Abstract
Background
In Europe, dietary management of isovaleric acidemia (IVA) may vary widely. There is limited collective information about dietetic management.
Aim
To describe European practice regarding the dietary management of IVA, prior to the availability of the E-IMD IVA guidelines (E-IMD 2014).
Methods
A cross-sectional questionnaire was sent to all European dietitians who were either members of the Society for the Study of Inborn Errors of Metabolism Dietitians Group (SSIEM-DG) or whom had responded to previous questionnaires on dietetic practice (n = 53). The questionnaire comprised 27 questions about the dietary management of IVA.
Results
Information on 140 patients with IVA from 39 centres was reported. 133 patients (38 centres) were given a protein restricted diet. Leucine-free amino acid supplements (LFAA) were routinely used to supplement protein intake in 58% of centres. The median total protein intake prescribed achieved the WHO/FAO/UNU [2007] safe levels of protein intake in all age groups. Centres that prescribed LFAA had lower natural protein intakes in most age groups except 1 to 10 y. In contrast, when centres were not using LFAA, the median natural protein intake met WHO/FAO/UNU [2007] safe levels of protein intake in all age groups. Enteral tube feeding was rarely prescribed.
Conclusions
This survey demonstrates wide differences in dietary practice in the management of IVA across European centres. It provides unique dietary data collectively representing European practices in IVA which can be used as a foundation to compare dietary management changes as a consequence of the first E-IMD IVA guidelines availability.
Keywords: Isovaleric acidemia, Protein restricted diet, Leucine, Leucine free l-amino acids, Natural protein
1. Introduction
Isovaleric acidemia (IVA) (McKusick 243500) is a rare inherited condition, caused by a deficiency of the mitochondrial enzyme isovaleryl-CoA dehydrogenase (EC 1.3.99.10), leading to accumulation of isovaleryl-CoA and its metabolites including free isovaleric acid, 3-hydroxyisovalerate and N-isovalerylglycine [1]. The major goal of IVA management is to reduce the production and increase excretion of isovaleryl-CoA. This is achieved by: 1) limiting leucine intake via protein restriction [2], [3], [4]; 2) enhancement of alternative metabolic pathways using carnitine [5], [6] and glycine [7], [8] which conjugate with isovaleryl-CoA to produce the non-toxic compounds isovalerylglycine and isovalerylcarnitine; and 3) application of an emergency management protocol at times of metabolic stress (e.g. illness and fasting). However, partly due to IVA's heterogeneity, its rarity, shortage of large, multi-centre longitudinal studies and long-term outcome data, there is disagreement regarding optimal dietary management. Almost all reports are from case studies or small case series only.
The first case studies of IVA reported signs of dietary protein intolerance typically with episodes of vomiting, lethargy and acidosis with ketonuria after increased intake of protein-rich foods [9], [10]. Many patients maintain long term metabolic stability on dietary protein restriction only [1], [11], [12], [13], [14], [15], [16], [17], [18]. In fact, the majority of IVA case studies advise some protein restriction [1], [2], [3], [4], [5], [6], [7], [9], [11], [14], [15], [16], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30] but with wide differences in the amount of natural protein given. Some centres prescribe less than the WHO/FAO/UNU 2007 safe levels of protein intake [31] and may specifically calculate and control leucine intake supplemented with leucine-free l-amino acid supplements (LFAA) [3], [5], [22], [23], [26], [27], [28], [29], [32], [33], [34], [35].
In September 2014, the web based E-IMD IVA guidelines [36] advocated that natural protein intake be restricted to reduce the isovaleric acid burden but should supply at least the WHO/FAO/UNU 2007 safe levels of protein intake [31]. The use of LFAA was not discussed [36] and some considered they may provide little clinical benefit and thus provide extra burden to patients and families and unnecessary expense to health services.
This paper aims to describe European practice regarding the dietary management of IVA prior to the introduction of the E-IMD IVA guidelines in 2014.
2. Material and methods
A cross-sectional questionnaire was sent to all European dietitians who were either members of the Society for the Study of Inborn Errors of Metabolism Dietitians Group (SSIEM-DG) or whom had responded to previous questionnaires on dietetic practice (n = 53) [37]. We requested dietitians cascade the questionnaire to other dietitians and/or physicians within their own country between July and August 2014. The questionnaire consisted of 27 multiple choice and short answer questions. The questions were aimed at patients on dietary treatment. The following data were collected by age group: total protein intake prescribed, amount of natural protein and use of LFAA. Specific questions were asked about: special low protein foods, energy, vitamin and mineral supplements, use of enteral feeding, monitoring, treatment criteria and treatment drugs. The results were divided into geographical regions to examine trends in protein prescription. The groupings were: Western Europe Group A (Netherlands, Belgium, France), Western Europe Group B (Germany, Switzerland, Austria), Eastern Europe (Poland), Southern Europe (Italy, Spain and Portugal), and Northern Europe (Denmark, Norway, Sweden and UK).
