Abstract
Purpose
This prospective study was conducted to evaluate the bone regeneration capacity of synthetic hydroxyapatite mixed with autogenous bone marrow aspirate when used as a bone graft substitute in maxillo-mandibular osseous defects.
Methods
This study included nine patients with histopathalogically proven benign osteolytic lesions in maxilla and mandible that were treated with enucleation or marginal resection followed by bone marrow aspirate coated synthetic biphasic hydroxyapatite (hydroxyapatite and beta tricalcium phosphate) graft placement. Incorporation of graft was assessed based on Irwin’s radiologic staging. The efficacy of graft to form new bone was radiologically evaluated by observing the sequential changes of density at grafted site using gray scale level histogram which was processed in adobe photoshop 7.0 elements. Clinical assessment of recipient and donor sites was done.
Results
Based on Irwin’s radiologic staging, at 6 month follow up period, obvious incorporation of graft with new bone was observed. Sequential changes in bone density measured by gray scale histogram revealed initial resorption followed by replacement of BMA coated hydroxyapatite with new bone formation. None of the patients eventually had complications like infection, wound dehiscence, graft loss at recipient sites at 6 months follow up period.
Conclusion
Autogenous bone marrow aspirate in combination with synthetic hydroxyapatite is an effective option for accelerating bone regeneration in small to moderate sized jaw bone defects. This mixture provides all the three critical elements needed for bone regeneration (osteogenesis, osteoinduction and osteoconduction) with an added advantage of obviating donor site morbidity.
Keywords: Benign bone tumor, Mandible, Bone graft substitute, Hydroxyapatite, Calcium phosphate ceramic, Bone marrow aspirate
Introduction
Bony defects after the removal of pathologies should be reconstructed to permit the placement of prosthesis thus restoring the function and esthetics of a patient. The healing of bone occurs mainly in three stages (1) Inflammatory phase (2) repair stage (3) late remodeling stage. The natural healing process takes up to 12 months for 43 % of bone filling to occur with a 48 % increase in bone density [1]. To accelerate the bone healing without compromising the quality of regenerated bone, bone grafts have been used.
Autogenous bone grafts remain the gold standard as they provide all the three critical elements for bone regeneration, that is, cells for osteogenesis, growth factors for osteoinduction and cortical bone as a scaffold for osteoconduction. But their inherent disadvantages are the need for a donor site and its added morbidity, increased operating time and cost, increased patient recuperation time.
Allografts and xenografts have been used as an alternative to autogenous bone grafts. But they are expensive, produce variable clinical results than autografts with potential risks of bacterial contamination, viral transmission and immunogenicity [2]. Synthetic bone grafts have been tried, including ceramics, collagen, non-collagenous proteins, bioactive glasses and biodegradable polymers. Though porous hydroxyapatite, (ceramic-based synthetic bone graft) is an excellent osteoconductive scaffold, its inherent drawbacks are slow resorption and brittleness [3]. To overcome these demerits, a mixture of hydroxyapatite, β-tricalcium phosphate (TCP) and calcium carbonate (multi-phasic) have been used that can provide better osteoconduction for bone formation, as well as long-term stability, causing successful incorporation into a bone fusion mass [4].
With years of experience and wide clinical use, bone marrow aspirate (BMA) is the most practical source of bone progenitor cells [5]. Aspiration of bone marrow cells is a minimally invasive procedure. Osteoprogenitor stem cells are prevalent in BMA and are able to differentiate into five other cell types (osteoblasts, osteoclasts, adipose cells, chondroblasts and fibroblasts) which modify their morphologic/functional attributes as needed [6]. In this respect, synthetic hydroxyapatite and bone marrow aspirate (BMA) composite may even be superior to the autograft, in which most cellular elements suffer from anoxic cell death due to delayed revascularization.
Hence, we conducted a study to assess the use of hydroxyapatite and beta-tricalcium phosphate mixed with autogenous bone marrow aspirate as a bone graft substitute in maxillary and mandibular osseous defects caused by removal of benign osteolytic lesions.
Methods
This prospective clinical study was done from October 2012–October 2014 in the department of Oral and Maxillofacial Surgery, Narayana Dental College and Hospital, Nellore, India. Approval from the institutional ethics committee was obtained for this clinical study. Patients who voluntarily gave their written consent to a structured informed consent for the study drafted as per the declaration of Helsinki were included in the study.
