Abstract
Background
Physicians are increasingly using point of care lung ultrasound (LUS) for diagnosing pneumonia, especially in critical situations as it represents relatively easy and immediately available tool. They also used it in many associated pathological conditions such as consolidation, pleural effusion, and interstitial syndrome with some reports of more accuracy than chest X-ray. This systematic review and meta-analysis are aimed to estimate the pooled diagnostic accuracy of ultrasound for the diagnosis of pneumonia versus the standard chest radiological imaging.
Methods and main results
A systematic literature search was conducted for all published studies comparing the diagnostic accuracy of LUS against a reference Chest radiological exam (C X-ray or Chest computed Tomography CT scan), combined with clinical criteria for pneumonia in all age groups. Eligible studies were required to have a Chest X-ray and/or CT scan at the time of clinical evaluation. The authors extracted qualitative and quantitative information from eligible studies, and calculated pooled sensitivity and specificity and pooled positive/negative likelihood ratios (LR). Twenty studies containing 2513 subjects were included in this meta-analysis. The pooled estimates for lung ultrasound in the diagnosis of pneumonia were, respectively, as follows: Overall pooled sensitivity and specificity for diagnosis of pneumonia by lung ultrasound were 0.85 (0.84–0.87) and 0.93 (0.92–0.95), respectively. Overall pooled positive and negative LRs were 11.05 (3.76–32.50) and 0.08 (0.04–0.15), pooled diagnostic Odds ratio was 173.64 (38.79–777.35), and area under the pooled ROC (AUC for SROC) was 0.978.
Conclusion
Point of care lung ultrasound is an accurate tool for the diagnosis of pneumonia. Considering being easy, readily availability, low cost, and free from radiological hazards, it can be considered as important diagnostic strategy in this condition.
Keywords: Systematic review, Ultrasound; Pneumonia; Point of care, lung, interstitial syndrome, and diagnosis
Background
Acute pneumonia or acute respiratory tract infection is considered the most common cause of mortality in children around the globe [1]. In adult, pneumonia also is a serious disease with increased rate of mortality and hospitalization [2, 3]. The diagnosis of pneumonia can be difficult and challenging in the emergency setting or in critically ill patients [4]. Many of the commonly used radiological signs are non-specific [5]. In daily practice, pneumonia diagnosis is based on clinical presentation through patient history and physical exam, plus radiological imaging commonly chest X-ray (and infrequently CT scan) that may help confirm the diagnosis particularly with equivocal clinical status. Early diagnosing of pneumonia is very important to promptly starting the treatment; otherwise, it can be life-threatening or associated with high morbidity particularly in critically ill patients who need immediate decision.
There are many diagnostic approaches to diagnose and evaluate pneumonia and every tool has its own diagnostic accuracy.
Flexible bronchoscopy or endotracheal aspiration usually is reserved for intubated patients. Blood samples, urinary antigens, and expectorate collections are among routine examinations that are performed once pneumonia is suspected. Collected specimens are sent to microbiology laboratories [6] which may take several days to have conclusive results. Bronchoscope can give useful information; however, it has its own limitations and contraindications such as patients with severe hypoxemia, recent myocardial infarctions, or significant cardiac arrhythmia. Being relatively invasive technique, it is also not possible to perform bronchoscope in all patients but only in selected cases [7].
Another diagnostic tool is computed tomography, which is considered as the gold standard in lung imaging in general. This tool is particularly useful in lung masses or cavitary abnormality and any changes in lung parenchyma either acute or chronic such as the cases of pneumonia, interstitial lung disease, emphysema, and malignancy. The limitations are several but most important are radiation hazards, cost, and logistics that limit its routine use. A major limitation is difficulty in transporting patients with critical conditions to imaging section which precludes markedly unstable patients either respiratory or hemodynamically [8, 9].
Nevertheless, chest radiography remains an important imaging tool that been used for long and still helping in diagnosing many abnormalities in the chest. Chest X-ray is considered as the most common diagnostic tool that has been used traditionally in daily practice for diagnosis of pneumonia, especially in critical conditions [10]. Many limitations in using portable chest X-ray have been well described and noticed such as quality of an X-ray film in addition to the risk of repetitive radiation exposure [11]. Some reports claim that removal of chest radiography from daily practice may not affect intensive care unit mortality [12].
Relatively recently, lung ultrasound was promoted as a modality that can overcome many of the above-mentioned limitations of other tools in the diagnosis of pneumonia in multiple settings [13]. Through the last 2 decade, the ultrasound has shown that it could play a major role in medicine and common practice in assessing the lung [14]. Traditionally, the accessibility of the lung by ultrasound was considered poor due to the air barrier. However, this position has been dramatically changed with tremendous amount of literature supporting the use of LUS in multiple conditions [15–17]. This diagnostic tool can be used easily and immediately as a bedside tool which give it a huge advantage [18]. Lung ultrasound was reported with high accuracy in many pathological lung conditions such as consolidation, pleural effusion, and interstitial syndrome compared to bedside chest radiography [19].
The aim of our study is to conduct systematic review (SR) followed by meta-analysis for the diagnostic power of lung ultrasound versus chest radiological imaging for the diagnosis of pneumonia in both adult and pediatric population through estimation of the pooled diagnostic accuracy measures.