Clinical outcome data or patient specific data were not included in this questionnaire. Therefore, ethical approval was not required.
2.1. Statistical analysis
Data were analysed using descriptive statistics (percentage of total responses, means or medians). Prior to analysis, responses to some open answer questions were grouped or categorised according to answers received.
3. Results
Dietitians from 53 centres, representing 14 countries returned questionnaires. Fourteen of 53 centres reported they had no patients with IVA. For each country a median of 3 centres responded (range 1–14).
3.1. Patient description
In total, 140 patients with IVA were reported. Seven of 140 patients were excluded from analysis as they were not prescribed a protein restriction. Therefore 133 patients from 38 centres were on protein restriction with a median of 2 patients (range 1–17) per centre.
The numbers of centres and patients in each geographical grouping were: Western Europe Group A, n = 14 centres, 44 patients; Western Europe Group B, n = 6 centres, 24 patients; Eastern Europe, n = 1 centre, 8 patients; Southern Europe, n = 4 centres, 9 patients; and Northern Europe, n = 13 centres, 48 patients. In the 133 IVA patients presentation age was: neonatal, n = 81 (61%); late, n = 48 (36%), and unknown, n = 4 (3%). Patients were distributed in the following age ranges at the time of questionnaire completion: < 1 y (n = 7), 1–10 y (n = 71), 11–16 y (n = 31) and > 16 y (n = 24).
3.2. Total protein prescription
All centres distributed by region and age range are presented in Fig. 1. Total protein intake (g/kg/day) in almost all centres (32 of 38, 84%) met WHO/FAO/UNU safe levels of protein intake [31].
Fig. 1.
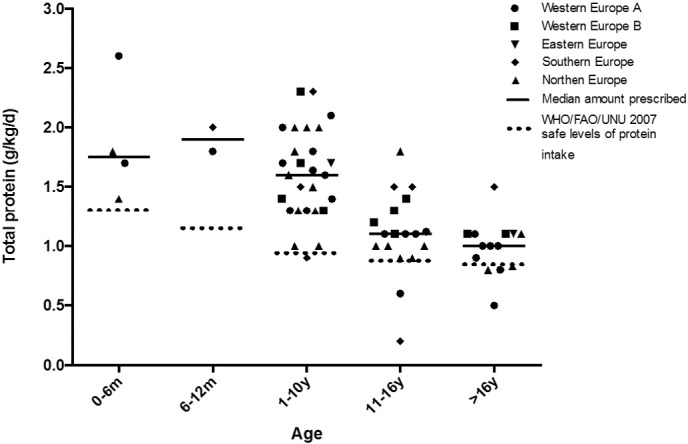
Mean total protein prescription to IVA patients by centre in each age range (n = 38 centres).
Twenty centres (53%) prescribed total protein prescription according to the WHO/FAO/UNU safe levels of protein intake [31], and 15 centres (39%) used the countries national reference protein intake as a guide for protein prescription. The median total protein prescription (g/kg/day) with and without LFAA is given in Table 1.
Table 1.
Descriptive statistics comparing centres (using/not using LFAA) for dietary prescription of: total protein (g/kg), natural protein (g/kg), LFAA (g/kg) and % of protein provided by LFAA compared with total protein prescription.
| Centres using LFAA |
Centres not using LFAA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age 0–6 m (n = 2) |
Age 7–12 m (n = 2) |
Age 1–10 y (n = 40) |
Age 11–16 y (n = 16) |
Age > 16 y (n = 5) |
Age 0–6 m (n = 3) |
Age 7–12 m (n = 0) |
Age 1–10 y (n = 31) |
Age 11–16 y (n = 15) |
Age > 16 y (n = 19) |
|
| Total protein (g/kg) | ||||||||||
| Median | 2.2 | 1.9 | 1.7 | 1.1 | 1.1 | 1.6 | None reported | 1.3 | 1.0 | 0.9 |
| Min | 1.7 | 1.8 | 0.9 | 1.0 | 1.0 | 1.4 | 1.0 | 0.2 | 0.5 | |
| Max | 2.6 | 2.0 | 2.3 | 1.5 | 1.5 | 1.8 | 2.0 | 1.8 | 1.1 | |
| Natural protein (g/kg) | ||||||||||
| Median | 1.1 | 0.4 | 1.0 | 0.7 | 0.7 | 1.6 | None reported | 1.3 | 1.0 | 0.9 |
| Min | 1.0 | 0.4 | 0.4 | 0.4 | 0.4 | 1.4 | 1.0 | 0.2 | 0.5 | |
| Max | 1.1 | 0.5 | 1.9 | 1.0 | 0.8 | 1.8 | 2.0 | 1.8 | 1.1 | |
| LFAA (g/kg) | ||||||||||
| Median | 1.1 | 1.5 | 0.7 | 0.5 | 0.6 | 0 | None reported | 0 | 0 | 0 |
| Min | 0.7 | 1.3 | 0.2 | 0.4 | 0.3 | 0 | 0 | 0 | 0 | |
| Max | 1.5 | 1.7 | 1.2 | 0.6 | 0.8 | 0 | 0 | 0 | 0 | |
| % amount of total protein from LFAA | ||||||||||
| Median | 50 | 77 | 44 | 42 | 48 | 0 | None reported | 0 | 0 | 0 |
| Min | 41 | 72 | 17 | 29 | 27 | 0 | 0 | 0 | 0 | |
| Max | 58 | 82 | 75 | 60 | 64 | 0 | 0 | 0 | 0 | |
LFAA: leucine free l-amino acids.