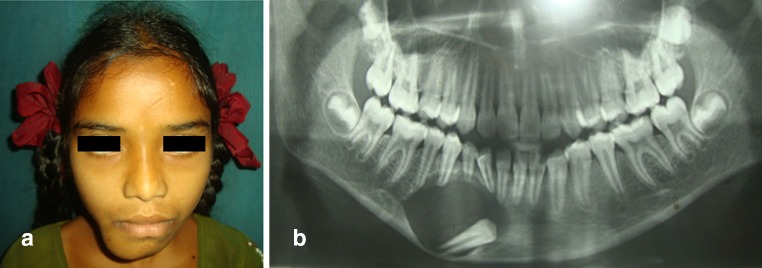
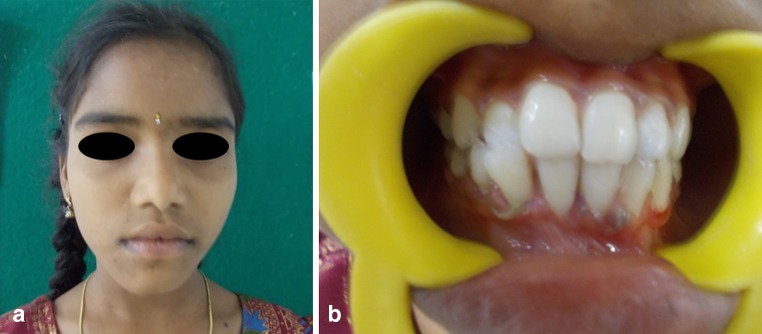
This study included nine patients. Among them four were male and five were female patients. Patients with histologically proven benign osteolytic lesions of mandible or maxilla of size less than or equal to 3–4 cm that required enucleation or marginal resection were included in the study (Fig. 1a, b; Table 1).
Fig. 1.
a Pre-operarive clinical picture showing expansile bony swelling in the right side of chin region. b Pre-operative orthopantomogram showing unilocular radioluscent lesion associated with an impacted tooth in the parasymphysis region of mandible
Table 1.
Patient characteristics
| S. no | Age/sex | Diagnosis | Site of occurrence | Size of defect (cm) | Treatment done | Follow up (months) | Outcome | Irwin’s radiologic staging at last follow-up visit |
|---|---|---|---|---|---|---|---|---|
| 1. | 12 years/F | Adenomatoid odontogenic tumor | Anterior mandible 32 | 4 | Enucleation + BMAC-hydroxyapatite | 13 | Good | Stage-III |
| 2. | 37 years/M | Odontogenic keartocyst-orthokeratinized | Angle, Ramus mandible 8 | 4 | Enucleation + BMAC-hydroxyapatite | 10 | Good | Stage-III |
| 3 | 22y/F | Periapical cyst | Anterior maxilla 123 | 3.5 | Enucleation + BMAC-hydroxyapatite | 8 | Good | Stage-III |
| 4 | 16 years/F | Adenomatoid odontogenic tumor | Anterior maxilla 43 | 4 | Enucleation + BMAC-hydroxyapatite | 6 | Good | Stage-III |
| 5 | 25 years/M | Periapical cyst | Anterior maxilla 12 | 3 | Enucleation + BMAC-hydroxyapatite | 8 | Good | Stage-III |
| 6 | 37 years/F | Periapical cyst | Anterior maxilla 43 | 3.5 | Enucleation + BMAC-hydroxyapatite | 7 | Good | Stage-III |
| 7 | 15 years/M | Dentigerous cyst | Anterior maxilla 3 | 4 | Enucleation + BMAC-hydroxyapatite | 8 | Good | Stage-III |
| 8 | 16 years/M | Complex odontoma | Posterior mandible 78 | 4 | Enucleation + BMAC-hydroxyapatite | 9 | Good | Stage-III |
| 9 | 13 years/M | Periapical cyst | Anterior maxilla 21 | 3 | Enucleation + BMAC-hydroxyapatite | 7 | Good | Stage-III |
Patients with malignant osteolytic lesions, benign osteolytic lesions of size more than 5 cm, medically compromised patients who are not fit for surgery, any previous surgery on iliac crest to obtain bone graft, when there is inadequate overlying mucosa to get primary closure, maxillary osseous defects involving maxillary sinus and nasal floor were excluded from the study.
