Abstract
Introduction
Odontogenic foci can rarely cause intracranial infection. Hematogenous spread is considered to be the most important pathophysiological mechanism of intracranial infection of odontogenic origin. To investigate the oral origin of intracranial infections, oral surgeons should understand the underlying mechanisms by which oral bacteria spread to the central nervous system. However, there have been very few reports of intracranial infection resulting from odontogenic infection.
Case reports
The authors report the cases of a 64-year-old man, a 68-year-old man, and a 64-year-old woman whose brain abscesses perhaps have arisen from odontogenic foci, because other sources of intracranial infection such as endocarditis and maxillary sinusitis were not found. Bacteriological examination of brain abscess specimens identified Staphylococcus aureus in case 1, Streptococcus constellatus, Fusobacterium nucleatum, and Parvimonas micra in case 2, and Lactobacillus catenaformis, Porphyromonas gingivalis, and F. nucleatum in case 3. All suspected causal teeth had no obvious signs of acute inflammation in all three cases.
Conclusions
Oral surgeons should understand these characteristics of odontogenic brain abscess, in which the potentially causal odontogenic foci often lack acute symptoms. If other origins of infection are not found, it would be better to eliminate the potentially causal odontogenic foci for improvement of oral hygiene, however, the decision making criteria to eliminate suspected causal teeth is needed to be elucidated.
Keywords: Brain abscess, Odontogenic foci, Magnetic resonance image, Drainage
Introduction
Brain abscesses are rare but life-threatening infections. There are very few case reports to date of brain abscess caused by a primary infection of odontogenic origin [1–11]. A recent systematic review investigating the pathogenesis, microbiology, interventions, and outcomes of brain abscesses of oral origin indicated four possible routes of odontogenic spread: (1) systemic bacteremia (hematogenous); (2) direct venous drainage via the facial and pterygoid vein systems to the cavernous sinus; (3) inoculation via contiguous extension or by introduction of foreign objects; and (4) lymphatic drainage [1]. If direct venous drainage played a predominant role in intracranial dissemination, the incidence of brain infection caused by odontogenic foci would likely be much higher. Therefore, hematogenous spread is considered to be the most important pathophysiological mechanism [1]. This hypothesis is supported by the previous finding that maxillary and mandibular foci were equally likely to be associated with intracranial infections [12]. Another report proposed three criteria for diagnosing odontogenic brain abscess: (1) no alternative source of bacteremia must be found; (2) microbiological studies reveal organisms typically found in the oral microflora; and (3) clinical or radiographic signs of active dental or periodontal disease must be present [13]. Moazzam et al. [1] added several caveats: (a) even in the absence of dental pathosis, dental procedures can potentially cause central nervous system (CNS) infections, if performed within 1–4 weeks before the onset; (b) standard culturing techniques may not reveal all bacteria present in a CNS lesion; (c) the presence of endocarditis should not deter investigation of the mouth.
This report presents three cases of brain abscesses potentially resulting from odontogenic infection. We discuss the role of oral surgeons in the management of intracranial infection suspected to arise from odontogenic foci and provide a literature review.
Case Reports
Between 2012 and 2015, three patients were referred from the Department of Neurosurgery to the Department of Oral and Maxillofacial Surgery at our institution to be screened for odontogenic foci after drainage of brain abscesses.
Case I
Four months after surgical clipping of a cerebral aneurysm, a 64-year-old man began to feel ill, with symptoms that included difficulty in speaking, gait instability, and memory impairment. Five months after the clipping, cranial computed tomography (CT) showed a 36-mm cystic lesion with extensive edema in the left temporal lobe. After urgent admission to the Department of Neurosurgery, magnetic resonance imaging (MRI) confirmed features suggestive of an intracranial abscess in the same region (Fig. 1a, b). Glycerol administration was initiated to treat cerebral edema, and blood and urine samples were collected for microbial culture. One day after admission, emergency drainage was performed under local anesthesia. Incising the skin revealed thickened subcutaneous tissue. Purulent material was not evacuated immediately after dural incision, but was drained through needle aspiration. After drainage, empirical intravenous antibiotic treatment was initiated with ampicillin-sulbactam (3 g every 6 h). Endocarditis was ruled out by echocardiography. Although the patient had a past history of noninfectious otitis media with effusion during previous hospitalization for surgical clipping of a cerebral aneurysm 4 months before, the results of medical examinations did not reveal the presence of acute middle ear infection. To evaluate bacterial sources in mouth, the patient was referred to our department 1 day after drainage. Although no evidence of acute inflammation was found on extra- or intra-oral examination, the right mandibular lateral incisor and canine tooth had localized advanced periodontitis (panoramic radiographs could not be taken, because the patient was bedridden). Five days after drainage, these teeth were extracted. Although blood and urine cultures were negative, the purulent material drained from the abscess contained Staphylococcus aureus. Serial imaging showed resolution of the intracranial inflammatory changes, and the patient’s neurologic deficits improved. Forty-four days postoperatively, the patient was able to walk without assistance and was discharged from the hospital.