n: number of patients.
The following criteria were used by all centres for adjusting protein prescription: quantitative plasma amino acid profiles, growth and severity of IVA. Protein prescription was a combined decision between medical doctors and dietitians in 24 centres (63%), medical doctors only in 11 centres (29%) and a dietitian's decision only in 2 centres (5%). One centre did not answer this question.
3.3. Natural protein prescription
The median natural protein prescription (g/kg/day) compared with WHO/FAO/UNU safe levels of protein, with and without LFAA is given in Table 2. In Fig. 2, natural protein prescription is presented by region and age. Almost half of the centres (n = 18; 47%) prescribed natural protein intakes below WHO/FAO/UNU 2007 safe levels of protein intake [27] in at least one age range. However, the use of LFAA in 12 (66%) centres improved protein intake allowing safe levels to be achieved. Five of 38 centres (13%) prescribed a low protein diet (without LFAA) that did not provide safe levels of protein intake [27] in patients above 11 y (centres from France n = 2; UK, n = 2; and Italy, n = 1).
Table 2.
Comparison of natural protein and total protein with WHO/FAO/UNU [2007] safe levels of protein intake for all centres.
| Centres using LFAA |
Centres not using LFAA |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 0–6 m (n = 2) |
7–12 m (n = 2) |
1–10 y (n = 40) |
11–16 y (n = 16) |
> 16 y (n = 5) |
0–6 m (n = 3) |
7–12 m (n = 0) |
1–10 y (n = 31) |
11–16 y (n = 15) |
> 16 y (n = 19) |
| Natural protein (g/kg) | ||||||||||
| Median | 1.1 | 0.4 | 1.0 | 0.7 | 0.7 | 1.6 | None reported | 1.3 | 1.0 | 0.9 |
| WHO/FAO/UNU 2007 | 1.31 | 1.14 | 0.94a | 0.89a | 0.84 | 1.31 | 1.14 | 0.94a | 0.89a | 0.84 |
| % intake compared to WHO reference value 2007 | 84 | 35 | 106 | 79 | 83 | 122 | – | 138 | 112 | 107 |
| Total protein (g/kg) | ||||||||||
| Median | 2.2 | 1.9 | 1.7 | 1.1 | 1.1 | 1.6 | None reported | 1.3 | 1.0 | 0.9 |
| WHO/FAO/UNU 2007 | 1.31 | 1.14 | 0.94a | 0.89a | 0.84 | 1.31 | 1.14 | 0.94a | 0.89a | 0.84 |
| % intake compared to WHO reference value 2007 | 168 | 167 | 181 | 124 | 131 | 122 | – | 138 | 112 | 107 |
LFAA: leucine free l-amino acids.
n: number of patients.
Mean protein values for ages 1–10 y, 11–16 y taken for WHO/FAO/UNU [2007] recommendations.
Fig. 2.
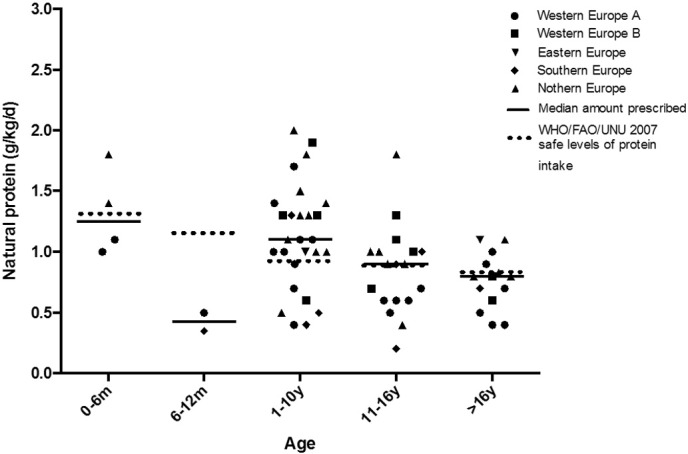
Mean natural protein prescription to IVA patients by centre in each age range (n = 38 centres).
