leu M, Stumm M, Dorchies OM, Ruegg UT, Schittny J, Muller U. Beta1 integrins regulate myoblast fusion and sarcomere assembly. Dev Cell. 2003;4(5):673–85. doi: 10.1016/s1534-5807(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 130.Schwander M, Shirasaki R, Pfaff SL, Muller U. Beta1 integrins in muscle, but not in motor neurons, are required for skeletal muscle innervation. J Neurosci. 2004;24(37):8181–91. doi: 10.1523/JNEUROSCI.1345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.van der Flier A, Gaspar AC, Thorsteinsdottir S, Baudoin C, Groeneveld E, Mummery CL, Sonnenberg A. Spatial and temporal expression of the beta1D integrin during mouse development. Dev Dyn. 1997;210(4):472–86. doi: 10.1002/(SICI)1097-0177(199712)210:4<472::AID-AJA10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 132.Brancaccio M, Cabodi S, Belkin AM, Collo G, Koteliansky VE, Tomatis D, Altruda F, Silengo L, Tarone G. Differential onset of expression of alpha 7 and beta 1D integrins during mouse heart and skeletal muscle development. Cell Adhes Commun. 1998;5(3):193–205. doi: 10.3109/15419069809040291. [DOI] [PubMed] [Google Scholar]
- 133.Cohen MW, Hoffstrom BG, DeSimone DW. Active zones on motor nerve terminals contain alpha 3beta 1 integrin. J Neurosci. 2000;20(13):4912–21. doi: 10.1523/JNEUROSCI.20-13-04912.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sunderland WJ, Son YJ, Miner JH, Sanes JR, Carlson SS. The presynaptic calcium channel is part of a transmembrane complex linking a synaptic laminin (alpha4beta2gamma1) with non-erythroid spectrin. J Neurosci. 2000;20(3):1009–19. doi: 10.1523/JNEUROSCI.20-03-01009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carlson SS, Valdez G, Sanes JR. Presynaptic calcium channels and alpha3-integrins are complexed with synaptic cleft laminins, cytoskeletal elements and active zone components. J Neurochem. 2010;115(3):654–66. doi: 10.1111/j.1471-4159.2010.06965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kortesmaa J, Yurchenco P, Tryggvason K. Recombinant laminin-8 (alpha(4)beta(1)gamma(1)). Production, purification,and interactions with integrins. J Biol Chem. 2000;275(20):14853–9. doi: 10.1074/jbc.275.20.14853. [DOI] [PubMed] [Google Scholar]
- 137.Fujiwara H, Kikkawa Y, Sanzen N, Sekiguchi K. Purification and characterization of human laminin-8. Laminin-8 stimulates cell adhesion and migration through alpha3beta1 and alpha6beta1 integrins. J Biol Chem. 2001;276(20):17550–8. doi: 10.1074/jbc.M010155200. [DOI] [PubMed] [Google Scholar]
- 138.von der Mark H, Williams I, Wendler O, Sorokin L, von der Mark K, Poschl E. Alternative splice variants of alpha 7 beta 1 integrin selectively recognize different laminin isoforms. J Biol Chem. 2002;277(8):6012–6. doi: 10.1074/jbc.M102188200. [DOI] [PubMed] [Google Scholar]
- 139.Hall ZW. An introduction to molecular neurobiology. Sinauer Associates, Inc; Sunderland, MA, USA: 1992. [Google Scholar]
- 140.Matejas V, Hinkes B, Alkandari F, Al-Gazali L, Annexstad E, Aytac MB, Barrow M, Blahova K, Bockenhauer D, Cheong HI, Maruniak-Chudek I, Cochat P, Dotsch J, Gajjar P, Hennekam RC, Janssen F, Kagan M, Kariminejad A, Kemper MJ, Koenig J, Kogan J, Kroes HY, Kuwertz-Broking E, Lewanda AF, Medeira A, Muscheites J, Niaudet P, Pierson M, Saggar A, Seaver L, Suri M, Tsygin A, Wuhl E, Zurowska A, Uebe S, Hildebrandt F, Antignac C, Zenker M. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum Mutat. 2010;31(9):992–1002. doi: 10.1002/humu.21304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pierson M, Cordier J, Hervouuet F, Rauber G. An Unusual Congenital and Familial Congenital Malformative Combination Involving the Eye and Kidney. J Genet Hum. 1963;12:184–213. [PubMed] [Google Scholar]
