Abstract
Background
Increasing intra-abdominal volume (IAV) can lead to intra-abdominal hypertension (IAH) or abdominal compartment syndrome. Both are associated with raised morbidity and mortality. IAH can increase airway pressures and impair ventilation. The relationship between increasing IAV and airway pressures is not known. We therefore assessed the effect of increasing IAV on airway and intra-abdominal pressures (IAP).
Methods
Seven pigs (41.4 +/−8.5 kg) received standardized anesthesia and mechanical ventilation. A latex balloon inserted in the peritoneal cavity was inflated in 1-L increments until IAP exceeded 40 cmH2O. Peak airway pressure (pPAW), respiratory compliance, and IAP (bladder pressure) were measured. Abdominal compliance was calculated. Different equations were tested that best described the measured pressure-volume curves.
Results
An exponential equation best described the measured pressure-volume curves. Raising IAV increased pPAW and IAP in an exponential manner. Increases in IAP were associated with parallel increases in pPAW with an approximate 40% transmission of IAP to pPAW. The higher the IAP, the greater IAV effected pPAW and IAP.
Conclusions
The exponential nature of the effect of IAV on pPAW and IAP implies that, in the presence of high grades of IAH, small reductions in IAV can lead to significant reductions in airway and abdominal pressures. Conversely, in the presence of normal IAP levels, large increases in IAV may not affect airway and abdominal pressures.
Electronic supplementary material
The online version of this article (doi:10.1186/s40635-017-0124-7) contains supplementary material, which is available to authorized users.
Keywords: Intra-abdominal pressure, Intra-abdominal hypertension, Abdominal compliance, Airway pressures, Respiratory compliance, Abdominal volume
Background
Intra-abdominal hypertension (IAH) is defined as a sustained intra-abdominal pressure (IAP) ≥ 12 mmHg [1]. IAH is common in critically ill patients [2] and is associated with an increased morbidity and mortality [3]. IAH is caused by additional intra-abdominal volume (IAV) within the confined abdominal cavity (e.g., retroperitoneal bleed, free fluid from massive fluid resuscitation, ascites, ileus with dilated bowel, etc.) or by reduced compliance of the abdominal wall (e.g., obesity or eschars in burns patients). If IAP is significantly increased or persists, an abdominal compartment syndrome may develop which is defined as a sustained IAP > 20 mmHg that is associated with new onset organ dysfunction [1]. Organ failure may include cardiac, respiratory, renal, and/or gastro-intestinal failure.
It has long been thought that a linear relationship exists between IAV and IAP [4–6]. However, in a recent review article, by extracting all available human IAV and IAP measurements from current literature, we were able to demonstrate an exponential relationship between IAV and IAP [7]. The exponential relationship between IAV and IAP is of interest because of the clinical consequences of IAH. Patients with IAH often have impaired lung function due to a cephaled displacement of the diaphragm, associated with impaired lung volumes and increased airway pressures resulting in difficulties in maintaining adequate ventilation [8].
Several therapeutic options exist to reduce IAP [9, 10]. These therapies are associated with small reductions in IAV and are therefore thought to have a small effect on IAP. However, due to the exponential relationship at higher IAP ranges, small reductions in IAV may indeed have significant effects on IAP.
The effect of changes in IAV on airway pressures is well known. We therefore aimed to characterize the influence of IAV on both IAP and airway pressures.
Methods
Seven pigs were studied in a protocol to measure IAP and airway pressures caused by incremental increases in IAV. The Animal Ethics Committee of the University of Western Australia approved the study protocol (UWA RA/3/100/688). The study conformed to the regulations of the Australian code of practice for the care and use of animals for scientific purposes. Anesthesia, mechanical ventilation, surgical preparation, and instrumentation were performed as previously described [11] and are briefly outlined below.
Animals
Seven Large White breed pigs [mean (SD) animal weight of 41.4 (+/−8.5) kg] received standardized anesthesia including initial sedation using intramuscular zolazepam/tiletamine (Zoletil ®) and xylazine followed by a combination of propofol, morphine, and ketamine for maintenance of anesthesia. At the end of the experimental protocol the pigs were euthanized with intravenous pentobarbitone. No neuromuscular blocking agents were used as they are infrequently used in our clinical practice and also to reduce the risk of awareness of pain in the animals. Adequacy of the depth of anesthesia was regularly assessed (lack of muscle tone, absence of spontaneous ventilatory effort).
Mechanical ventilation and airway parameters
Mechanical ventilation (Servo 900, Siemens, Berlin, Germany) was maintained using constant tidal volumes of 8 mL/kg. Initial PEEP was 5 cmH20. Respiratory rate was adjusted to maintain an end-tidal CO2 between 35 and 45 mmHg before the abdomen was inflated but not changed thereafter. Peak inspiratory pressure (pPAW) and dynamic respiratory system compliance (CRS) were obtained automatically from the ventilator. End-expiratory lung volume was measured at baseline IAP and PEEP of 5 cmH2O using the multiple breath nitrogen wash-out method as previously described [11]. Pressure-volume (P-V) curves were performed at PEEP 5 cmH2O and then at PEEP 15 cmH2O.