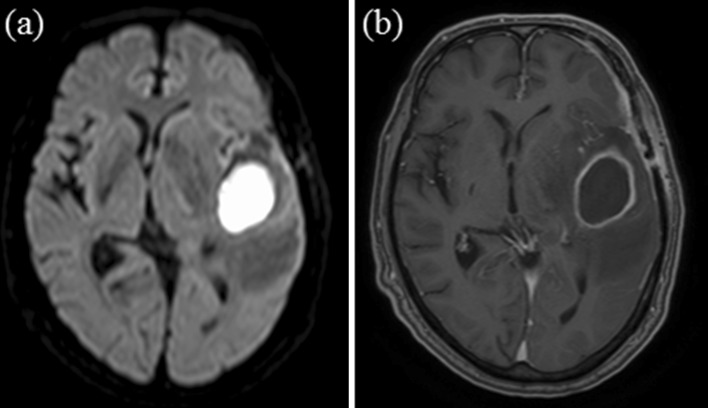
Fig. 1.
Case I a axial diffusion-weighted magnetic resonance image (DWI) showing high signal intensity in suspected abscess formation and b axial gadolinium-enhanced T1-weighted magnetic resonance image (Gd-T1WI) showing ring enhancing lesion
Case II
A 68-year-old man presented to the Department of Neurosurgery with a 3-day history of memory impairment, disequilibrium, and mild fever of 37.2 °C. The patient had right-sided hemiparesis resulting from lacunar infarction on the left side of his brain 7 years earlier. The patient did not have any visual disturbance or deterioration of hemiparesis. Brain MRI revealed features suggestive of an intracranial abscess with edema in the left lobe (Fig. 2a, b). One day after admission, drainage was performed under local anesthesia. Foul-smelling purulent material was drained with aspiration. Based on these findings, the Division of Infectious Diseases was consulted to determine the most appropriate antibiotic regimen. Culture of brain abscess samples was positive for Gram-positive cocci and Gram-negative bacilli, so administration of ceftriaxone (2 g every 12 h) and metronidazole (0.5 g every 8 h) were initiated. Three days after drainage, glycerol administration was initiated to treat cerebral edema. Blood, urine, and stool cultures were negative, and human T cell lymphotropic virus-I antibodies were also not found in blood samples. Bacteriological examination of brain abscess specimens identified Streptococcus constellatus, Fusobacterium nucleatum, and Parvimonas micra 10 days after drainage. Based on these results, ceftriaxone was switched to ampicillin (2 g every 6 h). Although echocardiography revealed no abnormal findings, including vegetation, enhanced CT of the chest revealed pulmonary embolism in the right pulmonary artery and lower-extremity venous duplex ultrasonography showed deep venous thromboses in the right peroneal and soleal veins 1 month after drainage. The Division of Cardiovascular Medicine was consulted, and anticoagulant therapy was begun. Because the results of medical examinations did not reveal the presence of maxillary sinusitis, otitis media, tonsillitis, and upper respiratory tract infection, the patient was referred to our department 42 days after drainage. Intraoral examination and panoramic radiographs revealed generalized advanced periodontitis without acute inflammation affecting all of the patient’s remaining teeth. All teeth were considered potential sources of intracranial infection, but the specific teeth causing the brain abscess could not be definitively determined (Fig. 2c). Extraction of all teeth with severe periodontitis and prosthetic rehabilitation were planned to improve oral hygiene. The patient was discharged from the hospital 3 days after consultation in our department because serial imaging showed resolution of the brain abscess and his neurologic deficits improved before tooth extraction. Therefore, information regarding elimination of all odontogenic foci was provided to other hospital.