- 142.Zenker M, Aigner T, Wendler O, Tralau T, Muntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wuhl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dotsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet. 2004;13(21):2625–32. doi: 10.1093/hmg/ddh284. [DOI] [PubMed] [Google Scholar]
- 143.Zenker M, Pierson M, Jonveaux P, Reis A. Demonstration of two novel LAMB2 mutations in the original Pierson syndrome family reported 42 years ago. Am J Med Genet A. 2005;138(1):73–4. doi: 10.1002/ajmg.a.30894. [DOI] [PubMed] [Google Scholar]
- 144.Maselli RA, Ng JJ, Anderson JA, Cagney O, Arredondo J, Williams C, Wessel HB, Abdel-Hamid H, Wollmann RL. Mutations in LAMB2 causing a severe form of synaptic congenital myasthenic syndrome. J Med Genet. 2009;46(3):203–8. doi: 10.1136/jmg.2008.063693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, Ozaltin F, Nurnberg G, Becker C, Hangan D, Pohl M, Kuwertz-Broking E, Griebel M, Schumacher V, Royer-Pokora B, Bakkaloglu A, Nurnberg P, Zenker M, Hildebrandt F. Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int. 2006;70(6):1008–12. doi: 10.1038/sj.ki.5001679. [DOI] [PubMed] [Google Scholar]
- 146.Vuolteenaho R, Nissinen M, Sainio K, Byers M, Eddy R, Hirvonen H, Shows TB, Sariola H, Engvall E, Tryggvason K. Human laminin M chain (merosin): complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal tissues. J Cell Biol. 1994;124(3):381–94. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hayashi YK, Engvall E, Arikawa-Hirasawa E, Goto K, Koga R, Nonaka I, Sugita H, Arahata K. Abnormal localization of laminin subunits in muscular dystrophies. J Neurol Sci. 1993;119(1):53–64. doi: 10.1016/0022-510x(93)90191-z. [DOI] [PubMed] [Google Scholar]
- 148.Allamand V, Sunada Y, Salih MA, Straub V, Ozo CO, Al-Turaiki MH, Akbar M, Kolo T, Colognato H, Zhang X, Sorokin LM, Yurchenco PD, Tryggvason K, Campbell KP. Mild congenital muscular dystrophy in two patients with an internally deleted laminin alpha2-chain. Hum Mol Genet. 1997;6(5):747–52. doi: 10.1093/hmg/6.5.747. [DOI] [PubMed] [Google Scholar]
- 149.Naom IS, D’Alessandro M, Topaloglu H, Sewry C, Ferlini A, Helbling-Leclerc A, Guicheney P, Weissenbach J, Schwartz K, Bushby K, Philpot J, Dubowitz V, Muntoni F. Refinement of the laminin alpha2 chain locus to human chromosome 6q2 in severe and mild merosin deficient congenital muscular dystrophy. J Med Genet. 1997;34(2):99–104. doi: 10.1136/jmg.34.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Di Blasi C, He Y, Morandi L, Cornelio F, Guicheney P, Mora M. Mild muscular dystrophy due to a nonsense mutation in the LAMA2 gene resulting in exon skipping. Brain. 2001;124(Pt 4):698–704. doi: 10.1093/brain/124.4.698. [DOI] [PubMed] [Google Scholar]
- 151.Vincent A, Beeson D, Lang B. Molecular targets for autoimmune and genetic disorders of neuromuscular transmission. Eur J Biochem. 2000;267(23):6717–28. doi: 10.1046/j.1432-1033.2000.01785.x. [DOI] [PubMed] [Google Scholar]
- 152.Elmqvist D, Lambert EH. Detailed analysis of neuromuscular transmission in a patient with the myasthenic syndrome sometimes associated with bronchogenic carcinoma. Mayo Clin Proc. 1968;43(10):689–713. [PubMed] [Google Scholar]
- 153.O’Neill JH, Murray NM, Newsom-Davis J. The Lambert-Eaton myasthenic syndrome. A review of 50 cases. Brain. 1988;111( Pt 3):577–96. doi: 10.1093/brain/111.3.577. [DOI] [PubMed] [Google Scholar]
- 154.Titulaer MJ, Verschuuren JJ. Lambert-Eaton myasthenic syndrome: tumor versus nontumor forms. Ann N Y Acad Sci. 2008;1132:129–34. doi: 10.1196/annals.1405.030. [DOI] [PubMed] [Google Scholar]
- 155.Titulaer MJ, Wirtz PW, Kuks JB, Schelhaas HJ, van der Kooi AJ, Faber CG, van der Pol WL, de Visser M, Sillevis Smitt PA, Verschuuren JJ. The Lambert-Eaton myasthenic syndrome 1988–2008: a clinical picture in 97 patients. J Neuroimmunol. 2008;201–202:153–8. doi: 10.1016/j.jneuroim.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 156.Oguro-Okano M, Griesmann GE, Wieben ED, Slaymaker SJ, Snutch TP, Lennon VA. Molecular diversity of neuronal-type calcium channels identified in small cell lung carcinoma. Mayo Clin Proc. 1992;67(12):1150–9. doi: 10.1016/s0025-6196(12)61144-6. [DOI] [PubMed] [Google Scholar]