Intra-abdominal pressure measurement
Urinary bladder pressure was used to assess IAP. For this, a 12F Foley catheter was placed in the urinary bladder via a caudal midline laparotomy and attached to a standard transducer system (Hospira, Lake Forest, IL). Mean pressures were measured from the mid-axillary line. Throughout the study, the animals remained in the supine position. A standardized injection volume of 25 mL of 0.9% NaCl (AbViser 300, Wolfe Tory Medical, Salt Lake City, UT) was used and 60 s relaxation time was allowed for before definitive measurement [1]. IAH was graded as recommended by the World Society of Abdominal Compartment Syndrome [1].
Abdominal pressure-volume curve
A large intra-abdominal balloon (200 g latex weather balloon, Scientific Sales, Lawrenceville, NJ) was placed in the peritoneal cavity via midline laparotomy. Even placement of the balloon in the abdomen was ensured by visual inspection and partial inflation. A 1-L precision syringe (Vitalograph, Hamburg, Germany) was used to add air to the IAV in 1-L incremental steps. After each addition to IAV, we waited 10 s to allow pressures to equilibrate before assessing all parameters. Abdominal inflation was not continued beyond an IAP of 40.8 cmH2O (30.0 mmHg).
Analysis and statistics
Absolute abdominal pressure-volume points were entered in a spreadsheet and analyzed using Excel (Microsoft, Redmond, WA, USA). All pressures were converted from millimeter of mercury into centimeter of water for better comparison between the IAP and pPAW (1 mmHg = 1.3595 cmH2O). The change in IAV following addition of air to the intra-abdominal balloon was pressure-corrected using the Boyle equation (pressure-corrected additional IAV = measured additional × 1033/(1033 + IAP in centimeter of water), to compensate for the compressibility of air.
Two different equations were assessed for their accuracy at describing the IAP-IAV curve. First, the Venegas equation, V = a + [b/(1 + e −(P − c)/d)], a logistic function was used. This has been used to describe the characteristic sigmoid shape of pulmonary [12] and other P-V curves [13]. In the original paper, V represents inflation or absolute lung volume, P represents airway opening or transpulmonary pressure, and a, b, c, and d represent fitting parameters. In the tested situation, V represented additional IAV, P represented absolute pressure (IAP or pPAW), and a, b, c, and d represent fitting parameters.
Second, an alternate exponential equation, V = v + k × Ln (P 0 − p) where V represented additional IAV, P 0 represented resting IAP (no additional IAV), and v, k, p represent fitting parameters, was tested. This equation has been used in the past to characterize lung elastic recoil and in other instances where pressures rise in near asymptotic fashion [14]. This exponential equation is single ended and exhibits near asymptotic shape at high volumes only whereas the Venegas equation exhibits true asymptotic shape at high and low volumes.
For each corresponding P-V data set, we used the Excel “Solver” function to find itting parameters that best described the measured P-V curve. The best fit was defined as a curve resulting in the smallest root mean square between the measured and calculated P-V points. Minimizing the residual sum of squares (RSS) is a standard method employed for fitting curves. The mean fitting parameter of all study subjects was used to plot a mean P-V curve.
Abdominal compliance (CAB) was defined as a measure of the ease of abdominal expansion, expressed as a change in intra-abdominal volume (IAV) per change in intra-abdominal pressure (IAP): AC = ΔIAV/ΔIAP [1]. CAB is given as milliliter/centimeter of water for easier comparison with respiratory compliance.
Mann-Whitney rank sum test or Wilcoxon signed rank test was used as appropriate. A p value of <0.5 was considered statistically significant.
Results
The subjects had a mean (SD) weight of 41.4 (+/−8.5) kg and end-expiratory lung volume of 1.68 (0.30) L. Baseline IAP was 5.0 cmH2O (3.7 mmHg). Expiratory tidal volume of 331 (68) mL and respiratory rate 34.1 (5.0) per minute were set at baseline (PEEP 5 cmH2O, no abdominal inflation). At 5 cmH2O PEEP, the highest applied IAP ranged from 42.1 to 55.7 cmH2O (31 to 41 mmHg), and additional IAV ranged from 7.7 to 14.3 L.
In comparison with the Venegas equation, the alternate exponential equation produced a P-V curve that better fitted the measured values (lower root mean square) (Additional file 1: Figure S1—P-V curves using Venegas and exponential equation, Additional file 2: Table S1—Equation parameters of intra-abdominal and airway pressure-volume curves).