Ten centres (26%) reported avoiding animal protein as part of the natural protein allowance. Seven of these centres used LFAA to supplement natural protein intake.
3.4. Prescription of LFAA
LFAA were used routinely to supplement protein intake by 23 of 38 (61%) centres (Fig. 3). The amount of LFAA prescribed per kg is given in Table 1. The median percentage of total protein provided by LFAA was over 40% (range17–82%) in all age groups. The numbers of centres prescribing LFAA declined from 10 years of age [aged 0 to 12 months, 67% (n = 4 centres); 1 to 10 y, 67% (n = 18 centres), 11 to 16 y, 50% (n = 9 centres) and > 16 y of age, 43% of centres (n = 6 centres)]. None of the UK centres prescribed LFAA for IVA. Centres within countries that varied in their approach to using LFAA were Austria, Belgium, France, Germany, Italy and Netherlands.
Fig. 3.
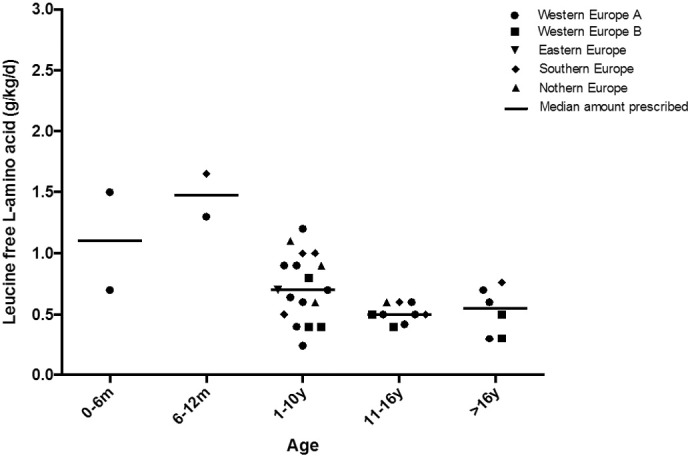
Mean leucine free l-amino acid prescription to IVA patients by centre in each age range (n = 23 centres).
LFAA were given in divided doses throughout the day mixed with water/fruit juice or as a puree. The preferred LFAA were for: infants, a leucine-free infant formula supplemented with carbohydrate, fats, vitamins and minerals, and patients over 1 y, a leucine-free l-amino acid supplement (powder/liquid) containing carbohydrate, vitamins and minerals. LFAA without added vitamins and minerals were rarely used.
3.5. Nutritional support
Only 8 of 133 patients (6%) were given tube feeds (for neurological dysfunction and feeding difficulties). Of this group all had gastrostomy tubes and 3 of 8 were on nocturnal enteral feeds only.
The majority of centres (34 of 38; 89%) used special low protein foods such as low protein pasta but the amount varied according to individual patient needs and the dietary practices of local centres. Seventy-four percent (28 of 38 centres) prescribed a low protein milk replacement instead of cow's milk. Only 11% (15 of 133) of patients were given additional energy supplements.
3.6. Monitoring
All centres monitored weight, height, quantitative plasma amino acids and nutritional blood markers. The frequency was age dependent (3–12 monthly) with infants and young children (pre-school age) assessed more frequently. Nutritional markers included: quantitative amino acids, zinc, selenium, haemoglobin, ferritin and vitamins B12 (and plasma MMA), D, A and E. Some centres reported monitoring essential fatty acids (23 centres; 60%), but at infrequent intervals.
3.7. Drugs treatment
Most centres reported using carnitine (37 centres, 97%) and glycine (29 centres, 76%) as a standard treatment approach. Nitrogen scavenger drugs where used by 2 centres (5%) but only to treat hyperammonemia at initial presentation. Laxatives for constipation were used by 7 centres (18%).
4. Discussion
This multicentre pan European survey describes the dietary management of 133 patients with IVA on protein restriction prior to the introduction of the E-IMD IVA guidelines. This provides a foundation to compare any changes in dietary approach as a consequence of the availability of the first European guidelines. Most centres managed only small numbers of IVA patients, constraining the development of dietetic expertise with this condition. There was little consensus in dietary approach. Centres that prescribed a natural protein restriction only, generally achieved the WHO/FAO/UNU [2007] safe levels of protein intake [31] but in 5 of these centres, particularly in patients ≥ 11 years of age, natural protein intakes were well below safe levels.