- 157.Benatar M, Blaes F, Johnston I, Wilson K, Vincent A, Beeson D, Lang B. Presynaptic neuronal antigens expressed by a small cell lung carcinoma cell line. J Neuroimmunol. 2001;113(1):153–62. doi: 10.1016/s0165-5728(00)00431-8. [DOI] [PubMed] [Google Scholar]
- 158.Lindquist S, Stangel M. Update on treatment options for Lambert-Eaton myasthenic syndrome: focus on use of amifampridine. Neuropsychiatr Dis Treat. 2011;7:341–9. doi: 10.2147/NDT.S10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tarr TB, Malick W, Liang M, Valdomir G, Frasso M, Lacomis D, Reddel SW, Garcia-Ocano A, Wipf P, Meriney SD. Evaluation of a novel calcium channel agonist for therapeutic potential in Lambert-Eaton myasthenic syndrome. J Neurosci. 2013;33(25):10559–67. doi: 10.1523/JNEUROSCI.4629-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tarr TB, Lacomis D, Reddel SW, Liang M, Valdomir G, Frasso M, Wipf P, Meriney SD. Complete reversal of Lambert-Eaton myasthenic syndrome synaptic impairment by the combined use of a K+ channel blocker and a Ca2+ channel agonist. J Physiol. 2014;592(16):3687–96. doi: 10.1113/jphysiol.2014.276493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Deschenes MR. Motor unit and neuromuscular junction remodeling with aging. Curr Aging Sci. 2011;4(3):209–20. doi: 10.2174/1874609811104030209. [DOI] [PubMed] [Google Scholar]
- 162.Nishimune H. Active zones of mammalian neuromuscular junctions: formation, density, and aging. Ann N Y Acad Sci. 2012;1274:24–32. doi: 10.1111/j.1749-6632.2012.06836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front Aging Neurosci. 2014;6:208. doi: 10.3389/fnagi.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fahim MA, Robbins N. Ultrastructural studies of young and old mouse neuromuscular junctions. J Neurocytol. 1982;11(4):641–56. doi: 10.1007/BF01262429. [DOI] [PubMed] [Google Scholar]
- 165.Smith DO, Rosenheimer JL. Decreased sprouting and degeneration of nerve terminals of active muscles in aged rats. J Neurophysiol. 1982;48(1):100–9. doi: 10.1152/jn.1982.48.1.100. [DOI] [PubMed] [Google Scholar]
- 166.Fahim MA, Holley JA, Robbins N. Scanning and light microscopic study of age changes at a neuromuscular junction in the mouse. J Neurocytol. 1983;12(1):13–25. doi: 10.1007/BF01148085. [DOI] [PubMed] [Google Scholar]
- 167.Arizono N, Koreto O, Iwai Y, Hidaka T, Takeoka O. Morphometric analysis of human neuromuscular junction in different ages. Acta Pathol Jpn. 1984;34(6):1243–9. doi: 10.1111/j.1440-1827.1984.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 168.Andonian MH, Fahim MA. Effects of endurance exercise on the morphology of mouse neuromuscular junctions during ageing. J Neurocytol. 1987;16(5):589–99. doi: 10.1007/BF01637652. [DOI] [PubMed] [Google Scholar]
- 169.Elkerdany MK, Fahim MA. Age changes in neuromuscular junctions of masseter muscle. Anat Rec. 1993;237(2):291–5. doi: 10.1002/ar.1092370215. [DOI] [PubMed] [Google Scholar]
- 170.Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45(5):389–93. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gutmann E, Hanzlikova V, Vysokocil F. Age changes in cross striated muscle of the rat. J Physiol. 1971;216(2):331–43. doi: 10.1113/jphysiol.1971.sp009528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Banker BQ, Kelly SS, Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. J Physiol. 1983;339:355–77. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kelly SS, Robbins N. Progression of age changes in synaptic transmission at mouse neuromuscular junctions. J Physiol. 1983;343:375–83. doi: 10.1113/jphysiol.1983.sp014898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fahim MA, Robbins N, Price R. Fixation effects on synaptic vesicle density in neuromuscular junctions of young and old mice. Neurobiol Aging. 1987;8(1):71–5. doi: 10.1016/0197-4580(87)90061-3. [DOI] [PubMed] [Google Scholar]