Methods
A systematic search of electronic databases was conducted, including MEDLINE, EMBASE, and Cochrane databases from 1990 to 2016 to identify the relevant articles in the effectiveness of ultrasound in the diagnosis of pneumonia. Hand search was then conducted on references of relevant studies. The search strategy followed Cochrane guidelines with using the terms “Ultrasonography, ultrasound, sonography, ultrasonographies, sonogram”; “pneumonia, Bronchopneumonia, Pleuropneumonia, severe Acute Respiratory Syndrome, pulmonary inflammation, bronchiolitis”; and “sensitivity or specificity” with its MeSH terms. No restriction for language or type of patients was made at the time of the search. We included in this systematic review all studies evaluating diagnostic accuracy of lung ultrasound as index test against chest radiological imaging (CXR or CT) as reference standard. We included in this SR patients with respiratory disease and symptoms of acute respiratory failure. The evaluation of pneumonia is a combination of clinical data, laboratory results, and chest imaging. In addition, articles that evaluated any sign of respiratory disease, symptoms, or acute respiratory failure were included. We included all types of patients’ pneumonia—both community- and hospital-acquired pneumonia—, children, adolescents, or adults. We have chosen to combine both adults and pediatric based on current literature suggesting that ultrasound findings in both are similar [17].
Two authors (SZ and WM) screened titles and abstracts for valid articles. Full-text articles were retrieved afterward. We developed an abstraction tables that includes year of publication, patients’ baseline characteristics, and diagnostic study data (numbers of true positive, false positive, false negative, and true negative test results). Disagreement in study selection and abstraction was resolved by discussion with the third reviewer (ME).
Two reviewers (ME and SZ) independently used the QUADAS-2 instrument to assess the quality assessment of the included studies [20]. This tool consists of key domains covering patient selection, index test, reference standard, flow of patients through the study, and timing of the index test(s) and reference standard. Each domain was assessed in terms of the risk of bias and the concerns regarding applicability.
Risk of bias was judged as “Low,” “High,” or “Unclear.” If all signaling questions for a domain are answered “Yes,” then risk of bias can be judged “Low.” If any signaling question is answered “No,” this flags the potential for bias.
The meta-analysis was conducted using Meta-Disc 1.4 [21]. Random effect model was used in all analyses. The diagnostic accuracy measures used in the analysis were sensitivity, specificity, and likelihood ratio for positive and negative test (LR+ and LR−). Heterogeneity was assessed using the I-squared statistic and Q test.
Results
We identified (431) studies that were relevant and fit our search strategy. After reviewing the articles and applying inclusion criteria and exclusion commentaries, we identified and enrolled 20 studies (see Fig. 1 flowchart). These 20 studies provided population of 2513 patients. The main reasons for exclusions were duplication of studies between the Pubmed and the Embase Databases and studies were not diagnostic.
Fig. 1.
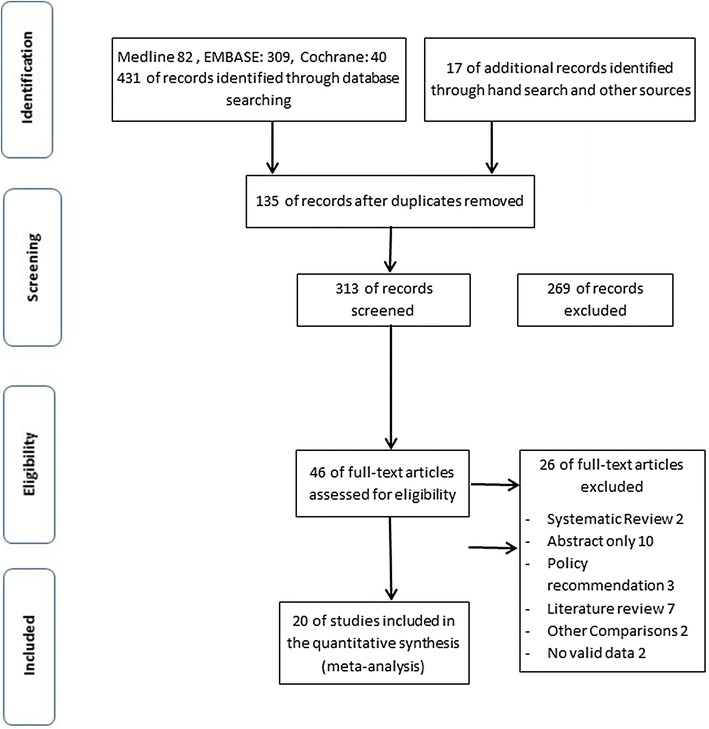
Flow chart for literature search process
Table 1 describes the basic characteristics of the 20 included studies. Among the included 20 studies, five of them are dealing with pediatrics patients [22–26]. Age of patients ranges from 1 month to 100 years. Some studies had comprehensive result of CT, clinical course, conventional tests, and follow-up outcomes as a diagnostic standard, which was considered clinical diagnosis. The quality of all studies was generally high, had low risk of bias, and satisfied the majority of the risk of bias criteria. Table 2 includes the chest imaging (reference standard) and other diagnostic criteria.