We therefore subsequently used the alternate exponential equation. Figure 1 depicts P-V curves derived from the exponential equation of the average and of each individual of these animals. To calculate an expected IAP from a given additional IAV, the alternate exponential equation V = v + k × Ln (P 0 − p) can be rearranged to P 0 = p + Exp (V − v)/k.
Fig. 1.

Pressure volume curves showing intra-abdominal pressure (IAP) in centimeter of water in function of increasing additional intra-abdominal volume in liters. An exponential equation was used to calculate IAP for individual animals (narrow dashed curve) and for the average of all animals (bold dashed curve)
After maximal abdominal inflation was achieved using on average 10.4 L (2.1) additional IAV, IAP after initial abdominal inflation at 5 cmH2O of positive end-expiratory pressure (PEEP) was higher than after subsequent abdominal re-inflation using the same additional IAV at 15 cmH2O of PEEP, 49.4 (4.1) vs 45.6 (2.3) cmH2O, respectively (p = 0.03) (Additional file 3: Figure S3—P-V curves at PEEP of 5 and 15 cmH2O). The abdominal compliance (CAB) at maximal IAV and IAP after initial (5 cmH2O PEEP) and repeat inflation (15 cmH2O PEEP) were 55.3 (5.0) mL/cmH2O and 62.0 (6.9) mL/cmH2O, respectively (p = 0.06).
With an increasing amount of additional IAV, IAP and to a lesser extent pPAW increased exponentially (Fig. 2). There was a directly proportional relationship between delta pPAW as a function of delta IAP, with a strong correlation (delta pPAW = 0.14 + 0.43 × delta IAP, R 2 = 0.83, p < 0.001). Hence, abdomino-thoracic transmission approximated 40% (Fig. 3). With increasing IAP, CAB and CRS decreased (Fig. 4).
Fig. 2.

Pressure volume curves showing intra-abdominal pressure (IAP) (dashed curve) and peak airway pressure (pPAW) (dotted curve) in centimeter of water in function of increasing additional intra-abdominal volume in liters. An exponential equation was used to calculate IAP and pPAW for the average of all animals
Fig. 3.
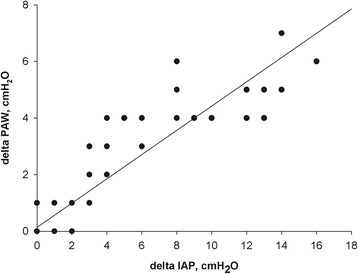
Delta peak airway pressure (pPAW) in centimeter of water as a function of delta IAP in centimeter of water. Delta pPAW = 0.14 + 0.43 × delta IAP, R 2 = 0.83, p < 0.001
Fig. 4.
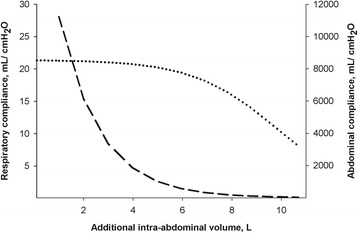
Average abdominal compliance in milliliter per centimeter of water (dashed curve) and dynamic respiratory system compliance in milliliter per centimeter of water (dotted curve) as a function of increasing additional intra-abdominal volume in liters. Abdominal compliance was calculated from the difference of additional intra-abdominal volume per difference of resulting intra-abdominal pressure. Dynamic respiratory compliance was taken from the ventilator
We calculated the effect of an additional IAV of 500 mL on pPAW and IAP (Table 1). The addition of 500 mL IAV increased pPAW and IAP to a greater extent at higher grades of IAH than at lower grades of IAH (i.e., reduced compliance at higher levels of IAH).
Table 1.
Effect of additional 500 mL intra-abdominal volume on intra-abdominal and peak airway pressure at different grades of intra-abdominal hypertension
| Given IAP, cmH2O (mmHg) |
IAH grade | PAW at given IAP, cmH2O |
Additional IAV at given IAP, L | IAV increased by 0.5 L, L |
IAP after addition of 0.5 L IAV, cmH2O (mmHg) |
Resulting increase in IAP, cmH2O (mmHg) | CAB, mL/mmHg | Resulting increase in PAW, cmH2O |
|---|---|---|---|---|---|---|---|---|
| 5.0 (3.7) | Baseline | 20.5 | 0.0 | 0.5 | 5.0 (3.7) | 0.0 (0.0) | – | 0.0 |
| 16.3 (12.0) | 1 | 24.8 | 7.7 | 8.2 | 20.4 (15.0) | 4.0 (3.0) | 168 | 1.5 |
| 21.8 (16.0) | 2 | 26.8 | 8.4 | 8.9 | 27.7 (20.4) | 6.0 (4.4) | 114 | 2.2 |
| 28.5 (21.0) | 3 | 29.3 | 8.9 | 9.4 | 36.9 (27.2) | 8.4 (6.2) | 81 | 3.0 |
| 35.3 (26.0) | 4 | 31.8 | 9.3 | 9.8 | 46.1 (33.9) | 10.8 (7.9) | 63 | 3.9 |
Values are given in mean (SD). Pigs weight was 41.4 (8.5) kg. IAP intra-abdominal pressure, IAH intra-abdominal hypertension, grading of IAH according to the World Society of Abdominal Compartment Syndrome [1], PAW peak airway pressure, IAV intra-abdominal volume, C AB abdominal compliance
Discussion
The main findings in this animal model were that (a) raising IAV increased pPAW and IAP in an exponential manner, (b) raising IAV decreased CAB and CRS, and (c) there was approximately a 40% transmission of IAP to pPAW.