- 175.Chen J, Mizushige T, Nishimune H. Active zone density is conserved during synaptic growth but impaired in aged mice. J Comp Neurol. 2012;520(2):434–52. doi: 10.1002/cne.22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Courtney J, Steinbach JH. Age changes in neuromuscular junction morphology and acetylcholine receptor distribution on rat skeletal muscle fibres. J Physiol. 1981;320:435–47. doi: 10.1113/jphysiol.1981.sp013960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oda K. Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. J Neurol Sci. 1984;66(2–3):327–38. doi: 10.1016/0022-510x(84)90021-2. [DOI] [PubMed] [Google Scholar]
- 178.Cardasis CA, LaFontaine DM. Aging rat neuromuscular junctions: a morphometric study of cholinesterase-stained whole mounts and ultrastructure. Muscle Nerve. 1987;10(3):200–13. doi: 10.1002/mus.880100303. [DOI] [PubMed] [Google Scholar]
- 179.Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. 1998;21(7):887–95. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 180.Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107(33):14863–8. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One. 2011;6(12):e28090. doi: 10.1371/journal.pone.0028090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li Y, Lee Y, Thompson WJ. Changes in aging mouse neuromuscular junctions are explained by degeneration and regeneration of muscle fiber segments at the synapse. J Neurosci. 2011;31(42):14910–9. doi: 10.1523/JNEUROSCI.3590-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Deschenes MR, Roby MA, Glass EK. Aging influences adaptations of the neuromuscular junction to endurance training. Neuroscience. 2011;190:56–66. doi: 10.1016/j.neuroscience.2011.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cheng A, Morsch M, Murata Y, Ghazanfari N, Reddel SW, Phillips WD. Sequence of age-associated changes to the mouse neuromuscular junction and the protective effects of voluntary exercise. PLoS One. 2013;8(7):e67970. doi: 10.1371/journal.pone.0067970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Deschenes MR, Sherman EG, Roby MA, Glass EK, Harris MB. Effect of resistance training on neuromuscular junctions of young and aged muscles featuring different recruitment patterns. J Neurosci Res. 2015;93(3):504–13. doi: 10.1002/jnr.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rosenheimer JL, Smith DO. Differential changes in the end-plate architecture of functionally diverse muscles during aging. J Neurophysiol. 1985;53(6):1567–81. doi: 10.1152/jn.1985.53.6.1567. [DOI] [PubMed] [Google Scholar]
- 187.Rosenheimer JL. Ultraterminal sprouting in innervated and partially denervated adult and aged rat muscle. Neuroscience. 1990;38(3):763–70. doi: 10.1016/0306-4522(90)90069-g. [DOI] [PubMed] [Google Scholar]
- 188.Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One. 2012;7(4):e34640. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nishimune H, Numata T, Chen J, Aoki Y, Wang Y, Starr MP, Mori Y, Stanford JA. Active zone protein Bassoon co-localizes with presynaptic calcium channel, modifies channel function, and recovers from aging related loss by exercise. PLoS One. 2012;7(6):e38029. doi: 10.1371/journal.pone.0038029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rosenheimer JL. Effects of chronic stress and exercise on age-related changes in end-plate architecture. J Neurophysiol. 1985;53(6):1582–9. doi: 10.1152/jn.1985.53.6.1582. [DOI] [PubMed] [Google Scholar]