All the nine patients were operated under general anesthesia after standard pre-anesthetic evaluation and work up. General anesthesia was administered through naso-endotracheal intubation after standard painting and draping the donor site (postero-superior iliac spine) and recipient sites (maxilla/mandible based on the location of the pathology) of the patient.
Harvesting Bone Marrow Aspirate
The patients were placed in a right or left lateral decubitus position, with the knee and hip flexed to about 45°. Overlying skin of posterior iliac crest region (donor site) was prepared with 5 % povidine iodine and draping was done exposing the surgical area. Then the skin, subcutaneous tissue and the periosteum were infiltrated with 2 % lidocaine with adrenaline (1:80,000).
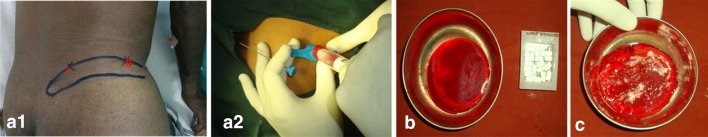
Posterior iliac crest spine was identified by palpating with the thumb and index finger and width of the posterior iliac crest spine was estimated (Fig. 2a1). Marking was placed on the center point of the posterior iliac crest spine, Jamshedi bone marrow aspiration needle with the stylet was advanced slowly through the dermal layer, pointing towards the posterior superior iliac spine. When the posterior iliac crest was reached, it was penetrated by gentle rotary motion of the needle. Once the cortex was penetrated and the needle was securely placed within the marrow cavity, the stylet was withdrawn and the aspiration was performed with a 20 cc syringe without withdrawing the needle (Fig. 2a2). A total volume of 10 ml was aspirated. After aspiration, the needle was withdrawn slowly and pressure dressing was placed on the injected area. Then the aspirated blood was mixed with 0.1 cc of heparin anticoagulant (Fig. 2b).
Fig. 2.
a1 Marking anatomical landmarks on iliac crest—* Posterior iliac spine, # anterior superior iliac spine. a2 Harvesting bone marrow aspirate from posterior ilium. b Aspirated and anticoagulant bone marrow aspirate and hydroxyapatite blocks. c: Mixture of bone marrow aspirate and hydroxyapatite
Recipient Site
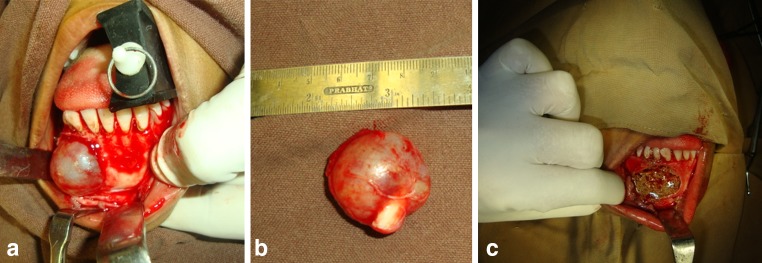
After the procurement of aspirate, patient was placed in supine position. Extra-oral and intraoral painting was done with povidine iodine solution. Xylocaine with adrenaline (vasoconstrictor) was administered at the site of lesion to be excised. Crevicular or vestibular incision was given (based on individual location and extent of the lesion), mucoperiosteal flap was elevated, exposing the lesion (Fig. 3a). Thinned out cortical plate was removed, the cystic lining was separated from underlying bony margins and was removed in toto (Fig. 3b). Sharp bony margins were smoothened and thorough irrigation was done with povidone iodine, isotonic saline solution. Hemostasis was achieved.
Fig. 3.