Table 1.
Characteristics of studies and patients enrolled from studies retrieved for meta-analysis
| Study | Year | Origin | Design | Sample size | Mean age (years) | M/F | True positive | False positive | False negative | True negative |
|---|---|---|---|---|---|---|---|---|---|---|
| Benci et al. [27] | 1996 | Italy | Prospective | 75 | 38.5 | 50/30 | 37 | 0 | 0 | 20 |
| Lichtenstein et al. [19] | 2004 | France | Prospective | 32 | 58 | Not mentioned | 111 | 0 | 8 | 265 |
| Lichtenstein et al. [28] | 2004 | France | Prospective | 117 | 53 | 37/23 | 59 | 1 | 6 | 51 |
| Lichtenstein et al. [29] | 2008 | France | Prospective | 260 | 68 | 140/120 | 74 | 10 | 9 | 167 |
| Parlamento et al. [30] | 2009 | Italy | Prospective | 49 | 60.9 | 31/81 | 31 | 0 | 1 | 17 |
| Cortellaro et al. [31] | 2010 | Italy | Prospective | 120 | 69 | 77/43 | 80 | 2 | 1 | 37 |
| Xirouchaki et al. [32] | 2011 | Greece | Prospective | 42 | 57.1 | 34/8 | 66 | 4 | 0 | 14 |
| Reissig et al. [33] | 2012 | Europe | Prospective | 356 | 63.8 | 228/134 | 211 | 3 | 15 | 127 |
| Testa et al. [34] | 2012 | Italy | Prospective | 67 | 55 | Not mentioned | 32 | 5 | 2 | 28 |
| Unluer et al. [24] | 2013 | China | Prospective | 72 | 66.3 | 35/37 | 27 | 7 | 1 | 37 |
| Luri [23] | 2009 | Italy | Prospective | 32 | 4.5 | 60 | 20 | 2 | 8 | 2 |
| Shah [35] | 2012 | US | Prospective | 200 | 3 | 112/88 | 31 | 18 | 146 | 5 |
| Hadeel and Dien [25] | 2013 | Egypt | Prospective | 75 | Neonates | Not mentioned | 64 | 4 | 7 | 0 |
| Copetti [36] | 2008 | Italy | Prospective | 144 | 77.6 | 72/72 | 60 | 0 | 0 | 19 |
| Caiulo VA [26] | 2013 | Italy | Prospective | 88 | 5.1 | 56/47 | 88 | 0 | 1 | 13 |
| Nafae [37] | 2013 | Egypt | Prospective | 100 | 50.6 | 56/44 | 61 | 1 | 1 | 17 |
| Liu [38] | 2014 | China | Prospective | 179 | 72 | 124/99 | 80 | 0 | 5 | 27 |
| Esposito [39] | 2014 | Italy | Prospective | 103 | 5.6 | 56/47 | 52 | 3 | 1 | 47 |
| Nazerian [40] | 2015 | Italy | Prospective | 285 | 71.14 | 133/152 | 72 | 9 | 15 | 189 |
| Bourcier [41] | 2014 | France | Prospective | 144 | 77.6 | 72/72 | 117 | 9 | 6 | 12 |
Table 2.
Chest imaging and diagnostic criteria of selected studies
| Study | Imaging | Pneumonia diagnosis | Patient type | Inclusion criteria | Ultrasound operator | Diagnostic criteria | Blinding |
|---|---|---|---|---|---|---|---|
| Benci et al. [27] | CXR + Chest CT if CXR/LUS discordance | Clinical diagnosis or imaging | Hospitalized | Pneumonia symptoms | Experienced physicians | Consolidation | Yes |
| Lichtenstein et al. [19] | Chest CT | Imaging only | Critically ill | Acute respiratory distress syndrome | Experienced physicians | Consolidation | Yes |
| Lichtenstein et al. [28] | Chest CT | Imaging only | Critically ill | Chest pain or severe thoracic diseases | Two ED physician sonographers | Consolidation | Yes |
| Lichtenstein et al. [29] | CXR + Chest CT if possible | Clinical diagnosis or imaging | Critically ill | Acute respiratory failure | Experienced physicians | Alveolar and interstitial | Yes |
| Parlamento et al. [30] | CXR + Chest CT if CXR/LUS discordance | Imaging only | Presented to ED | CAP symptoms | Experienced physicians | Alveolar and interstitial | Yes |