- 191.Andonian MH, Fahim MA. Endurance exercise alters the morphology of fast- and slow-twitch rat neuromuscular junctions. Int J Sports Med. 1988;9(3):218–23. doi: 10.1055/s-2007-1025009. [DOI] [PubMed] [Google Scholar]
- 192.Alshuaib WB, Fahim MA. Effect of exercise on physiological age-related change at mouse neuromuscular junctions. Neurobiol Aging. 1990;11(5):555–61. doi: 10.1016/0197-4580(90)90117-i. [DOI] [PubMed] [Google Scholar]
- 193.Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. J Appl Physiol (1985) 1997;83(1):59–66. doi: 10.1152/jappl.1997.83.1.59. [DOI] [PubMed] [Google Scholar]
- 194.Rudolf R, Khan MM, Labeit S, Deschenes MR. Degeneration of neuromuscular junction in age and dystrophy. Front Aging Neurosci. 2014;6:99. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Deschenes MR, Judelson DA, Kraemer WJ, Meskaitis VJ, Volek JS, Nindl BC, Harman FS, Deaver DR. Effects of resistance training on neuromuscular junction morphology. Muscle Nerve. 2000;23(10):1576–81. doi: 10.1002/1097-4598(200010)23:10<1576::aid-mus15>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 196.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem. 1996;271(44):27879–87. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 197.Rasmussen BB, Winder WW. Effect of exercise intensity on skeletal muscle malonyl-CoA and acetyl-CoA carboxylase. J Appl Physiol (1985) 1997;83(4):1104–9. doi: 10.1152/jappl.1997.83.4.1104. [DOI] [PubMed] [Google Scholar]
- 198.Birk JB, Wojtaszewski JF. Predominant alpha2/beta2/gamma3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577(Pt 3):1021–32. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134(3):405–15. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104(29):12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Handschin C, Kobayashi YM, Chin S, Seale P, Campbell KP, Spiegelman BM. PGC-1alpha regulates the neuromuscular junction program and ameliorates Duchenne muscular dystrophy. Genes Dev. 2007;21(7):770–83. doi: 10.1101/gad.1525107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gordon PM, Liu D, Sartor MA, IglayReger HB, Pistilli EE, Gutmann L, Nader GA, Hoffman EP. Resistance exercise training influences skeletal muscle immune activation: a microarray analysis. J Appl Physiol (1985) 2012;112(3):443–53. doi: 10.1152/japplphysiol.00860.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ogasawara R, Nakazato K, Sato K, Boppart MD, Fujita S. Resistance exercise increases active MMP and beta1-integrin protein expression in skeletal muscle. Physiol Rep. 2014;2(11) doi: 10.14814/phy2.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nishimune H. Molecular mechanism of active zone organization at vertebrate neuromuscular junctions. Mol Neurobiol. 2012;45(1):1–16. doi: 10.1007/s12035-011-8216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Torres-Benito L, Ruiz R, Tabares L. Synaptic defects in spinal muscular atrophy animal models. Dev Neurobiol. 2012;72(1):126–33. doi: 10.1002/dneu.20912. [DOI] [PubMed] [Google Scholar]
- 206.Cano R, Torres-Benito L, Tejero R, Biea AI, Ruiz R, Betz WJ, Tabares L. Structural and functional maturation of active zones in large synapses. Mol Neurobiol. 2013;47(1):209–19. doi: 10.1007/s12035-012-8347-9. [DOI] [PubMed] [Google Scholar]
- 207.Ackermann F, Waites CL, Garner CC. Presynaptic active zones in invertebrates and vertebrates. EMBO Rep. 2015;16(8):923–38. doi: 10.15252/embr.201540434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Gundelfinger ED, Reissner C, Garner CC. Role of Bassoon and Piccolo in Assembly and Molecular Organization of the Active Zone. Front Synaptic Neurosci. 2015;7:19. doi: 10.3389/fnsyn.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ellisman MH, Rash JE, Staehelin LA, Porter KR. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. J Cell Biol. 1976;68(3):752–74. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Walrond JP, Reese TS. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. J Neurosci. 1985;5(5):1118–31. doi: 10.1523/JNEUROSCI.05-05-01118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brose N, Hofmann K, Hata Y, Sudhof TC. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem. 1995;270(42):25273–80. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- 212.Cases-Langhoff C, Voss B, Garner AM, Appeltauer U, Takei K, Kindler S, Veh RW, De Camilli P, Gundelfinger ED, Garner CC. Piccolo a novel 420 kDa protein associated with the presynaptic cytomatrix. Eur J Cell Biol. 1996;69(3):214–23. [PubMed] [Google Scholar]