Intra-operative views. a Exposure of the lesion–Intra-follicular type of adenomatoid odontogenic tumor. b Enucleated lesion. c Mixture of bone marrow aspirate and hydroxyapatite placed in the post-enucleation mandibular bony defect
Graft Placement
G-Bone (Surgiwear, India) Synthetic Granules are of synthetic multi-phasic Calcium Hydroxyapatite in low crystalline form. It is a mixture of hydroxyapatite, Tri-calcium phosphate and other forms of calcium such as calcium carbonate and bi calcium phosphate. G-bone blocks were soaked in 4 ml of sterile normal saline for 1–3 min and were allowed to hydrate. Synthetic hydroxyapatite blocks were then broken into small crystals and were soaked in anti-coagulated bone marrow aspirate (Fig. 2c). This mixture with semi solid consistency was placed in the osseous defect (Fig. 3c). Watertight closure was done with 3-0 Vicryl.
Post-Operative Follow-Up
All the patients received inj. Cefotaxime.1 gm –intravenous twice daily, Inj.metronidazole.500 mg. intravenous twice daily for two post-operative days. Upon discharge they were advised Tab.Cefixime 200 mg orally twice daily, Tab.Metronidazole. 400 mg twice daily for 5 days. All the nine patients were evaluated for the minimum period of about 6 months. Every follow up was done in the intervals of 1, 3 and 6 months. During post-operative follow up, healing of overlying mucosa at grafted site was assessed. Assessment of post-operative complications like wound dehiscence over recipient site, leaching of graft, pain and infection in donor and recipient site was done. Postoperative pain was assessed and scored with the help of visual analog scale given by Pasqualini et al. [7]. All the preoperative and immediate, 1st, 3rd and 6th month postoperative radiographs were taken with equal exposure and magnification using PLANMECA DIAMAXIS PRO 4.4 DIGITAL SOFTWARE. The graft incorporation at postoperative follow up of 1, 3, 6 months intervals was assessed using Irwin’s radiologic staging [8] (Table 2).
Table 2.
Irwin’s radiologic staging
| Stage | Radiolucent zone between the bone cavity and the graft | Intrinsic graft indistinctiveness | Graft margins |
|---|---|---|---|
| I | Present | Present | Obvious |
| II | Indistinct | Indistinct | Hazy |
| III | Indistinct/disappearance | Indistinct/disappearance | Obvious incorporation |
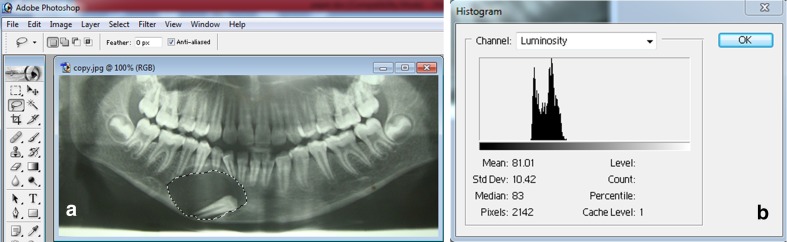
Digital panoramic radiograph was processed with the adobe photoshop elements 7.0 by selecting osseous defect area. To compare, serial radiographs of same patients, radiolucency of a tooth was used as a reference. The control region (tooth) and the regions of interest (cyst cavities) were defined through a gray scale of 255 tonalities using gray-level histograms [9] (Fig. 4a, b).
Fig. 4.
a Margins of the bony defect marked on the digitized orthopantomograph processed through adobe photoshop 7.0 elements. b Gray scale histogram
A single examiner demarcated the outline of the lesion. The control regions from radiographs taken at different times were matched, and the mean gray-level values of the regions of interests were calculated and then compared with each other. The mean and average values of density in histogram were noted pre operatively and at every follow up intervals of 1 week, 1, 3 and 6 months post operatively.
Post-operative clinical photographs and radiographs (orthopantamograph) were taken at 1 week, 1, 3 and 6 months intervals periodically. Graft incorporation was assessed based on Irwin’s radiologic staging (Table 2).
Statistical Analysis
Results were analyzed with the help of graph pad calculator and expressed as mean ± SD for continuous data. One way repeated measures non parametric Friedmans ANOVA was used for multiple group comparisons followed by ‘Post-hoc–Dunns’ test for group-wise comparisons.
Sample size was calculated assuming a standard deviation of measurements on gray scale histogram with 80 % power and two sided alpha error of 0.05. A total of nine patients were sufficient to detect 20 points on histogram.