| Cortellaro et al. [31] | CXR + Chest CT if possible | Clinical diagnosis or imaging | Presented to ED | CAP symptoms | Experienced physicians | Alveolar and interstitial | Yes |
| Xirouchaki et al. [32] | Chest CT scan | Imaging only | Critically ill | Mechanically ventilated patients scheduled for chest CT scan | Single physician (Expertise not mentioned) | Consolidation | Yes |
| Reissig et al. [33] | CXR + chest CT if CXR/LUS discordance | Clinical diagnosis or imaging | Presented to ED or hospitalized | CAP symptoms | Experienced physicians | Consolidation | Yes |
| Testa et al. [34] | CXR + chest CT if possible/indicated | Clinical diagnosis or imaging | Presented to ED | Suspected H1N1 infection | Experienced physicians | Alveolar and interstitial | Yes |
| Unluer et al. [24] | CXR + chest CT if possible/indicated | Imaging only | Presented to ED | CAP symptoms | Trained emergency physicians | Alveolar and interstitial | Yes |
| Nafae et al. [37] | Chest CT scan | Imaging only | Hospitalized | Pneumonia symptoms | Experienced physicians | Consolidation | No |
| Esposito et al. [39] | CR | Imaging only | Critically ill | CAP symptoms | Resident with limited experience | Alveolar and interstitial | Yes |
| Liu et al. [38] | CT scan | Imaging only | Presented to ED | CAP symptoms | Trained emergency physicians | Consolidation | Yes |
| Copetti et al. [36] | Electrocardiogram, Chest X-ray, and Color-Doppler echocardiography. | Imaging only | Critically ill | acute pulmonary edema | NA | Alveolar and interstitial | NA |
| Iuri [23] | Chest radiographs | Imaging only | admitted to the pediatric emergency ward | CAP symptoms | Two radiologists | Alveolar and interstitial | Yes |
| Shah [35] | Chest radiographs | Imaging only | patients had a routine clinical examination | Pneumonia symptoms | Trained physicians | Consolidation | Yes |
| Dien [25] | Chest radiographs | Imaging only | Critically ill | Pneumonia symptoms | One radiologist | Consolidation | NA |
| Caiulo [26] | Chest radiographs | Clinical diagnosis or imaging | Presented to ED | Pneumonia symptoms | One radiologist | Alveolar and interstitial | Yes |
| Nazerian [40] | Chest CT scan | Clinical diagnosis or imaging | Presented to ED | Any respiratory complaint | Trained emergency physicians | Consolidation | Yes |
| Bourcier [41] | Chest CT scan | Clinical diagnosis or imaging | Presented to ED | CAP pneumonia | Trained emergency physicians | Alveolar-interstitial syndrome | NA |
Overall pooled sensitivity and specificity for diagnosis of pneumonia by lung ultrasound were 0.85 (0.84–0.87) and 0.93 (0.92–0.95), respectively (Figs. 2, 3). Overall pooled positive and negative LRs (Fig. 4) were 11.05 (3.76–32.50) and 0.08 (0.04–0.15), pooled diagnostic Odds ratio (Fig. 5) was 173.64 (38.79–777.35), and area under the pooled ROC (AUC for SROC) was 0.978 (Fig. 6).
Fig. 2.
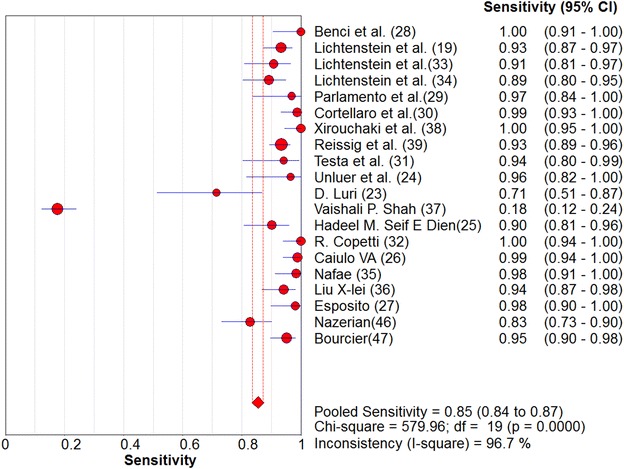
Pooled sensitivity of Ultrasound in ruling out pneumonia
Fig. 3.
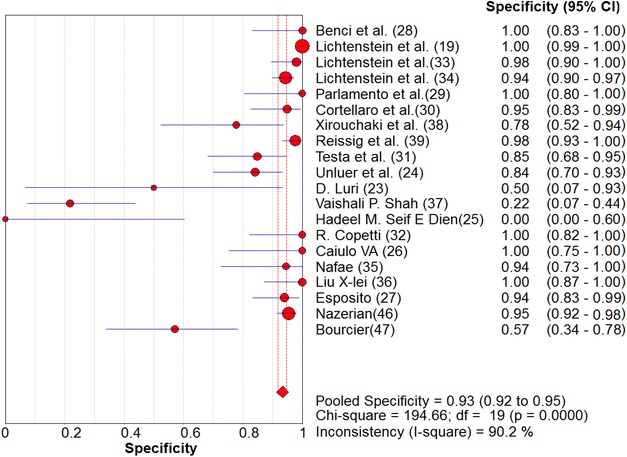
Pooled specificity of Ultrasound in ruling out pneumonia
Fig. 4.