LFAA were used by almost two thirds of European metabolic centres to compensate for the low natural protein prescription in order to meet safe levels of protein intake. The need for LFAA has been challenged [1], [11], [12], [13], [14], [15], [16], [17], [18] and the use of LFAA is not advocated by the IVA-EIMD guidelines [36]. There is some suggestion that LFAA may correct amino acid imbalance or deficiency [27], [30], [38], [39] but the effectiveness of this is unknown and remains unstudied with severe natural protein restriction appearing unnecessary. There is limited published IVA patient outcome data that describes dietary management in depth. In a recent study, data from a patient registry outlined 45 patients with IVA who had baseline height and weight z scores that were close to zero but dietary intake was unreported [40]. In 4 adults with IVA, managed with low protein diets only, had normal growth and no progressive disease [17]. In a group of 21 IVA patients, catabolic episodes mainly occurred in early childhood and protein intake did not appear to be a major precipitating factor [41]. Generally, long term treatment with carnitine, glycine and a low protein diet (with or without LFAA) appears to lead to relative metabolic stability, particularly after the early childhood years.
Geographical location appeared to have a large influence on the prescription of LFAA with more centres prescribing in Western Europe A (79% of centres), Southern Europe (75% of centres), Western Europe B (67% of centres), and Eastern Europe (the only centre participating used LFAA). Less LFAA was prescribed in Northern Europe (only 31% of centres), and no centres in the UK used LFAA (Fig. 3). In addition, companies that produce LFAA for IVA only supply a limited range. These are mainly unflavoured with narrow presentation choice (mainly powders). It is possible adherence may be sub-optimal but this question was not asked and remains unreported in IVA.
The IVA E-IMD guidelines [36] recommend that natural protein intake should be restricted to reduce the isovaleric acid burden but ought to supply at least the safe levels of protein intake advocated by WHO/FAO/UNU 2007 [31]. They emphasise that over-restriction of natural protein could lead to catabolism and metabolic instability [42]. From case reports, patients are able to maintain metabolic stability when consuming at least the WHO/FAO/UNU safe levels of protein/leucine intake although some younger patients have tolerated double this amount [1], [14], [15], [24]. In IVA, there are no reported differences in leucine/protein prescription for age, growth rate and severity of disorder. There are also no case reports describing protein intake in adolescents and adults or for patients with the milder 932C-T mutations. Although in our cohort some of the older patients, in particular, were prescribed less than safe levels of protein intake, it is possible that their actual natural protein intake was higher but we did not collect this data.
Leucine is an essential amino acid; it plays an important role in the regulation of metabolism, promotes global protein synthesis by signalling an increase in translation [43], [44], promotes insulin release [45] and inhibits autophagic protein degradation [46]. Over restriction will lead to anorexia, triglyceride lipolysis [47], weight loss [48], and amino acid imbalance. Leucine provides 10% of the amino acid content of animal protein but only 6% of vegetable protein [49]. In this study, almost one third of centres used only low biological food sources to provide natural protein intake. This may potentially lead to leucine deficiency, which has been reported in IVA children [50].
Generally, in IVA, emphasis has been placed on protein intake but maintenance of energy intake may be as important as protein restriction. In an early study, Millington et al. [51] demonstrated that the turnover of endogenous protein, rather than dietary protein intake, is the main cause for the production of toxic metabolites in IVA. He suggested that suppression of endogenous catabolism is more effective than exogenous dietary protein restriction. Therefore, although there was low use of tube feeding (8 of 133 patients) in this survey, overall evidence would suggest that attention should be paid to adequate energy intake when patients are both stable and during metabolic stress.
There are some limitations in this survey. Initially participant interpretation of some questions was inconsistent, but all answers were quality checked with individual participants until we were certain data was interpreted correctly. No data was collected about patient clinical outcome, disease severity or detailed information about drug treatments which may have impacted on centres practices with protein prescription. Also data was collected about dietary prescriptions rather than actual dietary intake.
There remains much to be done to define the optimum dietary management of IVA considering all age groups and disorder severity. The prescription of median natural protein intake was below safe levels of protein intake when centres were giving LFAA. Overall, there may be ‘over restriction’ of natural protein and unnecessary use of LFAA creating a diet that is burdensome and expensive [51], [52], particularly in older patients who have fewer episodes of metabolic instability. It is also important that further guidelines should differentiate dietary management for the “classic” IVA and the “mild” phenotype [52]. This study provides baseline data from a large cohort of IVA patients and will help enable the development of controlled trials to further investigate dietary management and help standardise treatment.