Results
Clinical Evaluation
Recipient Site
In all the nine patients, healing of overlying mucosa was eventually good at 6 months follow up (Fig. 5a, b). Only one patient (second case) had minor area of wound dehiscence and partial leaching of graft on 7th postoperative day and it subsided with local wound care, re-suturing the wound and antibiotic management for 5 days. Apart from this no other complications were noted.
Fig. 5.
a Eight months post-operative clinical photograph showing restoration of symmetry and normal contour of chin. b 8 months post-operative view of grafted site showing normal overlying mucosa
Donor Site
Donor site healing was good in all the patients. Regarding pain assessment at donor site, among nine patients only three patients were scored with mild pain on immediate postoperative day and remaining six patients were scored with no pain. After 7 days postoperative follow up, none of the patients were scored with pain. Other donor site complications like swelling, hematoma formation, infection were not present in all the nine patients.
Radiologic Analysis
Graft incorporation was assessed in all the patients based on Irwin’s radiologic staging. Margins of the granules appeared to be slightly hazy (Irwin’s stage II) at 3 months follow up period and at 6-month follow-up there was obvious incorporation (Irwin’s stage III) with a homogenous mass (Fig. 6a, b, c).
Fig. 6.
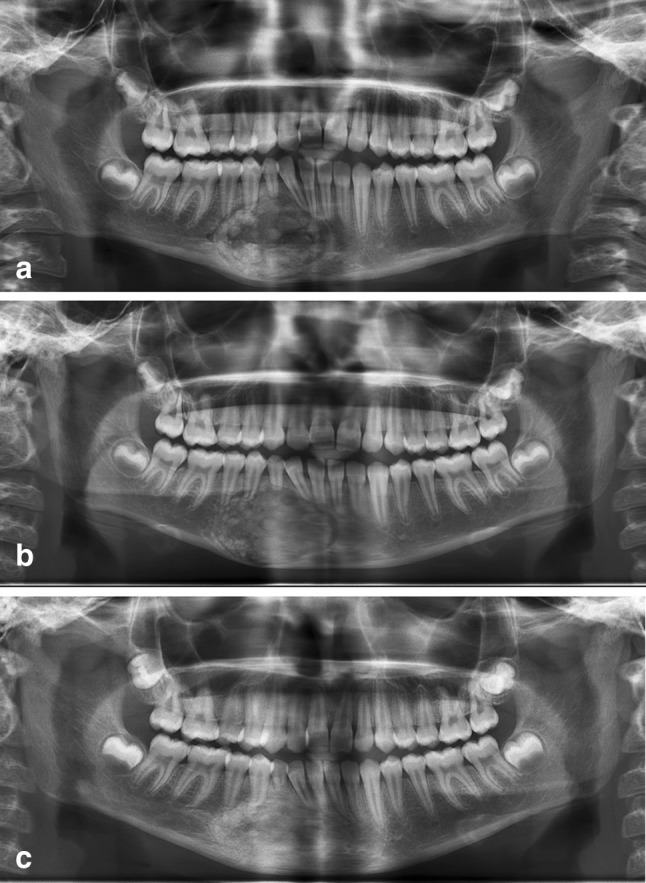
a Immediate post-operative orthopantomogram showing radio-opacity in the grafted site due to hydroxyapatite granules. b 1 month post-operative orthopantomogram showing slightly reduced radiodensity at grafted site suggesting resorption of hydroxyapatite. c Six months post-operative orthopantomogram showing homogenous radiodensity at the grafted site comparable to that of adjacent normal bone, suggestive of replacement of hydroxyapatite by normal new bone formation
Sequential changes of density at grafted site were measured using gray scale level histogram which was processed in adobe photoshop 7.0 elements (Table 3).
Table 3.