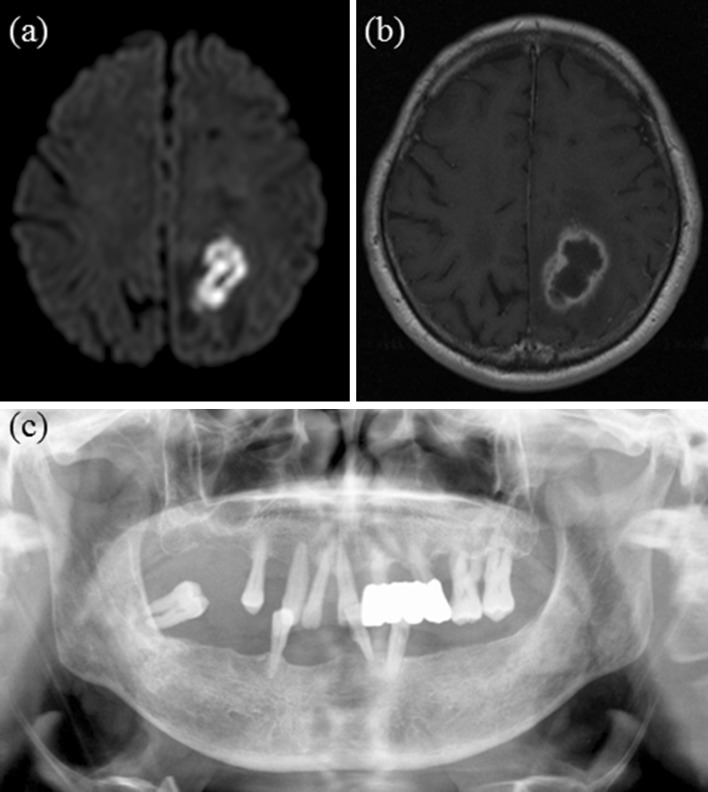
Fig. 2.
Case II a axial DWI showing suspected abscess formation, b axial Gd-T1WI image and c panoramic radiograph showing generalized advanced periodontitis of all residual teeth
Case III
A 64-year-old woman presented to the Department of Neurosurgery with seizure episodes lasting from 5 to 15 min. The patient had ventricular septal defect and Eisenmenger’s syndrome. Brain MRI revealed features suggestive of an intracranial abscess with edema in the right lobe (Fig. 3a, b). The Division of Infectious Diseases was consulted to determine the most appropriate antibiotic regimen (ceftriaxone, metronidazole, and vancomycin). One day after admission, left hemiplegia occurred. Two days after admission, drainage was performed under local anesthesia. Purulent material was drained with aspiration. Culture of brain abscess samples was positive for anaerobic Gram-negative bacilli, so antibiotics were switched to sulbactam/ampicillin (3 g every 6 h). Blood, urine, and stool cultures were negative. Bacteriological examination of brain abscess specimens identified Lactobacillus catenaformis, Porphyromonas gingivalis, and F. nucleatum. The patient was referred to our department 2 days after drainage. Intraoral examination and panoramic radiographs revealed that the right maxillary second molar had localized advanced periodontitis with moderate gingival tenderness (Fig. 3c). Extraction of causal tooth with severe periodontitis was planned. Patient’s symptoms improved, however, the brain edema and size of abscess deteriorated (Fig. 3d, e). Twenty-six days after drainage, open craniotomy for excision of brain abscess under general anesthesia was performed. Serial imaging showed resolution of the brain abscess and his symptoms improved. The patient was discharged from the hospital 27 days after craniotomy with information regarding removal of causal odontogenic foci to other hospital.