IAV increased pPAW and IAP in an exponential manner
We aimed to characterize how abdominal volumes influenced airway pressures and IAP. The IAV that produced an IAP >40 cmH2O (30 mmHg) varied between subjects. We therefore explored functions that could describe generic P-V curves of all the animals studied.
Functions have the advantage of allowing extrapolations even in the setting of a non-linear curve if a certain number of pressure-volume (P-V) values are known. We first tested the Venegas equation for the additional IAV—pPAW and IAP relationship frequently used to describe a respiratory P-V curve [12]. The Venegas equation has been used to describe P-V curves other than respiratory [13]. We found that our alternative exponential function characterized the changes in pPAW and IAP with additional IAV more accurately than the Venegas equation. Exponential equations have been used to describe respiratory P-V curves [15].
An exponential relationship between IAV and IAP has been found in a previous animal study [16]. In a recent review article, we extracted all available human IAV and IAP measurements from the current literature [7]. In contrast to previous studies, we analyzed multiple IAV and IAP measurements that included multiple measurements and/or were derived from a larger IAP range (greater than 15 mmHg, the upper limit during laparoscopy). We found an exponential relationship between IAV and IAP. The pseudo-linear relationship between IAV and IAP found in previous studies can be explained by the relatively low IAP range and/or small number of measurements examined [4–6]. To our knowledge, we are the first to report an exponential relationship between IAV and pPAW.
Abdominal compliance
After completion of the first P-V curves at 5 cmH2O PEEP, we performed a second set of P-V curves at 15 cmH2O PEEP levels. The higher CAB at 15 cmH2O of PEEP indicates a left shift of the P-V curves. This was opposite to what we anticipated and suggests that a considerable amount of “pre-stretching” occurred during the initial abdominal inflation rather than being a result of PEEP itself. We therefore only presented data obtained at PEEP of 5 cmH2O. Data from the literature suggests that stretching of the abdominal wall can lead to long-term changes in the elastic properties of the abdominal wall thereby improving abdominal compliance [7]. Unfortunately, we did not perform a third P-V curve at 5 cmH2O PEEP following the P-V curve at 15 cmH2O PEEP to confirm our hypothesis of the occurrence of pre-stretching.
Conceptually, three phases of abdominal pressure-volume behavior exist that occurs to some degree in parallel: the initial reshaping phase (minimal change in IAP despite large IAV change), the subsequent stretching phase, and finally, the pressurization phase (large IAP changes as a result of small IAV changes) [7]. Pre-stretching regularly occurs as an adaptive response to a chronic disease process (e.g., growing ascites or pregnancy), but it has also been shown to occur in the acute setting within a short period of time (e.g., during laparoscopy) [7].
The WSACS (www.wsacs.org) defines abdominal compliance as a measure of the ease of abdominal expansion, determined by the elasticity of the abdominal wall and diaphragm and expressed as a change in IAV per change in IAP (L/mmHg) [1, 17, 18]. Not surprisingly additional IAV decreased CAB and CRS.
Abdomino-thoracic transmission
We found approximately 40% of abdomino-thoracic transmission, whereby pPAW increased due to raising IAP. There is a paucity of literature examining the effect of various IAP on airway pressures. When averaging the results of three porcine studies, we found an approximate 40% abdomino-thoracic transmission [11, 19, 20]. In keeping with this, Cortes-Puentes and colleges found an approximate 50% abdomino-thoracic transmission in pigs [21]. These results should be treated with caution as abdomino-thoracic transmission is likely to be different in critically ill patients. Factors such as obesity, presence of pleural effusions, and lung compliance may well substantially influence abdomino-thoracic transmission [22]. We could only locate one study in human subjects from which abdomino-thoracic transmission can be derived. Torquato et al. placed 5 kg weights on the abdomen of mechanically ventilated, critically ill patients. The average IAP increased from 10.5 to 15.6 cmH2O and plateau airway pressures from 22.4 to 23.6 cmH2O equating to an approximate 20% abdomino-thoracic transmission [23].