Mean gray level values indicating bone density of the defect area
| S. no | Pre operative | Immediate post operative | 1 month post operative | 3 months postoperative | 6 months Postoperative |
|---|---|---|---|---|---|
| 1 | 81.75 | 108.65 | 94.77 | 106.27 | 115.27 |
| 2 | 94.22 | 115.78 | 92.21 | 107.80 | 124.24 |
| 3 | 102.36 | 136.84 | 117.53 | 110.97 | 126.38 |
| 4 | 60.22 | 107.93 | 72.02 | 81.03 | 98.79 |
| 5 | 64.94 | 110.87 | 89.80 | 92.07 | 102.81 |
| 6 | 83.70 | 105.25 | 68.89 | 98.09 | 118.65 |
| 7 | 87.07 | 155.09 | 139.62 | 142.54 | 146.77 |
| 8 | 104.86 | 131.13 | 113.28 | 121.82 | 126.27 |
| 9 | 72.51 | 110.65 | 92.54 | 116.34 | 132.21 |
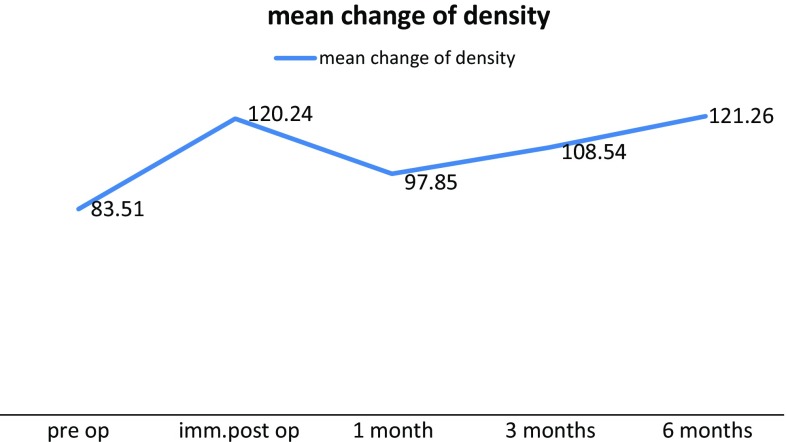
| Mean | 83.51 | 120.24 | 97.85 | 108.54 | 121.26 |
| SD | 14.72 | 16.05 | 21.11 | 16.81 | 13.82 |
0 versus 1 week = P < 0.001, 0 versus 6 Month = P < 0.001, 1 week versus 1 month = P < 0.01, 1 versus 6 Month = P < 0.01
Initial mean values were lower in preoperative period followed by much higher value in immediate postoperative period. This increased value indicated the presence of radio-opaque graft material. Then the change in mean gray level values in 1st and 3rd month postoperative period indicates that the graft resorption followed by replacement with new bone at defect site (Fig. 7). Increased 6 months postoperative mean value showed 45.2 % increase in bone formation when compared to preoperative values. Statistical analysis has shown significant p value.
Fig. 7.
Graphical representation of mean change of bone density measured by changes in gray scale values on histogram
Discussion
Reconstruction of post ablative jaw bone defects is a challenging task faced by oral and maxillofacial surgeons. Though autogenous bone grafts are presently the gold standard, the need for a donor site, increased operative time, regenerative capacity of bone has initiated a diverse spectrum of modalities to correct these bony defects.
A fully non-resorbable graft material may impede remodeling, newly formed bone will have insufficient strength causing permanent stress in the fusion mass [10]. Commercially available hydroxyapatite resorbs very slowly under normal physiological conditions, whereas beta-tricalcium phosphate is generally resorbed within 6 weeks after implantation [11]. When a mixture of biodegradable hydroxyapatite/beta-tricalcium phosphate ceramics is used, it dissolves, breaks down and allows new bone formation and remodeling to attain optimal mechanical strength without interference.
The acceptance of these substitutes by host tissues is determined by two important features: pore diameter and the porosity or interconnectivity. Optimum pore size for bone ingrowth is 100 μm, whereas pore size more than 200 μm facilitates development of mature osteon [12]. Level of porosity should be larger than 50–60 %, the minimal interconnection size should be larger than 50–100 μm and the mean pore size should be between 100 and 500 μm [13]. Inter-connectivity is essential because dead-end pockets limit vascular supply to the in-growing bone. In the present study, the pore size was about 300–400 μm. This pore size was favorable to bone formation. The form of hydroxyapatite (blocks or granules), determines the clinical utility and the size of the bone defect determines the appropriate size of the implant. In the present study, block form was used as the size of the defect was large.