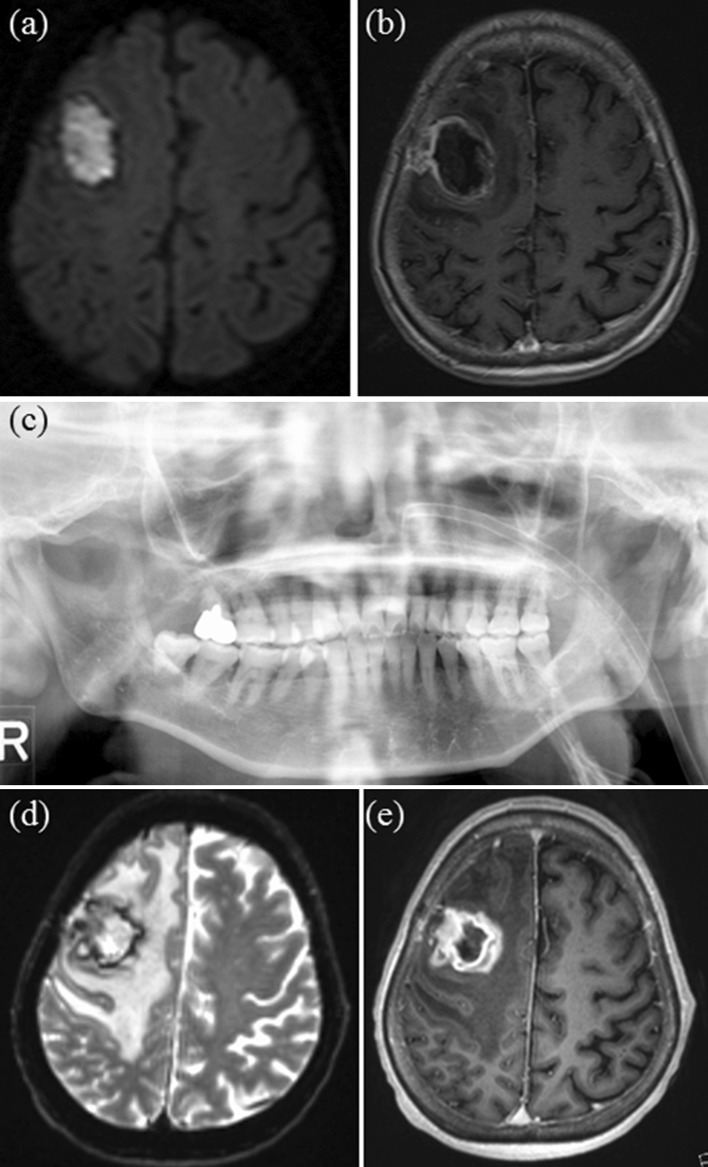
Fig. 3.
Case III a axial DWI showing suspected abscess formation, b axial Gd-T1WI, c panoramic radiograph showing localized advanced periodontitis of the right maxillary second molar, d axial DWI showing deteriorated abscess and e axial Gd-T1WI image showing deteriorated abscess
Discussion
There are few reports of brain abscesses of odontogenic origin [1, 7]. Corson et al. [3] reviewed 17 reports from the 1940s to the 1990s, in which the mortality rate was 30 % (6/20). Antunes et al. [7] described the location of brain abscesses and their treatment in 11 cases from the 1960s to the 1980s, and in five cases from 2001 through 2007. A systematic review by Moazzam et al. [1] included 60 individual cases, 40 of which were published since 2000. They reported the precipitating dental pathology or procedures, the pathogenic microorganisms, the locations of CNS infections, and clinical outcomes [1]. The mortality rate of patients with intracranial bacterial infections of oral origin in that study was improved to 8.3 % (5/60) [1]. Ewald et al. [13] reported six cases of pyogenic infection of the CNS secondary to dental infection. They indicated that there was no definable origin for intracerebral or intraspinal infection in up to 25 % of cases, and these are termed “cryptic abscesses” [13, 14]. They noted that a dental focus should always be considered in the evaluation and treatment of “cryptic” CNS infections, and recommended clinical evaluation by a dentist and oral pantomogram [13]. In the report described by Neidert et al. [15], regarding the suspected origin of infection, most cases could be linked to an oral/dental source (23 %) followed by sinusitis (14 %) or a cardiac source (14 %), and no specific origin of infection could be determined in other cases (43 %).
To determine the oral origin of intracranial infections, oral surgeons should know the underlying mechanism of oral bacterial spread into the CNS. Hematogenous spread via bacteremia is probably a more common route than direct venous drainage. Therefore, there appears to be no predilection for the location of odontogenic foci [1]. There may be no apparent past history or symptoms of acute inflammation caused by odontogenic foci or dental procedures prior to development of brain abscess, as shown in our cases [3]. Microbes can spread as a result of acute infection with a large number of involved pathogens or highly virulent microorganisms, as well as chronic infection with recurrent bacteremia [13]. Table 1 shows the locations of brain abscesses, causal teeth, microorganisms identified, and the past and current state of dentition in case reports since 2011 [7–11, 16, 17]. Our literature review, including our cases, revealed that the occurrence of so-called “cryptic abscess” was 30 % (3/10). We note that there may be no predilection of the laterality of the odontogenic foci, because odontogenic foci can cause intracranial infection on the contralateral side, as shown in Case I in the present report and as reported by Yang et al. [10] in one case. In addition, the apparent causal teeth cannot be identified in cases of “cryptic abscess.” We must also note the difficulty of pathogen identification. It has been reported that brain abscesses are poly-microbial [1]. Many oral bacteria are fastidious pathogens which are difficult to culture and have to be taken to the laboratory immediately under strict anaerobic conditions, therefore, standard bacterial culturing may not serve as pathogen identification [1]. As shown in Case I in the present report and as reported by Greenstein et al. [17] and Ben Hadj Hassine et al. [11], bacterial culture revealed the presence of only Staphylococcus. Mueller et al. [18] attempted to correlate brain abscesses with subgingival microflora by use of conventional culturing techniques, and indicated the underestimation of oral infective sources in brain abscesses in view of the bacterial match between oral infective source and brain abscesses in their retrospective analyses. They commented that a future study using DNA analysis of bacterial samples is needed [18].