Thoraco-abdominal transmission
Thoraco-abdominal transmission can be explored by assessing the effect of either different PEEP levels or different tidal volumes on IAP. We attempted to examine the influence of PEEP on IAP. However, as we did not randomize the levels of PEEP and as described above, we believe that the unexpected findings may be the result of pre-stretching rather than the effect of different levels of PEEP. Therefore, we were unable to examine the influence of PEEP on thoraco-abdominal P-V curves.
Published reports suggest that PEEP has either no or minimal effect on IAP in animals and in humans [24]. We found in an animal experiment that PEEP did not influence IAP [8]. In humans, PEEP appears to increase IAP and the calculated thoraco-abdominal transmission ranges between 0.2 and 0.4 cmH2O increase in IAP for each centimeter of water of PEEP [23, 25, 26]. Other studies have found tidal volume to have a significant impact on IAP [15].
The thoraco-abdominal transmission has been suggested to provide an estimate of the CAB by measuring the influence of different tidal volumes on the changes in IAP [18].
How is this study useful?
It is important for the critical care physician to be aware of the exponential nature of respiratory and abdominal pressure—IAV curve. An exponential pressure-volume relationship is the well-known Monro-Kellie doctrine applied in patients with intra-cranial hypertension [27].
At the lower IAV spectrum (i.e., patients with normal IAP), the respiratory and abdominal pressure—IAV curve is flat. This means that the abdominal cavity can accommodate several liters of additional IAV (i.e., intra- or retro-peritoneal hemorrhage) with little effect on airway or abdominal pressures. In using our pigs of around 40 kg as examples, an additional IAV of 4 L increased IAP by only 0.7 cmH2O (0.5 mmHg) and the effect on airway pressures was negligible. Therefore, an absent rise in IAP does not exclude an intra-abdominal or retroperitoneal hemorrhage in the lower IAV spectrum.
In the high IAV spectrum (i.e., patients with IAH), the respiratory and abdominal pressure—IAV curve is steep. Small changes in IAV can significantly affect airway pressure and IAP. Therefore, it is important to measure IAP regularly in patients at risk of developing IAH. Especially in patients with impending ACS, a small increase in IAV can easily progress to an ACS.
There is a high incidence of IAH in patients with respiratory failure [28]. IAH contributes to morbidity and mortality in patients with acute respiratory distress syndrome (ARDS) [15, 25, 29, 30]. Airway pressures are often high when ventilating patients with ARDS, and it has been suggested that plateau pressure should be limited to 30 cmH2O [31]. These recommendations do not take IAP into account even though IAH has been shown to increase airway pressures in this current study and in previous animal and human studies [8, 32]. At least in patients with ARDS, recent studies suggest it is more important to limit the driving airway pressure than it is to limit plateau airway pressure [33]. Of note is that a sudden rise in airway pressures may reflect an acute increase in IAV and should prompt an abdominal examination to exclude an intra-abdominal pathology.
When aiming to reduce airway pressures and/or IAP in patients with IAH/abdominal compartment syndrome, it is useful to understand that small reductions in IAV can significantly improve airway pressure and IAP. In example, in this study, at grade IV IAH, a 500-mL reduction in IAV reduced pPAW by 4 cmH2O and IAP by 11 cmH2O (8 mmHg). This observation is similar to the applied Monro-Kellie principle where drainage of small amount of cerebral spinal fluid can significantly reduce intra-cranial pressure in patients with intra-cranial hypertension [27].
There are multiple methods of reducing IAV, and the best management depends largely on the etiology of IAH [1]. Apart from diuresis (e.g., furosemide), renal replacement therapy, and surgery (e.g., removal of hematoma or decompressive laparotomy), percutaneous drainage of peritoneal fluid has been shown to be equally effective in reducing IAP [34–36]. In a case series, Reed et al. present 12 patients in which percutaneous drains were inserted in patients with IAH [35]. In the patients with higher pre-drainage IAP of smaller amount fluid removal led to greater decreases in IAP than in patients with smaller pre-drainage IAP.
Limitations
This study has several limitations: (a) These findings have been obtained in an animal model, limiting the transfer of our results into clinical practice. (b) We used a healthy lung model but critically ill patients frequently have injured lungs with reduced lung compliance. (c) This model assessed the effect of increasing IAV but not that of decreasing abdominal wall compliance on pPAW and IAP. Although decreased abdominal wall compliance does occur, increased IAV is the more dominant process in critically ill patients [7]. The abdominal closure may have decreased abdominal wall compliance [7]. (d) In clinical practice IAH arises more often on the basis of excess in intra-abdominal fluid than of an excess in intra-abdominal gas. We used air to increase IAV but corrected the additional IAV to account for the compressibility of gas under pressure using the Boyle’s equation. Although we visually ensured even distribution of the abdominal balloon we cannot exclude potential asymmetrical IAP distribution. (e) We measured mean IAP and not end-expiratory IAP as recommended by the WSACS [1]. (f) We measured dynamic respiratory compliance and peak airway pressure and not plateau airway pressure and static respiratory compliance. We assume that the same principles apply for plateau pressure. In a previous animal experiment [19], we found that plateau pressure paralleled peak airway pressure (data not published). (g) We did not observe any spontaneous diaphragmatic activity. However, we cannot totally rule out diaphragmatic activity potentially influencing our results as we did not use neuromuscular blocking agents. (h) We did not perform a third P-V curve at 5 cmH2O PEEP following the P-V curve at 15 cmH2O PEEP to confirm our hypothesis that pre-stretching had occurred.