- 213.Wang Y, Okamoto M, Schmitz F, Hofmann K, Sudhof TC. Rim is a putative Rab3 effector in regulating synaptic-vesicle fusion. Nature. 1997;388(6642):593–8. doi: 10.1038/41580. [DOI] [PubMed] [Google Scholar]
- 214.Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Sudhof TC, Rettig J, Brose N. Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21(1):123–36. doi: 10.1016/s0896-6273(00)80520-6. [DOI] [PubMed] [Google Scholar]
- 215.tom Dieck S, Sanmarti-Vila L, Langnaese K, Richter K, Kindler S, Soyke A, Wex H, Smalla KH, Kampf U, Franzer JT, Stumm M, Garner CC, Gundelfinger ED. Bassoon, a novel zinc-finger CAG/glutamine-repeat protein selectively localized at the active zone of presynaptic nerve terminals. J Cell Biol. 1998;142(2):499–509. doi: 10.1083/jcb.142.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dresbach T, Qualmann B, Kessels MM, Garner CC, Gundelfinger ED. The presynaptic cytomatrix of brain synapses. Cell Mol Life Sci. 2001;58(1):94–116. doi: 10.1007/PL00000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13–1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400(6743):457–61. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- 218.Ohtsuka T, Takao-Rikitsu E, Inoue E, Inoue M, Takeuchi M, Matsubara K, Deguchi-Tawarada M, Satoh K, Morimoto K, Nakanishi H, Takai Y. Cast: a novel protein of the cytomatrix at the active zone of synapses that forms a ternary complex with RIM1 and munc13–1. J Cell Biol. 2002;158(3):577–90. doi: 10.1083/jcb.200202083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wang Y, Liu X, Biederer T, Sudhof TC. A family of RIM-binding proteins regulated by alternative splicing: Implications for the genesis of synaptic active zones. Proc Natl Acad Sci U S A. 2002;99(22):14464–9. doi: 10.1073/pnas.182532999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Deguchi-Tawarada M, Inoue E, Takao-Rikitsu E, Inoue M, Ohtsuka T, Takai Y. CAST2: identification and characterization of a protein structurally related to the presynaptic cytomatrix protein CAST. Genes Cells. 2004;9(1):15–23. doi: 10.1111/j.1356-9597.2004.00697.x. [DOI] [PubMed] [Google Scholar]
- 221.Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, Hell SW, Buchner E, Heckmann M, Sigrist SJ. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312(5776):1051–4. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- 222.Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49(6):833–44. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 223.Davydova D, Marini C, King C, Klueva J, Bischof F, Romorini S, Montenegro-Venegas C, Heine M, Schneider R, Schroder MS, Altrock WD, Henneberger C, Rusakov DA, Gundelfinger ED, Fejtova A. Bassoon specifically controls presynaptic P/Q-type Ca(2+) channels via RIM-binding protein. Neuron. 2014;82(1):181–94. doi: 10.1016/j.neuron.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 224.Misgeld T, Burgess RW, Lewis RM, Cunningham JM, Lichtman JW, Sanes JR. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36(4):635–48. doi: 10.1016/s0896-6273(02)01020-6. [DOI] [PubMed] [Google Scholar]
- 225.Fenster SD, Chung WJ, Zhai R, Cases-Langhoff C, Voss B, Garner AM, Kaempf U, Kindler S, Gundelfinger ED, Garner CC. Piccolo, a presynaptic zinc finger protein structurally related to bassoon. Neuron. 2000;25(1):203–14. doi: 10.1016/s0896-6273(00)80883-1. [DOI] [PubMed] [Google Scholar]