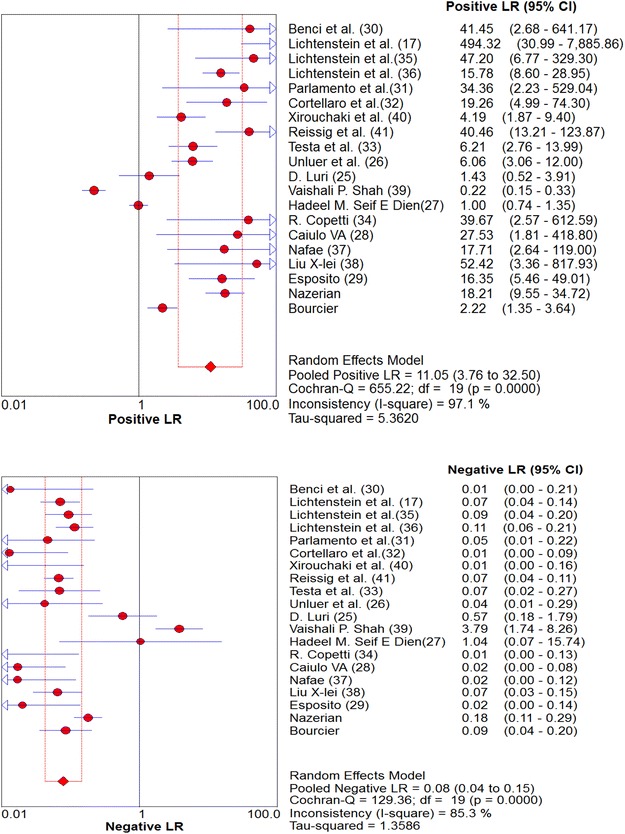
Pooled likelihood ratios of Ultrasound in diagnosing pneumonia
Fig. 5.
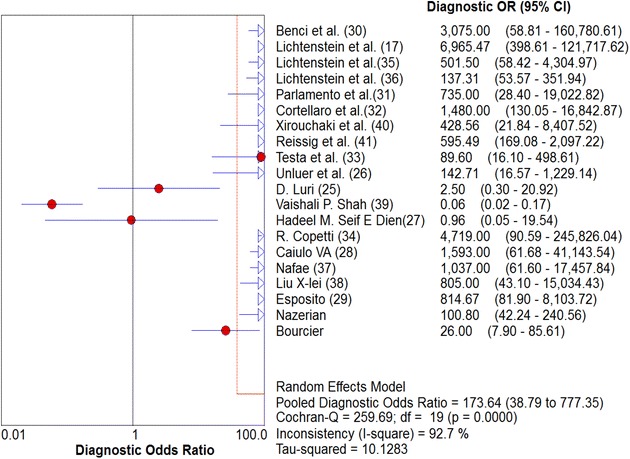
Pooled diagnostic Odds Ratio of Ultrasound in diagnosing pneumonia
Fig. 6.
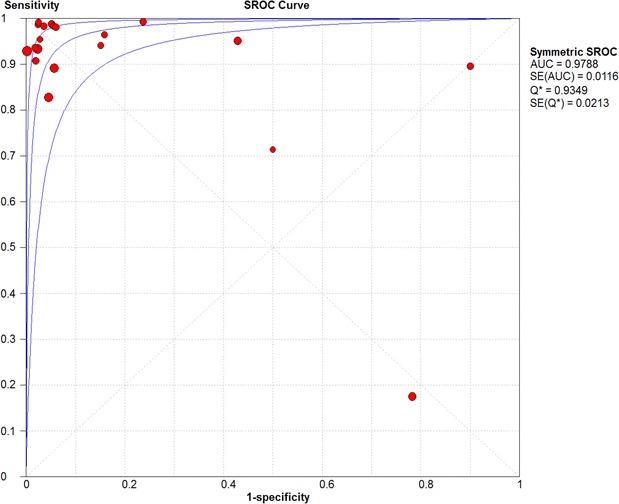
Pooled receiver operator characteristic curve of ultrasound in diagnosing pneumonia
Discussion
Pneumonia commonly leads to significant pulmonary consolidation that is demonstrated with a complete loss of aeration in the concerned lung region. On CXR, pulmonary consolidation is defined as a homogeneous opacity that may have effacement of blood vessel shadows and the presence of air bronchograms.
In lung ultrasound, the normal lung displays the “lung sliding” and A-lines. Lung sliding indicates sliding of the visceral pleura against the parietal pleura and A-lines are repetitive horizontal reverberation artifacts parallel to the pleural line generated by normally present subpleural air in the alveoli.
On ultrasound examination, consolidation is defined as tissue-like pattern reminiscent of the liver, sometimes called “hepatization,” with boundaries that may be formed from the pleural line or a pleural effusion if present and the aerated lung, potentially forming an irregular scattered line if the consolidation is limited (shred sign) or a regular line if the whole lobe is involved. The LUS is logically capable in detecting superficial pneumonia, but it remains, however, doubtful in detecting deep alveolar lesions [39]. Consolidation is defined as an isoechoic tissue-like structure, which is caused by the loss of lung aeration. [4, 27] Power Doppler sometimes is used in order to differentiate tissue-like structures (e.g., echoic pleural effusion) from consolidation. The shred sign is specific for consolidation. B-lines are well-defined hyperechoic comet-tail artifacts, arising from pleural line and spreading vertically indefinitely, erasing A-lines and moving with the lung sliding when lung sliding is present. It indicates partial loss of lung aeration. Lung ultrasound using Doppler or contrast-enhanced sonography visualizes regional pulmonary blood flow within lung consolidations, thereby providing critical information about the etiology of the disease [27]. CXR does not provide any information about regional vascularization. The ultrasound detection of a dynamic air bronchogram is reported to be useful for differentiating obstructive atelectasis from pneumonia [27]. Several studies have demonstrated the superiority of lung ultrasound over CXR for diagnosing lung consolidation, particularly when portable CXR technique is used [30]. Therefore, the use of lung ultrasound can significantly reduce the number of chest radiographs and CT scans and decreases patients’ radiation exposure. It is easily repeatable at the bedside and provides more accurate diagnostic information than CXR in critically ill and emergency patients with lung consolidation.