Author's roles
All authors were involved in data collection, interpretation of data, critical revision of the paper for important intellectual content and final approval of the version to be published. Alex Pinto was involved in quality check of data, data analysis and writing of the manuscript. Anne Daly was involved in data analysis and manuscript development. Sharon Evans was involved in questionnaire design, collection of data and manuscript development and Anita MacDonald was involved in questionnaire development, interpretation of data and writing of the manuscript.
Source of funding
There was no need of funding to develop this study.
Conflict of interest
Alex Pinto has received an educational grant from Cambrooke Therapeutics and grants from Vitaflo, Merck Serono and Biomarin to attend scientific meetings.
Anita MacDonald has received research funding and honoraria from Nutricia, Vitaflo International and Merck Serono. She is a member of the European Nutrition Expert Panel (Biomarin), member of Sapropterin Advisory Board (Biomarin), member of the Advisory Board entitled ELEMENT (Danone-Nutricia), and member of an Advisory Board for Arla and Applied Pharma Research.
Anne Daly has undertaken evaluation work for the nutritional companies – Vitaflo Ltd, Nutricia Ltd and Metax.
Liesbeth van der Ploeg received several grants from Nutricia and Vitaflo to visit congresses and education.
A. van Wegberg is a member of the SSIEM Council and received honoraria as a speaker from Excemed.
Gudrun Elise Kahrs received support from Vitaflo and SHS/Nutricia to attend meetings.
Sharon Evans is a research dietitian funded by Nutricia; financial support from Nutricia and Vitaflo to attend study days and conferences.
Corrie Timmer received financial support from Nutricia and Vitaflo to attend conferences.
Manuela Ferreira Almeida received grants from Glutamine, Nutricia, Merck Serono, Biomarin, Orphan and Lifediet to attend congress and for education.
Júlio César Rocha is member of the European Nutrition Expert Panel (Biomarin) and member of an Advisory Board for Applied Pharma Research.
Amaya Bélanger-Quintana, Martine Robert, Esther van Dam, Carina Heidenborg, Margreet van Rijn, Katharina Dokoupil are members of the European Nutrition Expert Panel (Biomarin).
Fiona White has received honoria from Alexion, Nutricia Metabolics and Vitaflo as well as educational and travel grants from Nutricia Metabolics and Vitaflo.
Marjorie Dixon has received research honoraria and congress travel allowances from Nutricia and Vitaflo International.
Acknowledgments
The authors would like to thank the following people for the assistance in data collection: Louise Robertson (University Hospitals Birmingham NHS Foundation Trust, UK); Sharan Lowry (Sheffield Children's Hospital, UK); Rychelle Winstone, Kate Stonstreet & Karen van Wyk (Evelina London Children's Hospital, Guy's and St Thomas' NHS Foundation Trust, London, UK); Charlotte Ellerton & Rachel Carruthers (Charles Dent Metabolic Unit National Hospital for Neurology and Surgery, London, UK); Isabelle Nedellec (CHU Angers, France); Skadi Beblo (Hospital of Children's & Adolescents, University of Leipzig, Germany), Ulrike Och (University Children's Hospital, Munster, Germany); Anne-Kathrin Neugebauer (Heinrich-Heine-University, Department of General Pediatrics, Neonatology and Paediatric Cardiology, Dusseldorf, Germany); Karen Corthouts & Marianne Diels (Metabolic Center, University Hospitals Leuven and KU Leuven, Belgium); Sophie Defouny (Hôpital Universitaire des Enfants, Reine fabiola, Bruxelles, Belgium); Esmeralda Martins & Anabela Bandeira (Unidade de Doenças Metabólicas, Centro Hospitalar do Porto - CHP, Porto, Portugal); Elisa Leão Teles & Esmeralda Rodrigues (Centro Hospitalar São João - Unidade de Doenças Metabólicas, Porto, Portugal); Mercedes Martinez-Pardo (Unidad de Enfermedades Metabolicas, Servicio de Pediatria, Hospital Ramon y Cajal Madrid, Spain) and Camilla Caroe & Ann Roskjaer (National University Hospital, Copenhagen, Denmark). We thank the Association “La Vita e’ un Dono” for supporting the fellowship of Giorgia Gallo.
