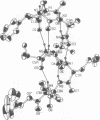
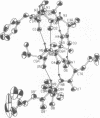
Abstract
The presence of multiple alpha,alpha-dialkyl amino acids such as alpha-methylalanine (alpha-aminoisobutyric acid, Aib) leads to predominantly helical structures, either with alpha-helical or 3(10)-helical hydrogen bonding patterns. The crystal structure of emerimicin-(1-9) benzyl ester (Ac-Phe-Aib-Aib-Aib-Val-Gly-Leu-Aib-Aib-OBzl) reported here shows essentially pure alpha-helical character, whereas other similar compounds show predominantly 3(10)-helical structures. The factors that govern helical preference include the inherent relative stability of the alpha-helix compared with the 3(10)-helix, the extra hydrogen bond seen with 3(10)-helices, and the enhanced electrostatic dipolar interaction of the 3(10)-helix when packed in a crystalline lattice. The balance of these forces, when combined with the steric requirements of the amino acid side chains, determines the relative stability of the two helical conformations under a given set of experimental conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barlow D. J., Thornton J. M. Helix geometry in proteins. J Mol Biol. 1988 Jun 5;201(3):601–619. doi: 10.1016/0022-2836(88)90641-9. [DOI] [PubMed] [Google Scholar]
- Bavoso A., Benedetti E., Di Blasio B., Pavone V., Pedone C., Toniolo C., Bonora G. M., Formaggio F., Crisman M. Long, chiral polypeptide 3(10)-helices at atomic resolution. J Biomol Struct Dyn. 1988 Feb;5(4):803–817. doi: 10.1080/07391102.1988.10506428. [DOI] [PubMed] [Google Scholar]
- Bavoso A., Benedetti E., Di Blasio B., Pavone V., Pedone C., Toniolo C., Bonora G. M. Long polypeptide 3(10)-helices at atomic resolution. Proc Natl Acad Sci U S A. 1986 Apr;83(7):1988–1992. doi: 10.1073/pnas.83.7.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess A. W., Leach S. J. An obligatory alpha-helical amino acid residue. Biopolymers. 1973 Nov;12(11):2599–2605. doi: 10.1002/bip.1973.360121112. [DOI] [PubMed] [Google Scholar]
- Esposito G., Carver J. A., Boyd J., Campbell I. D. High-resolution 1H NMR study of the solution structure of alamethicin. Biochemistry. 1987 Feb 24;26(4):1043–1050. doi: 10.1021/bi00378a010. [DOI] [PubMed] [Google Scholar]
- Fox R. O., Jr, Richards F. M. A voltage-gated ion channel model inferred from the crystal structure of alamethicin at 1.5-A resolution. Nature. 1982 Nov 25;300(5890):325–330. doi: 10.1038/300325a0. [DOI] [PubMed] [Google Scholar]
- Furois-Corbin S., Pullman A. Theoretical study of the packing of alpha-helices into possible transmembrane bundles. Sequences including alanines, leucines and serines. Biochim Biophys Acta. 1987 Aug 7;902(1):31–45. doi: 10.1016/0005-2736(87)90133-7. [DOI] [PubMed] [Google Scholar]
- Hall J. E., Vodyanoy I., Balasubramanian T. M., Marshall G. R. Alamethicin. A rich model for channel behavior. Biophys J. 1984 Jan;45(1):233–247. doi: 10.1016/S0006-3495(84)84151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima H., Dunbar J. B., Jr, Marshall G. R. Calibration of effective van der Waals atomic contact radii for proteins and peptides. Proteins. 1987;2(4):330–339. doi: 10.1002/prot.340020408. [DOI] [PubMed] [Google Scholar]
- Karle I. L. Conformational characteristics of peptides and unanticipated results from crystal structure analyses. Biopolymers. 1989 Jan;28(1):1–14. doi: 10.1002/bip.360280104. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J. L., Sukumar M., Balaram P. Monoclinic polymorph of Boc-Trp-Ile-Ala-Aib-Ile-Val-Aib-Leu-Aib-Pro-OMe(anhydrous). Parallel packing of 3(10)-/alpha-helices and a transition of helix type. Int J Pept Protein Res. 1988 Jun;31(6):567–576. doi: 10.1111/j.1399-3011.1988.tb00915.x. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J. L., Uma K., Balaram H., Balaram P. Alpha-helix and mixed 3(10)/alpha-helix in cocrystallized conformers of Boc-Aib-Val-Aib-Aib-Val-Val-Val-Aib-Val-Aib-OMe. Proc Natl Acad Sci U S A. 1989 Feb;86(3):765–769. doi: 10.1073/pnas.86.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J. L., Uma K., Balaram P. Aggregation studies in crystals of apolar helical peptides: Boc-Aib-Val-Ala-Leu-Aib-Val-Ala-Leu-Aib-OMe. Int J Pept Protein Res. 1988 Dec;32(6):536–543. doi: 10.1111/j.1399-3011.1988.tb01385.x. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J., Sukumar M., Balaram P. Conformation of a 16-residue zervamicin IIA analog peptide containing three different structural features: 3(10)-helix, alpha-helix, and beta-bend ribbon. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5087–5091. doi: 10.1073/pnas.84.15.5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J., Uma K., Balaram P. Aqueous channels within apolar peptide aggregates: solvated helix of the alpha-aminoisobutyric acid (Aib)-containing peptide Boc-(Aib-Ala-Leu)3-Aib-OMe.2H2O.CH3OH in crystals. Proc Natl Acad Sci U S A. 1988 Jan;85(2):299–303. doi: 10.1073/pnas.85.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karle I. L., Sukumar M., Balaram P. Parallel packing of alpha-helices in crystals of the zervamicin IIA analog Boc-Trp-Ile-Ala-Aib-Ile-Val-Aib-Leu-Aib-Pro-OMe.2H2O. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9284–9288. doi: 10.1073/pnas.83.24.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm B. R. A 310-helix in poly(alpha-aminoisobutyric acid) Biopolymers. 1977 Nov;16(11):2591–2592. doi: 10.1002/bip.1977.360161122. [DOI] [PubMed] [Google Scholar]
- Marshall G. R., Bosshard H. E. Angiotensin II. Studies on the biologically active conformation. Circ Res. 1972 Sep;31(9 Suppl):143–150. [PubMed] [Google Scholar]
- Marshall G. R., Clark J. D., Dunbar J. B., Jr, Smith G. D., Zabrocki J., Redlinski A. S., Leplawy M. T. Conformational effects of chiral alpha,alpha-dialkyl amino acids. I. C-terminal tetrapeptides of emerimicin containing alpha-ethylalanine. Int J Pept Protein Res. 1988 Dec;32(6):544–555. doi: 10.1111/j.1399-3011.1988.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Menestrina G., Voges K. P., Jung G., Boheim G. Voltage-dependent channel formation by rods of helical polypeptides. J Membr Biol. 1986;93(2):111–132. doi: 10.1007/BF01870804. [DOI] [PubMed] [Google Scholar]
- Pandey R. C., Cook J. C., Jr, Rinehart K. L., Jr Structures of the peptide antibiotics. Emerimicins III and IV 1,2. J Am Chem Soc. 1977 Jul 20;99(15):5205–5206. doi: 10.1021/ja00457a064. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Balaram P. The stereochemistry of peptides containing alpha-aminoisobutyric acid. CRC Crit Rev Biochem. 1984;16(4):307–348. doi: 10.3109/10409238409108718. [DOI] [PubMed] [Google Scholar]
- Presta L. G., Rose G. D. Helix signals in proteins. Science. 1988 Jun 17;240(4859):1632–1641. doi: 10.1126/science.2837824. [DOI] [PubMed] [Google Scholar]
- Presta L. G., Rose G. D. Helix signals in proteins. Science. 1988 Jun 17;240(4859):1632–1641. doi: 10.1126/science.2837824. [DOI] [PubMed] [Google Scholar]
- Toniolo C., Bonora G. M., Bavoso A., Benedetti E., di Blasio B., Pavone V., Pedone C. Molecular structure of peptaibol antibiotics: solution conformation and crystal structure of the octapeptide corresponding to the 2-9 sequence of emerimicins III and IV. J Biomol Struct Dyn. 1985 Dec;3(3):585–598. doi: 10.1080/07391102.1985.10508446. [DOI] [PubMed] [Google Scholar]
- Tosteson M. T., Auld D. S., Tosteson D. C. Voltage-gated channels formed in lipid bilayers by a positively charged segment of the Na-channel polypeptide. Proc Natl Acad Sci U S A. 1989 Jan;86(2):707–710. doi: 10.1073/pnas.86.2.707. [DOI] [PMC free article] [PubMed] [Google Scholar]