Table 1.
Recently reported cases of brain abscess of odontogenic origin
| Study | Age (years) | Sex | Location of brain abscess | Location of causal odontogenic focus | Pathogenic microorganisms | Previous and current state of dental health |
|---|---|---|---|---|---|---|
| Antunes et al. [7] | 56 | M | Left temporal region | Upper left quadrant with deep caries |
Streptococcus viridans
Actinobacillus actinomycetemcomitans Staphylococcus |
Patient had toothache in upper left dental arch and trismus <14 mm at the initial visit |
| Corre et al. [16] | 63 | F | Left temporal region | – |
Fusobacterium nucleatum
Staphylococcus epidermidis |
Patient had hereditary hemorrhagic telangiectasia, and had 10 teeth extracted 1 week previously |
| Haggerty et al. [8] | 68 | M | Left parietal region | Two anterior maxillary teeth | Actinomyces | Patient underwent endodontic therapy for a localized abscess in anterior maxillary teeth 2 weeks previously |
| Yang et al. [10] | 50 | F | Left frontoparietal region | Right maxillary first molar and canine | Streptococcus anginosus | Causal teeth had apical periodontitis with chronic pain but no acute inflammation |
| Park et al. [9] | 53 | F | Right temporal region | Right maxillary second molar | – | Patient had facial swelling and trismus <20 mm 2 weeks previously |
| Greenstein et al. [17] | 43 | M | Left temporal region | Maxillary third molar | Staphylococcus epidermidis | Patient underwent tooth extraction more than 4 weeks previously, and had left-sided facial swelling, trismus, and pain before drainage |
| Ben Hadj Hassine et al. [11] | 46 | M | Cerebellum | – | Staphylococcus aureus | Patient underwent right mandibular third molar extraction 1 month previously |
F female, M male, – not clearly described
Finally, the limitation of this report should be mentioned. First, there was no direct evidence indicating that brain abscess arose from odontogenic foci. Especially, Case I had a past history of otitis media. It has been known that maxillary sinusitis [15] and otitis media [19] can cause intracranial infection. A recent report noted that 80 % of intracranial complications were secondary to chronic otitis media of which the incidence of intracranial complication was 0.8 %; 20 % were due to acute otitis media [20]. This reveals that otitis media causing intracranial infection often lack acute symptoms as well as odontogenic foci. Second, this report cannot show the decision making criteria for the treatment of suspected causal teeth. In Case I, apparent diseased teeth were extracted. In Case II, all of the remaining teeth with severe periodontitis were considered potential sources of intracranial infection, but the specific causal teeth could not be determined. We planned the extraction of these teeth for improvement of oral hygiene. In Case III, causal tooth was planned to be extracted. Like Cases I and III, apparent causal teeth which are usually localized marginal or apical periodontitis with tenderness should be extracted. However, in cases in which causal tooth is not apparent like Case II, further studies for the decision making criteria to eliminate suspected causal teeth will be needed.
As conclusions, oral surgeons should understand these characteristics of odontogenic brain abscess, in which the potentially causal odontogenic foci often lack acute symptoms. If other origins of infection such as sinusitis [15] and otitis media [19] are not found, it would be better to eliminate the potentially causal odontogenic foci for improvement of oral hygiene.
Compliance with Ethical Standards
Conflict of interest
None declared.