In this study, we did a systematic review and meta-analysis for the diagnostic accuracy of radiological exam (CXR/CT) and lung ultrasound in relation to diagnosis of pneumonia. In comparison with previous systematic review published addressing this issue [4, 42], our study included more primary studies and subjects compared to previously published systematic reviews.
In our study, we found that lung ultrasound had a high LR, sensitivity, and specificity for the diagnosis of pneumonia. That represents a strong diagnostic accuracy measure with high precision as expressed by the relatively narrow 95% CI. It is important to emphasize that this high diagnostic accuracy can be operator-dependent [34]. The lung scan should be performed by well-trained operators in at least 6 zones to be able to achieve such high diagnostic accuracy [36]. However, in relation to CXR, previous 2 meta-analyses agrees about the superiority of ultrasound over portable CXR [4, 42].
This study emphasizes the role of lung ultrasound as an accurate technique for diagnosing pneumonia compared to chest radiological imaging. This comes in agreement with the multiple reports published for LUS use in multiple settings and new indication [43–47]. In addition, it can help in reducing the movement of patients to the radiology department for CT particularly in unstable mechanical ventilated patient.
Limitation
Moderate-to-high degree of inconsistency/heterogeneity was observed which puts some caution for the interpretation of this study. The reason of heterogeneity can be due to differences in the population or in the reference standard (CXR and CT scan).
The study did not aim to investigate clinical end-point to prove/disprove LUS as a useful diagnostic strategy. That requires another SR of preferably RCT to elicit potential benefits of using the strategy of ultrasound diagnosis over radiological diagnosis. It will require examining several clinical outcomes such as earlier start of treatment, more effective management, reducing costs, reducing need for endoscope, and reducing complication such as cross-infection. These clinical endpoints were not addressed, as the focus was to establish pooled diagnostic accuracy rather than estimating effectiveness between comparative diagnostic strategies. However, our study managed to estimate high pooled diagnostic accuracy of this tool, which may justify its use.
In addition, we did not do comparison between LUS and chest X-ray in the general population (adults and children). That will require individual patient data (IPD) which are not available in the published studies. However, IPD meta-analysis has a robust methodology and peculiar characteristics that can be considered in this topic as potential future research.
Conclusion
Lung ultrasound can play a major and valuable role in the diagnosis of pneumonia with high diagnostic accuracy. Moreover, it can be an alternative to chest X-ray and thoracic CT in several conditions. LUS can be used at the bedside easily, safely, and repetitively. Using LUS in Emergency department, ICUs, and medical wards after adequate training can be considered as a disruptive technology in this field.
Authors' contributions
ME: study protocol, methods, discussion, and overview of completion of the manuscript. MAAS: contribution in introduction, results, and discussion. WHAM: method and result. SAA: introduction, statistical analysis. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
No ethical approval was needed for a systematic review.
Abbreviations
- LUS
lung ultrasound
- CT
computerized axial tomography
- X-Ray
X-radiation
- LR
likelihood ratio
- DF
degree of freedom
- MeSH
medical subheadings
- CXR
chest computerized axial tomography scan
- QUADAS
quality assessment of primary diagnostic accuracy studies
Contributor Information
Saeed Ali Alzahrani, Email: drss2020sss@gmail.com.
Majid Abdulatief Al-Salamah, Email: malsalamah@gmail.com.
Wedad Hussain Al-Madani, Email: Madaniw@ksau-hs.edu.sa.