References
- 1.Chalmers R.A., de Sousa C., Tracey B.M., Stacey T.E., Weaver C., Bradley D. l-Carnitine and glycine therapy in isovaleric acidaemia. J. Inherit. Metab. Dis. 1985;8(Suppl. 2):141–142. doi: 10.1007/BF01811499. [DOI] [PubMed] [Google Scholar]
- 2.Berry G.T., Yudkoff M., Segal S. Isovaleric acidemia: medical and neurodevelopmental effects of long-term therapy. J. Pediatr. 1988;113:58–64. doi: 10.1016/s0022-3476(88)80528-6. [DOI] [PubMed] [Google Scholar]
- 3.Levy H.L., Erickson A.M., Lott I.T., Kurtz D.J. Isovaleric acidemia: results of family study and dietary treatment. Pediatrics. 1973;52:83–94. [PubMed] [Google Scholar]
- 4.Lott I.T., Erickson A.M., Levy H.L. Dietary treatment of an infant with isovaleric acidemia. Pediatrics. 1972;49:616–618. [PubMed] [Google Scholar]
- 5.Fries M.H., Rinaldo P., Schmidt-Sommerfeld E., Jurecki E., Packman S. Isovaleric acidemia: response to a leucine load after three weeks of supplementation with glycine, l-carnitine, and combined glycine-carnitine therapy. J. Pediatr. 1996;129:449–452. doi: 10.1016/s0022-3476(96)70081-1. [DOI] [PubMed] [Google Scholar]
- 6.Mayatepek E., Kurczynski T.W., Hoppel C.L. Long-term l-carnitine treatment in isovaleric acidemia. Pediatr. Neurol. 1991;7:137–140. doi: 10.1016/0887-8994(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 7.Krieger I., Tanaka K. Therapeutic effects of glycine in isovaleric acidemia. Pediatr. Res. 1976;10:25–29. doi: 10.1203/00006450-197601000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Shigematsu Y., Sudo M., Momoi T., Inoue Y., Suzuki Y., Kameyama J. Changing plasma and urinary organic acid levels in a patient with isovaleric acidemia during an attack. Pediatr. Res. 1982;16:771–775. doi: 10.1203/00006450-198209000-00013. [DOI] [PubMed] [Google Scholar]
- 9.Budd M.A., Tanaka K., Holmes L.B., Efron M.L., Crawford J.D., Isselbacher K.J. Isovaleric acidemia. Clinical features of a new genetic defect of leucine metabolism. N. Engl. J. Med. 1967;277:321–327. doi: 10.1056/NEJM196708172770701. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka K., Budd M.A., Efron M.L., Isselbacher K.J. Isovaleric acidemia: a new genetic defect of leucine metabolism. Proc. Natl. Acad. Sci. U. S. A. 1966;56:236–242. doi: 10.1073/pnas.56.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Sousa C., Chalmers R.A., Stacey T.E., Tracey B.M., Weaver C.M., Bradley D. The response to l-carnitine and glycine therapy in isovaleric acidaemia. Eur. J. Pediatr. 1986;144:451–456. doi: 10.1007/BF00441737. [DOI] [PubMed] [Google Scholar]
- 12.de Baulny H.O., Dionisi-Vici C., Wendel U., van den Berghe G. Branched-Chain organic acidurias/acidemias. In: Saudubray J.M., van den Berghe G., Walter J.H., editors. Maple Suryp Urine Disease, Isovaleric Aciemia, Propionic Acidemia, Methylmalonic Acidemia. fifth edition. Springer-Verlag; Berlin Heidelberg: 2012. pp. 279–288. (Inborn Metabolic Diseases: Diagnosis and Treatment). [Google Scholar]
- 13.Dixon M., S. V., editors. Clinical Paediatric Dietetics. Blackwell; Oxford: 2014. Organic acidaemias and urea cycle disorders. General principles of low protein diets for the management of urea cycle disorders and organic acidaemias; pp. 456–506. [Google Scholar]
- 14.Duran M., Bruinvis L., Ketting D., Wadman S.K., van Pelt B.C., Batenburg-Plenter A.M. Isovaleric acidaemia presenting with dwarfism, cataract and congenital abnormalities. J. Inherit. Metab. Dis. 1982;5:125–127. doi: 10.1007/BF01800006. [DOI] [PubMed] [Google Scholar]
- 15.Duran M., van Sprang F.J., Drewes J.G., Bruinvis L., Ketting D., Wadman S.K. Two sisters with isovaleric acidaemia, multiple attacks of ketoacidosis and normal development. Eur. J. Pediatr. 1979;131:205–211. doi: 10.1007/BF00538944. [DOI] [PubMed] [Google Scholar]