Conclusions
In conclusion, in an animal model, we found that raising IAV increased pPAW and IAP in an exponential manner. The exponential nature of IAV on pPAW and IAP suggests that the effect of a given change in IAV on pPAW and IAP will be greater at high than at low levels of IAP. In other words, in subjects with normal IAP, large increases in IAV will not affect airway pressure or IAP. But at high grades of IAH, small reductions in IAV can significantly improve airway and abdominal pressures.
Acknowledgements
The work was performed at the Large Animal Facility of the University of Western Australia, Perth, Australia. Bench work and data analysis were carried out at the Sir Charles Gairdner Hospital.
We thank the Department of Medical Technology and Physics at the Sir Charles Gairdner Hospital as well as the team of the Large Animal Facility of the University of Western Australia and Brigit Roberts from the Intensive Care Unit of the Sir Charles Gardner Hospital for the technical assistance.
Funding
This study was supported by the Sir Charles Gairdner Hospital Research Fund (No 0810) and by local research funds of the Intensive Care Unit at the Sir Charles Gairdner Hospital.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on a reasonable request.
Authors’ contributions
AR, BN, and PVH participated in the conception, hypothesis delineation, and design of the study. AR and LH contributed to data acquisition. AR and BN performed data and statistical analyses. AR drafted the manuscript. BDK, BS, and PVH performed the first revision. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval
The Animal Ethics Committee of the University of Western Australia approved the study protocol (UWA RA/3/100/688). The study conformed to the regulations of the Australian code of practice for the care and use of animals for scientific purposes.
Abbreviations
- CAB
Abdominal compliance
- CRS
Dynamic respiratory system compliance
- IAH
Intra-abdominal hypertension
- IAP
Intra-abdominal pressure
- IAV
Intra-abdominal volume
- PEEP
Positive end-expiratory pressure
- pPAW
Peak airway pressure
- P-V
Pressure-volume
Additional files
Intra-abdominal pressure (IAP) in centimeter of water in function of increasing additional intra-abdominal volume (IAV) in liters. Example of one pig showing measured IAP values (crosses), calculated IAP values using Venegas equation (dotted curve) and exponential equation (dashed curve). Venegas equation: V = a + [b/(1 + e −(P − c)/d)] [12], V represents additional IAV, P represents absolute IAP, and a, b, c, and d represents fitting parameters. Exponential equation: V = v + k × Ln (P − p) where V represents additional IAV, P represents absolute IAP, and v, k, p represents fitting parameters. (TIF 384 kb)
Equation parameters of intra-abdominal and airway pressure-volume curves. (DOCX 55 kb)
Pressure-volume curves showing intra-abdominal pressure in centimeter of water at the initial PEEP level of 5 cmH2O (dashed curve) and the subsequent PEEP level of 15 cmH2O (solid curve) in function of increasing additional intra-abdominal volume in liters. (TIF 445 kb)
Contributor Information
Adrian Regli, Phone: +61 8 6152 2222, Email: adrian.regli@gmail.com, Email: adrian.regli@health.wa.gov.au.
Bart Leon De Keulenaer, Phone: +61 8 6152 2222, Email: bdekeul@hotmail.com.
Bhajan Singh, Phone: +61 8 9346 3333, Email: bhajan.singh@health.wa.gov.au.
Lisen Emma Hockings, Phone: +61 3 9076 2000, Email: lehockings@gmail.com.
Bill Noffsinger, Phone: +61 8 9346 3333, Email: bill.noffsinger@health.wa.gov.au.
Peter Vernon van Heerden, Phone: +972 50 5172864, Email: vernon@hadassah.org.il.