Mahmoud A. Elbarbary, Email: barbarym@ngha.med.sa, Email: elbarbary.mahmoud@yahoo.com
References
- 1.Peden M (2008) World report on child injury prevention: World Health Organization [PubMed]
- 2.Almirall J, Bolibar I, Vidal J, Sauca G, Coll P, Niklasson B, et al. Epidemiology of community-acquired pneumonia in adults: a population-based study. Eur Respir J. 2000;15(4):757–763. doi: 10.1034/j.1399-3003.2000.15d21.x. [DOI] [PubMed] [Google Scholar]
- 3.Meehan TP, Fine MJ, Krumholz HM, Scinto JD, Galusha DH, Mockalis JT, et al. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA. 1997;278(23):2080–2084. doi: 10.1001/jama.1997.03550230056037. [DOI] [PubMed] [Google Scholar]
- 4.Chavez MA, Shams N, Ellington LE, Naithani N, Gilman RH, Steinhoff MC, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15(50):1465–9921. doi: 10.1186/1465-9921-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayaud C. Pneumonia is the leading cause of death of infectious origin. La Revue du praticien. 2011;61(8):1061. [PubMed] [Google Scholar]
- 6.Van der Eerden M, Vlaspolder F, De Graaff C, Groot T, Jansen H, Boersma W. Value of intensive diagnostic microbiological investigation in low-and high-risk patients with community-acquired pneumonia. Eur J Clin Microbiol Infect Dis. 2005;24(4):241–249. doi: 10.1007/s10096-005-1316-8. [DOI] [PubMed] [Google Scholar]
- 7.Wahidi MM, Rocha AT, Hollingsworth JW, Govert JA, Feller-Kopman D, Ernst A. Contraindications and safety of transbronchial lung biopsy via flexible bronchoscopy. Respiration. 2005;72(3):285–295. doi: 10.1159/000085370. [DOI] [PubMed] [Google Scholar]
- 8.Henschke CI, Yankelevitz DF, Wand A, Davis SD, Shiau M. Accuracy and efficacy of chest radiography in the intensive care unit. Radiol Clini North Am. 1996;34(1):21–31. [PubMed] [Google Scholar]
- 9.Lichtenstein D, Peyrouset O. Is lung ultrasound superior to CT? The example of a CT occult necrotizing pneumonia. Intensive Care Med. 2006;32(2):334–335. doi: 10.1007/s00134-005-0004-6. [DOI] [PubMed] [Google Scholar]
- 10.Rubinowitz AN, Siegel MD, Tocino I. Thoracic imaging in the ICU. Crit Care Clin. 2007;23(3):539–573. doi: 10.1016/j.ccc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Yu C-J, Yang P-C, Chang D, Luh K. Diagnostic and therapeutic use of chest sonography: value in critically ill patients. AJR Am J Roentgenol. 1992;159(4):695–701. doi: 10.2214/ajr.159.4.1529829. [DOI] [PubMed] [Google Scholar]
- 12.Oba Y, Zaza T. Abandoning daily routine chest radiography in the intensive care unit: meta-analysis 1. Radiology. 2010;255(2):386–395. doi: 10.1148/radiol.10090946. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein DA. Ultrasound examination of the lungs in the intensive care unit. Pediatric Crit Care Medi. 2009;10(6):693–698. doi: 10.1097/PCC.0b013e3181b7f637. [DOI] [PubMed] [Google Scholar]
- 14.Beckh S, Bolcskei PL, Lessnau K-D. Real-time chest ultrasonographya comprehensive review for the pulmonologist. CHEST J. 2002;122(5):1759–1773. doi: 10.1378/chest.122.5.1759. [DOI] [PubMed] [Google Scholar]
- 15.Gryminski J, Krakówka P, Lypacewicz G. The diagnosis of pleural effusion by ultrasonic and radiologic techniques. CHEST J. 1976;70(1):33–37. doi: 10.1378/chest.70.1.33. [DOI] [PubMed] [Google Scholar]
- 16.Reißig A, Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease the role of comet tail artifacts. J Ultrasound Med. 2003;22(2):173–180. doi: 10.7863/jum.2003.22.2.173. [DOI] [PubMed] [Google Scholar]
- 17.Volpicelli G, Elbarbary M, Blaivas M, Lichtenstein DA, Mathis G, Kirkpatrick AW, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012;38(4):577–591. doi: 10.1007/s00134-012-2513-4. [DOI] [PubMed] [Google Scholar]
- 18.Koenig SJ, Narasimhan M, Mayo PH. Thoracic ultrasonography for the pulmonary specialist thoracic ultrasonography. CHEST J. 2011;140(5):1332–1341. doi: 10.1378/chest.11-0348. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby J-J. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100(1):9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 21.Zamora J, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol. 2005;58(9):882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Iuri D, De Candia A, Bazzocchi M. Evaluation of the lung in children with suspected pneumonia: usefulness of ultrasonography. Radiol Med (Torino) 2009;114(2):321–330. doi: 10.1007/s11547-008-0336-8. [DOI] [PubMed] [Google Scholar]
- 24.Unluer E, Karagoz A, Senturk G, Karaman M, Olow K, Bayata S. Bedside lung ultrasonography for diagnosis of pneumonia. Hong Kong J Emerg Med. 2013;20(2):98. [Google Scholar]
- 25.El Dien HMS, ElLatif DAA. The value of bedside lung ultrasonography in diagnosis of neonatal pneumonia. Egypt J Radiol Nuclear Med. 2013;44(2):339–347. doi: 10.1016/j.ejrnm.2013.02.005. [DOI] [Google Scholar]
- 26.Caiulo VA, Gargani L, Caiulo S, Fisicaro A, Moramarco F, Latini G, et al. Lung ultrasound characteristics of community-acquired pneumonia in hospitalized children. Pediatric Pulmonol. 2013;48(3):280–287. doi: 10.1002/ppul.22585. [DOI] [PubMed] [Google Scholar]