Synthetic hydroxyapatite, a commercially available graft material is a combination of early resorbing beta tricalcium phosphate (b-TCP) and dense hydroxyapatite. These particles are becoming an increasingly important matrix for bone reconstruction and tissue engineering [14]. Synthetic hydroxyapatite when seeded with bone progenitor cells can provide the three essential elements for a successful bone graft, thus providing a competitive alternative to autografts.
Autogenous bone marrow aspirate (BMA) provides direct implantation of osteoprogenitor cells that lead to accelerated healing of bone defects [15]. It may bypass the time-consuming and technically difficult process of cell expansion and differentiation, permitting both harvesting and transplanting of BMA during the same surgical procedure. Harvesting sites include iliac bone, sternum, tibia, calcaneus bone. The posterior iliac crest has become the preferred site from which to obtain bone marrow because it is safer, psychologically less traumatic and ideal for aspirating large volumes [16]. However in the present study, bone marrow aspirate was taken from posterior iliac crest bone and immediate postoperative pain was very minimal, as assessed with visual analog scale (VAS) score and completely resolved in third postoperative day. No other donor site complications were observed during follow up period.
Based on Irwin’s radiologic staging, graft incorporation was obvious with uniform mass, this was in correlation with the study conducted by Bansal et al. [17]. Through computer analysed gray scale histogram, we observed that preoperative density values were lower suggesting a radiolucent lesion in the area of interest. After graft placement, we found statistically significant increase of density values between preoperative and immediate postoperative value, indicating the presence of graft in the defect.
At 1 month postoperative period the density values were reduced when compared to immediate postoperative period. These findings were similar to the study conducted by Pradel et al [9] in which a decrease of density values at 3 month postoperative period than preoperative values was reported, but in our study the density values were decreased at one month itself. This early change in values may be due to differences in resorption rates of different graft materials. In a study conducted by Nagahara et al. [18] they evaluated the resorption rates of hydroxyapatite and beta - tricalcium phosphate. Results showed that the resorption rate of beta tricalcium phosphate was faster than hydroxyapatite.
At 3, 6 month postoperative period, high statistically significant increase of density values was observed indicating bone formation. A study by Pradel et al has shown percentage increase of density values from 3rd month to 6th month postoperative period [9]. Clinically, the defect site have shown trabeculae pattern simulating to normal bone. Zijderveld et al. [19] compared the autogenous chin graft with pure beta-tricalcium phosphate in sinus floor augmentation and found a mean of 17 % new bone formation after 6 months in beta tricalcium phosphate grafted patients. In our study, 45.2 % of bone density was observed radiologically after 6 months which is not in accordance with Zijderveld et al. study. This increase of bone density may be due to placement of combined autogenous bone marrow aspirate and beta tricalcium phosphate which accelerated bone formation.
This combination of bone marrow aspirate and hydroxyapatite as a bone graft substitute has been successfully and extensively used in orthopedics (for spinal fusion, to prevent avascular necrosis of femoral head, etc.) [17]. But its use in maxillofacial surgery has been limited to maxillary sinus lift procedures and bone grafting in the peri-implant region [18, 19]. To best of our knowledge this is the first study to report the use of BMA and hydroxyapapatite as a bone graft substitute in small to moderate maxillo-mandibular osseous defects caused by removal of osteolytic lesions. Although there was a clinical and radiological evidence of new bone formation; it can only be confirmed on histology; we consider this as the drawback of our study, as we could not correlate the radiological findings with histopathological findings.
Conclusion
Our results support that the clinical application of autogenous bone marrow aspirate in combination with synthetic hydroxyapatite is an effective option for accelerating bone regeneration in small to moderate sized jaw bone defects. This mixture provides all the three critical elements needed for bone regeneration (Osteogenesis, Osteoinduction and Osteoconduction) with an added advantage of obviating donor site morbidity. However further long term studies should be directed towards its use as a bone graft material in larger defects involving studies with larger sample size and with control groups.
Compliance with Ethical Standards
Conflict of interest
None.