- 226.Altrock WD, tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C, Fassler R, Richter K, Boeckers TM, Potschka H, Brandt C, Loscher W, Grimberg D, Dresbach T, Hempelmann A, Hassan H, Balschun D, Frey JU, Brandstatter JH, Garner CC, Rosenmund C, Gundelfinger ED. Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron. 2003;37(5):787–800. doi: 10.1016/s0896-6273(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 227.Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37(5):775–86. doi: 10.1016/s0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 228.Leal-Ortiz S, Waites CL, Terry-Lorenzo R, Zamorano P, Gundelfinger ED, Garner CC. Piccolo modulation of Synapsin1a dynamics regulates synaptic vesicle exocytosis. J Cell Biol. 2008;181(5):831–46. doi: 10.1083/jcb.200711167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tokoro T, Higa S, Deguchi-Tawarada M, Inoue E, Kitajima I, Ohtsuka T. Localization of the active zone proteins CAST, ELKS, and Piccolo at neuromuscular junctions. Neuroreport. 2007;18(4):313–6. doi: 10.1097/WNR.0b013e3280287abe. [DOI] [PubMed] [Google Scholar]
- 230.Kiyonaka S, Nakajima H, Takada Y, Hida Y, Yoshioka T, Hagiwara A, Kitajima I, Mori Y, Ohtsuka T. Physical and functional interaction of the active zone protein CAST/ERC2 and the beta-subunit of the voltage-dependent Ca(2+) channel. J Biochem. 2012;152(2):149–59. doi: 10.1093/jb/mvs054. [DOI] [PubMed] [Google Scholar]
- 231.Kaeser PS, Deng L, Chavez AE, Liu X, Castillo PE, Sudhof TC. ELKS2alpha/CAST deletion selectively increases neurotransmitter release at inhibitory synapses. Neuron. 2009;64(2):227–39. doi: 10.1016/j.neuron.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.tom Dieck S, Specht D, Strenzke N, Hida Y, Krishnamoorthy V, Schmidt KF, Inoue E, Ishizaki H, Tanaka-Okamoto M, Miyoshi J, Hagiwara A, Brandstatter JH, Lowel S, Gollisch T, Ohtsuka T, Moser T. Deletion of the presynaptic scaffold CAST reduces active zone size in rod photoreceptors and impairs visual processing. J Neurosci. 2012;32(35):12192–203. doi: 10.1523/JNEUROSCI.0752-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Aravamudan B, Fergestad T, Davis WS, Rodesch CK, Broadie K. Drosophila UNC-13 is essential for synaptic transmission. Nat Neurosci. 1999;2(11):965–71. doi: 10.1038/14764. [DOI] [PubMed] [Google Scholar]
- 234.Richmond JE, Davis WS, Jorgensen EM. UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat Neurosci. 1999;2(11):959–64. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99(13):9037–42. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Varoqueaux F, Sons MS, Plomp JJ, Brose N. Aberrant morphology and residual transmitter release at the Munc13-deficient mouse neuromuscular synapse. Mol Cell Biol. 2005;25(14):5973–84. doi: 10.1128/MCB.25.14.5973-5984.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol. 2000;10(3):303–11. doi: 10.1016/s0959-4388(00)00105-7. [DOI] [PubMed] [Google Scholar]
- 238.Koushika SP, Richmond JE, Hadwiger G, Weimer RM, Jorgensen EM, Nonet ML. A post-docking role for active zone protein Rim. Nat Neurosci. 2001;4(10):997–1005. doi: 10.1038/nn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, Schmitz F, Malenka RC, Sudhof TC. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature. 2002;415(6869):321–6. doi: 10.1038/415321a. [DOI] [PubMed] [Google Scholar]