References
- 1.Kirkpatrick AW, Roberts DJ, De Waele J, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malbrain ML, Chiumello D, Pelosi P, et al. Prevalence of intra-abdominal hypertension in critically ill patients: a multicentre epidemiological study. Intensive Care Med. 2004;30:822–829. doi: 10.1007/s00134-004-2169-9. [DOI] [PubMed] [Google Scholar]
- 3.Malbrain ML, Chiumello D, Pelosi P, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: a multiple-center epidemiological study. Crit Care Med. 2005;33:315–322. doi: 10.1097/01.CCM.0000153408.09806.1B. [DOI] [PubMed] [Google Scholar]
- 4.Dejardin A, Robert A, Goffin E. Intraperitoneal pressure in PD patients: relationship to intraperitoneal volume, body size and PD-related complications. Nephrol Dial Transplant. 2007;22:1437–1444. doi: 10.1093/ndt/gfl745. [DOI] [PubMed] [Google Scholar]
- 5.Papavramidis TS, Michalopoulos NA, Mistriotis G, Pliakos IG, Kesisoglou II, Papavramidis ST. Abdominal compliance, linearity between abdominal pressure and ascitic fluid volume. J Emerg Trauma Shock. 2011;4:194–197. doi: 10.4103/0974-2700.82205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischbach M, Terzic J, Gaugler C, et al. Impact of increased intraperitoneal fill volume on tolerance and dialysis effectiveness in children. Adv Perit Dial. 1998;14:258–264. [PubMed] [Google Scholar]
- 7.Blaser AR, Bjorck M, De Keulenaer B, Regli A. Abdominal compliance: a bench-to-bedside review. J Trauma Acute Care Surg. 2015;78:1044–1053. doi: 10.1097/TA.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 8.Regli A, Hockings LE, Musk GC, et al. The Impact of positive end-expiratory pressure at different intra-abdominal pressures on functional residual capacity and oxygen delivery in a pig model. Intensive Care Med. 2009;35:S227. [Google Scholar]
- 9.De Keulenaer B, Regli A, De Laet I, Roberts D, Malbrain ML. What’s new in medical management strategies for raised intra-abdominal pressure: evacuating intra-abdominal contents, improving abdominal wall compliance, pharmacotherapy, and continuous negative extra-abdominal pressure. Anaesthesiol Intensive Ther. 2015;47:54–62. doi: 10.5603/AIT.a2014.0065. [DOI] [PubMed] [Google Scholar]
- 10.Regli A, De Keulenaer B, De Laet I, Roberts D, Dabrowski W, Malbrain ML. Fluid therapy and perfusional considerations during resuscitation in critically ill patients with intra-abdominal hypertension. Anaesthesiol Intensive Ther. 2015;47:45–53. doi: 10.5603/AIT.a2014.0067. [DOI] [PubMed] [Google Scholar]
- 11.Regli A, Hockings LE, Musk GC, et al. Commonly applied positive end-expiratory pressures do not prevent functional residual capacity decline in the setting of intra-abdominal hypertension: a pig model. Crit Care. 2010;14:R128. doi: 10.1186/cc9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venegas JG, Harris RS, Simon BA. A comprehensive equation for the pulmonary pressure-volume curve. J Appl Physiol. 1998;84:389–395. doi: 10.1152/jappl.1998.84.1.389. [DOI] [PubMed] [Google Scholar]
- 13.Williamson JP, McLaughlin RA, Noffsinger WJ, et al. Elastic properties of the central airways in obstructive lung diseases measured using anatomical optical coherence tomography. Am J Respir Crit Care Med. 2011;183:612–619. doi: 10.1164/rccm.201002-0178OC. [DOI] [PubMed] [Google Scholar]
- 14.Colebatch HJ, Ng CK, Nikov N. Use of an exponential function for elastic recoil. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:387–393. doi: 10.1152/jappl.1979.46.2.387. [DOI] [PubMed] [Google Scholar]
- 15.Ranieri VM, Brienza N, Santostasi S, et al. Impairment of lung and chest wall mechanics in patients with acute respiratory distress syndrome: role of abdominal distension. Am J Respir Crit Care Med. 1997;156:1082–1091. doi: 10.1164/ajrccm.156.4.97-01052. [DOI] [PubMed] [Google Scholar]
- 16.Yoshino O, Quail A, Oldmeadow C, Balogh ZJ. The interpretation of intra-abdominal pressures from animal models: the rabbit to human example. Injury. 2012;43:169–173. doi: 10.1016/j.injury.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Malbrain ML, Roberts DJ, De Laet I, et al. The role of abdominal compliance, the neglected parameter in critically ill patients—a consensus review of 16. Part 1: definitions and pathophysiology. Anaesthesiol Intensive Ther. 2014;46:392–405. doi: 10.5603/AIT.2014.0062. [DOI] [PubMed] [Google Scholar]
- 18.Malbrain ML, De Laet I, De Waele JJ, et al. The role of abdominal compliance, the neglected parameter in critically ill patients—a consensus review of 16. Part 2: measurement techniques and management recommendations. Anaesthesiol Intensive Ther. 2014;46:406–432. doi: 10.5603/AIT.2014.0063. [DOI] [PubMed] [Google Scholar]