References
- 1.Moazzam AA, Rajagopal SM, Sedghizadeh PP, Zada G, Habibian M. Intracranial bacterial infections of oral origin. J Clin Neurosci. 2015 doi: 10.1016/j.jocn.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Chandy B, Todd J, Stucker FJ, Nathan CO. Pott’s puffy tumor and epidural abscess arising from dental sepsis: a case report. Laryngoscope. 2001;111:1732–1734. doi: 10.1097/00005537-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Corson MA, Postlethwaite KP, Seymour RA. Are dental infections a cause of brain abscess? Case report and review of the literature. Oral Dis. 2001;7:61–65. doi: 10.1034/j.1601-0825.2001.70112.x. [DOI] [PubMed] [Google Scholar]
- 4.Iida Y, Honda K, Suzuki T, Matsukawa S, Kawai T, Shimahara T, Chiba H. Brain abscess in which Porphyromonas gingivalis was detected in cerebrospinal fluid. Br J Oral Maxillofac Surg. 2004;42:180. doi: 10.1016/S0266-4356(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 5.Mylonas AI, Tzerbos FH, Mihalaki M, Rologis D, Boutsikakis I. Cerebral abscess of odontogenic origin. J Craniomaxillofac Surg. 2007;35:63–67. doi: 10.1016/j.jcms.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Ulivieri S, Oliveri G, Filosomi G. Brain abscess following dental procedures. Case report. Minerva Stomatol. 2007;56:303–305. [PubMed] [Google Scholar]
- 7.Antunes AA, de Santana ST, de Carvalho RW, Avelar RL, Pereira CU, Pereira JC. Brain abscess of odontogenic origin. J Craniofac Surg. 2011;22:2363–2365. doi: 10.1097/SCS.0b013e318231e585. [DOI] [PubMed] [Google Scholar]
- 8.Haggerty CJ, Tender GC. Actinomycotic brain abscess and subdural empyema of odontogenic origin: case report and review of the literature. J Oral Maxillofac Surg. 2012;70:e210–e213. doi: 10.1016/j.joms.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 9.Park SY, Suh DW, Park CM, Oh MS, Lee DK. Brain abscess due to odontogenic infection: a case report. J Korean Assoc Oral Maxillofac Surg. 2014;40:147–151. doi: 10.5125/jkaoms.2014.40.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J, Liu SY, Hossaini-Zadeh M, Pogrel MA. Brain abscess potentially secondary to odontogenic infection: case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117:e108–e111. doi: 10.1016/j.oooo.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Ben Hadj Hassine M, Oualha L, Derbel A, Douki N. Cerebral abscess potentially of odontogenic origin. Case Rep Dent. 2015;2015:267625. doi: 10.1155/2015/267625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haymaker W. Fatal infections of the central nervous system and meninges after tooth extraction: with an analysis of twenty-eight cases. Am J Orthod Oral Surg. 1945;31:A117–A188. doi: 10.1016/0096-6347(45)90098-6. [DOI] [Google Scholar]
- 13.Ewald C, Kuhn S, Kalff R. Pyogenic infections of the central nervous system secondary to dental affections—a report of six cases. Neurosurg Rev. 2006;29:163–166. doi: 10.1007/s10143-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 14.Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;1997(25):763–779. doi: 10.1086/515541. [DOI] [PubMed] [Google Scholar]
- 15.Neidert MC, Karlin K, Actor B, Regli L, Bozinov O, Burkhardt JK. Preoperative C-reactive protein predicts the need for repeated intracerebral brain abscess drainage. Clin Neurol Neurosurg. 2015;131:26–30. doi: 10.1016/j.clineuro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Corre P, Perret C, Isidor B, Khonsari RH. A brain abscess following dental extractions in a patient with hereditary hemorrhagic telangiectasia. Br J Oral Maxillofac Surg. 2011;49:e9–e11. doi: 10.1016/j.bjoms.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Greenstein A, Witherspoon R, Leinkram D, Malandreni M. An unusual case of a brain abscess arising from an odontgenic infection. Aust Dent J. 2014 doi: 10.1111/adj.12266. [DOI] [PubMed] [Google Scholar]
- 18.Mueller AA, Saldamli B, Stübinger S, Walter C, Flückiger U, Merlo A, et al. Oral bacterial cultures in nontraumatic brain abscesses: results of a first-line study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:469–476. doi: 10.1016/j.tripleo.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Sun J, Sun J. Intracranial complications of chronic otitis media. Eur Arch Otorhinolaryngol. 2014;271:2923–2926. doi: 10.1007/s00405-013-2778-4. [DOI] [PubMed] [Google Scholar]
- 20.Penido NO, Chandrasekhar SS, Borin A, Maranhão AS, Gurgel Testa JR. Complications of otitis media—a potentially lethal problem still present. Braz J Otorhinolaryngol. 2015 doi: 10.1016/j.bjorl.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]