References
- 1.Chiapasco M, Rossi A, Motta JJ, Crescentini M. Spontaneous bone regeneration after enucleation of large mandibular cysts: a radiographic computed analysis of 27 consecutive cases. J Oral Maxillofac Surg. 2000;58:942–948. doi: 10.1053/joms.2000.8732. [DOI] [PubMed] [Google Scholar]
- 2.Friedlaender GE, Strong DM, Tomford WW, Mankin HJ. Long-term follow-up of patients with osteochondral allografts: a correlation between immunologic responses and clinical outcome. Orthop Clin N Am. 1999;30:583–588. doi: 10.1016/S0030-5898(05)70111-5. [DOI] [PubMed] [Google Scholar]
- 3.Morisue H, Matsumoto M, Chiba K, et al. Novel hydroxyapatite fiber mesh as a carrier for recombinant human bone morphogenetic protein-2 enhances bone union in rat posterolateral fusion model. Spine. 2006;31:1194–1200. doi: 10.1097/01.brs.0000217679.46489.1b. [DOI] [PubMed] [Google Scholar]
- 4.Brandoff JF, Siber JS, Vaccaro AR. Contemporary alternatives to synthetic bone grafts for spine surgery. Am J Orthop. 2008;37:410–414. [PubMed] [Google Scholar]
- 5.Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79:1699. doi: 10.2106/00004623-199711000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Ross MH, Romrell LJ, Kaye GI. Histology: a text and atlas. Baltimore: Williams and Wilkins; 1995. pp. 150–154. [Google Scholar]
- 7.Pasqualini D, Mollo L, Scotti N, Cantatore G, Castellucci A, Migliaretti G, Berutti E. Postoperative pain after manual and mechanical glide path: a randomized clinical trial. J Endod. 2012;38:1. doi: 10.1111/j.1747-4477.2012.00347.x. [DOI] [PubMed] [Google Scholar]
- 8.Irwin RB. Coralline hydroxyapatite as a bone graft substitute in orthopedic oncology. Am J Orthop. 2001;30:544–550. [PubMed] [Google Scholar]
- 9.Pradel W, Eckelt U, Lauer G. Bone regeneration after enucleation of mandibular cysts: comparing autogenous grafts from tissue-engineered bone and iliac bone. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:285–290. doi: 10.1016/j.tripleo.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Boden SD, Schimandle JH. Biologic enhancement of spinal fusion. Spine. 1995;20:113S–123S. doi: 10.1097/00007632-199512151-00007. [DOI] [PubMed] [Google Scholar]
- 11.Spivak JM, Hasharoni A. Use of hydroxyapatite in spine surgery. Eur Spine J. 2001;10:S197–S204. doi: 10.1007/s005860100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hulbert SF, Cooke FW, Klawitter JJ, Leonard RB, Sauer BW, et al. Attachment of prosthesis to musculoskeletal system by tissue ingrowth and mechanical inter locking. J Biomed Mater Res. 1973;7:1–23. doi: 10.1002/jbm.820070303. [DOI] [PubMed] [Google Scholar]
- 13.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26(27):5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Hirano S, Shoji K, Kojima H, Omori K. Use of hydroxyapatite for reconstruction after surgical removal of intraosseous hemangioma in the zygomatic bone. Plast Reconst Surg. 1997;100:86–90. doi: 10.1097/00006534-199707000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Bierman HR. Bone marrow aspiration of the posterior iliac crest, an additional safe site. Calif Med. 1952;77(2):138–139. [PMC free article] [PubMed] [Google Scholar]
- 16.Benzel Edward C. Spine surgery: techniques, complications, avoidance and management. 3. Philadelphia: Saunders; 2012. pp. 1–18. [Google Scholar]
- 17.Bansal S, Chauhan V, Sharma S, Maheshwari R, Juyal A, Raghuvanshi S. Evaluation of hydroxyapatite and beta-tricalcium phosphate mixed with bone marrow aspirate as a bone graft substitute for posterolateral spinal fusion. Ind J Orthop. 2009;43:3. doi: 10.4103/0019-5413.45317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagahara K, et al. Osteogenesis of hydroxyapatite and tricalcium phosphate used as a bone substitute. Int J Oral Maxillofac Implants. 1992;7:72–79. [PubMed] [Google Scholar]
- 19.Zijderveld SA, Zerbo IR, van den Bergh JP, et al. Maxillary sinus floor augmentation using beta-tricalcium phosphate (Cerasorb) alone compared to autogenous bone grafts. Int J Oral Maxillofac Implants. 2005;20(3):432–440. [PubMed] [Google Scholar]