- 19.Regli A, Chakera J, De Keulenaer BL, et al. Matching positive end-expiratory pressure to intra-abdominal pressure prevents end-expiratory lung volume decline in a pig model of intra-abdominal hypertension. Crit Care Med. 2012;40:1879–1886. doi: 10.1097/CCM.0b013e31824e0e80. [DOI] [PubMed] [Google Scholar]
- 20.Regli A, Mahendran R, Fysh ET, et al. Matching positive end-expiratory pressure to intra-abdominal pressure improves oxygenation in a porcine sick lung model of intra-abdominal hypertension. Crit Care. 2012;16:R208. doi: 10.1186/cc11840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cortes-Puentes GA, Cortes-Puentes LA, Adams AB, Anderson CP, Marini JJ, Dries DJ. Experimental intra-abdominal hypertension influences airway pressure limits for lung protective mechanical ventilation. J Trauma Acute Care Surg. 2013;74:1468–1473. doi: 10.1097/TA.0b013e31829243a7. [DOI] [PubMed] [Google Scholar]
- 22.O’Quin RJ, Marini JJ, Culver BH, Butler J. Transmission of airway pressure to pleural space during lung edema and chest wall restriction. J Appl Physiol (1985) 1985;59:1171–1177. doi: 10.1152/jappl.1985.59.4.1171. [DOI] [PubMed] [Google Scholar]
- 23.Torquato JA, Lucato JJ, Antunes T, Barbas CV. Interaction between intra-abdominal pressure and positive-end expiratory pressure. Clinics (Sao Paulo) 2009;64:105–112. doi: 10.1590/S1807-59322009000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Keulenaer BL, De Waele JJ, Powell B, Malbrain ML. What is normal intra-abdominal pressure and how is it affected by positioning, body mass and positive end-expiratory pressure? Intensive Care Med. 2009;35:969–976. doi: 10.1007/s00134-009-1445-0. [DOI] [PubMed] [Google Scholar]
- 25.Krebs J, Pelosi P, Tsagogiorgas C, Alb M, Luecke T. Effects of positive end-expiratory pressure on respiratory function and hemodynamics in patients with acute respiratory failure with and without intra-abdominal hypertension: a pilot study. Crit Care. 2009;13:R160. doi: 10.1186/cc8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verzilli D, Constantin JM, Sebbane M, et al. Positive end-expiratory pressure affects the value of intra-abdominal pressure in acute lung injury/acute respiratory distress syndrome patients: a pilot study. Crit Care. 2010;14:R137. doi: 10.1186/cc9193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson MH (2016) Monro-Kellie 2.0: The dynamic vascular and venous pathophysiological components of intracranial pressure. J Cereb Blood Flow Metab 36:1338-1350 [DOI] [PMC free article] [PubMed]
- 28.Holodinsky JK, Roberts DJ, Ball CG, et al. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: a systematic review and meta-analysis. Crit Care. 2013;17:R249. doi: 10.1186/cc13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L, Pelosi P, Suter PM, Pedoto A, Vercesi P, Lissoni A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am J Respir Crit Care Med. 1998;158:3–11. doi: 10.1164/ajrccm.158.1.9708031. [DOI] [PubMed] [Google Scholar]
- 30.Rouby JJ, Puybasset L, Nieszkowska A, Lu Q. Acute respiratory distress syndrome: lessons from computed tomography of the whole lung. Crit Care Med. 2003;31:S285–S295. doi: 10.1097/01.CCM.0000057905.74813.BC. [DOI] [PubMed] [Google Scholar]
- 31.Marini JJ, Gattinoni L. Ventilatory management of acute respiratory distress syndrome: a consensus of two. Crit Care Med. 2004;32:250–255. doi: 10.1097/01.CCM.0000104946.66723.A8. [DOI] [PubMed] [Google Scholar]
- 32.Hedenstierna G, Larsson A. Influence of abdominal pressure on respiratory and abdominal organ function. Curr Opin Crit Care. 2012;18:80–85. doi: 10.1097/MCC.0b013e32834e7c3a. [DOI] [PubMed] [Google Scholar]
- 33.Laffey JG, Bellani G, Pham T et al (2016) Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med 42:1865-1876. [DOI] [PubMed]
- 34.Sun ZX, Huang HR, Zhou H. Indwelling catheter and conservative measures in the treatment of abdominal compartment syndrome in fulminant acute pancreatitis. World J Gastroenterol. 2006;12:5068–5070. doi: 10.3748/wjg.v12.i31.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reed SF, Britt RC, Collins J, Weireter L, Cole F, Britt LD. Aggressive surveillance and early catheter-directed therapy in the management of intra-abdominal hypertension. J Trauma. 2006;61:1359–1363. doi: 10.1097/01.ta.0000245975.68317.5a. [DOI] [PubMed] [Google Scholar]
- 36.Cheatham ML, Safcsak K. Percutaneous catheter decompression in the treatment of elevated intraabdominal pressure. Chest. 2011;140:1428–1435. doi: 10.1378/chest.10-2789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on a reasonable request.