- 240.Calakos N, Schoch S, Sudhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron. 2004;42(6):889–96. doi: 10.1016/j.neuron.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 241.Schoch S, Mittelstaedt T, Kaeser PS, Padgett D, Feldmann N, Chevaleyre V, Castillo PE, Hammer RE, Han W, Schmitz F, Lin W, Sudhof TC. Redundant functions of RIM1alpha and RIM2alpha in Ca(2+)-triggered neurotransmitter release. EMBO J. 2006;25(24):5852–63. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kiyonaka S, Wakamori M, Miki T, Uriu Y, Nonaka M, Bito H, Beedle AM, Mori E, Hara Y, De Waard M, Kanagawa M, Itakura M, Takahashi M, Campbell KP, Mori Y. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nat Neurosci. 2007;10(6):691–701. doi: 10.1038/nn1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Uriu Y, Kiyonaka S, Miki T, Yagi M, Akiyama S, Mori E, Nakao A, Beedle AM, Campbell KP, Wakamori M, Mori Y. Rab3-interacting molecule gamma isoforms lacking the Rab3-binding domain induce long lasting currents but block neurotransmitter vesicle anchoring in voltage-dependent P/Q-type Ca2+ channels. J Biol Chem. 2010;285(28):21750–67. doi: 10.1074/jbc.M110.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kaeser PS, Deng L, Wang Y, Dulubova I, Liu X, Rizo J, Sudhof TC. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144(2):282–95. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Han Y, Kaeser PS, Sudhof TC, Schneggenburger R. RIM determines Ca(2)+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69(2):304–16. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Catterall WA. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- 247.Urbano FJ, Rosato-Siri MD, Uchitel OD. Calcium channels involved in neurotransmitter release at adult, neonatal and P/Q-type deficient neuromuscular junctions (Review) Mol Membr Biol. 2002;19(4):293–300. doi: 10.1080/0968768021000035087. [DOI] [PubMed] [Google Scholar]
- 248.Spafford JD, Zamponi GW. Functional interactions between presynaptic calcium channels and the neurotransmitter release machinery. Curr Opin Neurobiol. 2003;13(3):308–14. doi: 10.1016/s0959-4388(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 249.Wu J, Yan Z, Li Z, Yan C, Lu S, Dong M, Yan N. Structure of the voltage-gated calcium channel Cav1.1 complex. Science. 2015;350(6267):aad2395. doi: 10.1126/science.aad2395. [DOI] [PubMed] [Google Scholar]
- 250.Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378(6557):623–6. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 251.Sheng ZH, Rettig J, Takahashi M, Catterall WA. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13(6):1303–13. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 252.Martin-Moutot N, Charvin N, Leveque C, Sato K, Nishiki T, Kozaki S, Takahashi M, Seagar M. Interaction of SNARE complexes with P/Q-type calcium channels in rat cerebellar synaptosomes. J Biol Chem. 1996;271(12):6567–70. doi: 10.1074/jbc.271.12.6567. [DOI] [PubMed] [Google Scholar]
- 253.Sheng ZH, Rettig J, Cook T, Catterall WA. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature. 1996;379(6564):451–4. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- 254.Wiser O, Bennett MK, Atlas D. Functional interaction of syntaxin and SNAP-25 with voltage-sensitive L- and N-type Ca2+ channels. EMBO J. 1996;15(16):4100–10. [PMC free article] [PubMed] [Google Scholar]
- 255.Sheng ZH, Yokoyama CT, Catterall WA. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proc Natl Acad Sci U S A. 1997;94(10):5405–10. doi: 10.1073/pnas.94.10.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Coppola T, Magnin-Luthi S, Perret-Menoud V, Gattesco S, Schiavo G, Regazzi R. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. J Biol Chem. 2001;276(35):32756–62. doi: 10.1074/jbc.M100929200. [DOI] [PubMed] [Google Scholar]
- 257.Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+ sensor, and voltage-dependent Ca2+ channel in insulin granule exocytosis. J Biol Chem. 2004;279(9):7956–61. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 258.Billings SE, Clarke GL, Nishimune H. ELKS1 and Ca(2+) channel subunit beta4 interact and colocalize at cerebellar synapses. Neuroreport. 2012;23(1):49–54. doi: 10.1097/WNR.0b013e32834e7deb. [DOI] [PMC free article] [PubMed] [Google Scholar]