- 27.Benci A, Caremani M, Menchetti D, Magnolfi A. Sonographic diagnosis of pneumonia and bronchopneumonia. Eur J Ultrasound. 1996;4(3):169–176. doi: 10.1016/S0929-8266(96)00195-4. [DOI] [Google Scholar]
- 28.Lichtenstein DA, Lascols N, Mezière G, Gepner A. Ultrasound diagnosis of alveolar consolidation in the critically ill. Intensive Care Med. 2004;30(2):276–281. doi: 10.1007/s00134-003-2075-6. [DOI] [PubMed] [Google Scholar]
- 29.Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure. CHEST J. 2008;134(1):117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parlamento S, Copetti R, Di Bartolomeo S. Evaluation of lung ultrasound for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2009;27(4):379–384. doi: 10.1016/j.ajem.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Cortellaro F, Colombo S, Coen D, Duca PG. Lung ultrasound is an accurate diagnostic tool for the diagnosis of pneumonia in the emergency department. Emerg Med J. 2012;29(1):19–23. doi: 10.1136/emj.2010.101584. [DOI] [PubMed] [Google Scholar]
- 32.Xirouchaki N, Magkanas E, Vaporidi K, Kondili E, Plataki M, Patrianakos A, et al. Lung ultrasound in critically ill patients: comparison with bedside chest radiography. Intensive Care Med. 2011;37(9):1488–1493. doi: 10.1007/s00134-011-2317-y. [DOI] [PubMed] [Google Scholar]
- 33.Reissig A, Copetti R, Mathis G, Mempel C, Schuler A, Zechner P, et al. Lung ultrasound in the diagnosis and follow-up of community-acquired pneumonia: a prospective, multicenter, diagnostic accuracy study. CHEST J. 2012;142(4):965–972. doi: 10.1378/chest.12-0364. [DOI] [PubMed] [Google Scholar]
- 34.Testa A, Soldati G, Copetti R, Giannuzzi R, Portale G, Gentiloni-Silveri N. Early recognition of the 2009 pandemic influenza A (H1N1) pneumonia by chest ultrasound. Crit Care. 2012;16(1):R30. doi: 10.1186/cc11201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah VP, Tunik MG, Tsung JW. Prospective evaluation of point-of-care ultrasonography for the diagnosis of pneumonia in children and young adults. JAMA Pediatr. 2013;167(2):119–125. doi: 10.1001/2013.jamapediatrics.107. [DOI] [PubMed] [Google Scholar]
- 36.Copetti R, Cattarossi L. Ultrasound diagnosis of pneumonia in children. Radiol Med (Torino) 2008;113(2):190–198. doi: 10.1007/s11547-008-0247-8. [DOI] [PubMed] [Google Scholar]
- 37.Nafae R, Eman SR, Mohamad NA, El-Ghamry R, Ragheb AS. Adjuvant role of lung ultrasound in the diagnosis of pneumonia in intensive care unit-patients. Egypt J Chest Dis Tuberc. 2013;62(2):281–285. doi: 10.1016/j.ejcdt.2013.04.007. [DOI] [Google Scholar]
- 38.Liu X-L, Lian R, Tao Y-K, Gu C-D, Zhang G-Q (2014) Lung ultrasonography: an effective way to diagnose community-acquired pneumonia. Emerg Med J (emermed-2013-203039) [DOI] [PubMed]
- 39.Esposito S, Papa SS, Borzani I, Pinzani R, Giannitto C, Consonni D, et al. Performance of lung ultrasonography in children with community-acquired pneumonia. Ital J Pediatr. 2014;40:37. doi: 10.1186/1824-7288-40-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nazerian P, Volpicelli G, Vanni S, Gigli C, Betti L, Bartolucci M, et al. Accuracy of lung ultrasound for the diagnosis of consolidations when compared to chest computed tomography. Am J Emerg Med. 2015;33(5):620–625. doi: 10.1016/j.ajem.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 41.Bourcier J-E, Paquet J, Seinger M, Gallard E, Redonnet J-P, Cheddadi F, et al. Performance comparison of lung ultrasound and chest X-ray for the diagnosis of pneumonia in the ED. Am J Emerg Med. 2014;32(2):115–118. doi: 10.1016/j.ajem.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Ye X, Xiao H, Chen B, Zhang S. Accuracy of lung ultrasonography versus chest radiography for the diagnosis of adult community-acquired pneumonia: review of the literature and meta-analysis. PLoS ONE. 2015;10(6):e0130066. doi: 10.1371/journal.pone.0130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sartori S, Tombesi P. Emerging roles for transthoracic ultrasonography in pulmonary diseases. World J Radiol. 2010;2(6):203. doi: 10.4329/wjr.v2.i6.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lichtenstein DA. Lung ultrasound in the critically ill. Ann Intensive Care. 2014;4(1):2110–5820. doi: 10.1186/2110-5820-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volpicelli G, Zanobetti M. Lung ultrasound and pulmonary consolidations. Am J Emerg Med. 2015;33(9):1307–1308. doi: 10.1016/j.ajem.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 46.Lichtenstein D. Lung ultrasound in the critically ill. Curr Opin Crit Care. 2014;20(3):315–322. doi: 10.1097/MCC.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 47.Remérand F, Dellamonica J, Mao Z, Ferrari F, Bouhemad B, Jianxin Y, et al. Multiplane ultrasound approach to quantify pleural effusion at the bedside. Intensive Care Med. 2010;36(4):656–664. doi: 10.1007/s00134-010-1769-9. [DOI] [PubMed] [Google Scholar]