- 16.Ito T., Kidouchi K., Sugiyama N., Kajita M., Chiba T., Niwa T., Wada Y. Liquid chromatographic-atmospheric pressure chemical ionization mass spectrometric analysis of glycine conjugates and urinary isovalerylglycine in isovaleric acidemia. J. Chromatogr. B Biomed. Appl. 1995;670:317–322. doi: 10.1016/0378-4347(95)00174-3. [DOI] [PubMed] [Google Scholar]
- 17.Martin-Hernandez E., Lee P.J., Micciche A., Grunewald S., Lachmann R.H. Long-term needs of adult patients with organic acidaemias: outcome and prognostic factors. J. Inherit. Metab. Dis. 2009;32:523–533. doi: 10.1007/s10545-009-1191-12. [DOI] [PubMed] [Google Scholar]
- 18.Velazquez A., Prieto E.C. Glycine in acute management of isovalericacidaemia. Lancet (Lond. Engl.) 1980;1:313–314. doi: 10.1016/s0140-6736(80)90810-7. [DOI] [PubMed] [Google Scholar]
- 19.Bakkeren J.A., Sengers R.C., Ruitenbeek W., Trijbels J.M., Houben M.L., Van der Zee S.P. Isovaleric acidemia: identical biochemical picture in 3 patients with variable clinical manifestations. Tijdschr. Kindergeneesk. 1982;50:153–159. [PubMed] [Google Scholar]
- 20.Cohn R.M., Yudkoff M., Rothman R., Segal S. Isovaleric acidemia: use of glycine therapy in neonates. N. Engl. J. Med. 1978;299:996–999. doi: 10.1056/NEJM197811022991807. [DOI] [PubMed] [Google Scholar]
- 21.Dercksen M., Duran M., Ijlst L., Mienie L.J., Reinecke C.J., Ruiter J.P., Waterham H.R., Wanders R.J. Clinical variability of isovaleric acidemia in a genetically homogeneous population. J. Inherit. Metab. Dis. 2012;35:1021–1029. doi: 10.1007/s10545-012-9457-2. [DOI] [PubMed] [Google Scholar]
- 22.Elsas L.J., 2nd, Naglak M. Acute and chronic-intermittent isovaleric acidemia: diagnosis and glycine therapy. Acta Paediatr. Jpn. Overseas Ed. 1988;30:442–451. doi: 10.1111/j.1442-200x.1988.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 23.Erdem E., Cayonu N., Uysalol E., Yildirmak Z.Y. Chronic intermittent form of isovaleric acidemia mimicking diabetic ketoacidosis. J. Pediatr. Endocrinol. Metab. 2010;23:503–505. doi: 10.1515/jpem.2010.082. [DOI] [PubMed] [Google Scholar]
- 24.Gabriel M., Behbehant A.W., Hunneman D.H. Comparison of glycine and carnitine effect in neonatal isovaleric acidemia. Pediatr. Res. 1984;18:806. [Google Scholar]
- 25.Gilbert-Barness E., Barness L.A. Isovaleric acidemia with promyelocytic myeloproliferative syndrome. Pediatr. Dev. Pathol. 1999;2:286–291. doi: 10.1007/s100249900125. [DOI] [PubMed] [Google Scholar]
- 26.Habets D.D.J., Schaper N.C., Rogozinski H., van Spronsen F.J., van Rijn M., Bierau J., Bakker J.A. Biochemical monitoring and management during pregnancy in patients with isovaleric acidaemia is helpful to prevent metabolic decompensation. JIMD Rep. 2012;3:83–89. doi: 10.1007/8904_2011_66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Loots D.T., Mienie L.J., Erasmus E. Amino-acid depletion induced by abnormal amino-acid conjugation and protein restriction in isovaleric acidemia. Eur. J. Clin. Nutr. 2007;61:1323–1327. doi: 10.1038/sj.ejcn.1602648. [DOI] [PubMed] [Google Scholar]
- 28.Mehta K.C., Zsolway K., Osterhoudt K.C., Krantz I., Henretig F.M., Kaplan P. Lessons from the late diagnosis of isovaleric acidemia in a five-year-old boy. J. Pediatr. 1996;129:309–310. doi: 10.1016/s0022-3476(96)70261-5. [DOI] [PubMed] [Google Scholar]
- 29.Rakheja D., Bober M.B., Fisher S.L., Jones P.M. A neonate with hyperammonemia. Lab. Med. 2005;36:292–295. [Google Scholar]
- 30.Wolff J.A., Kelts D.G., Algert S., Prodanos C., Nyhan W.L. Alanine decreases the protein requirements of infants with inborn errors of amino acid metabolism. J. Neurogenet. 1985;2:41–49. doi: 10.3109/01677068509100142. [DOI] [PubMed] [Google Scholar]
- 31.Protein and amino acid requirements in human nutrition. 2007. pp. 1–265. (World Health Organization Technical Report series). (back cover) [PubMed] [Google Scholar]
- 32.Dionisi-Vici C., Deodato F., Roschinger W., Rhead W., Wilcken B. ‘Classical’ organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J. Inherit. Metab. Dis. 2006;29:383–389. doi: 10.1007/s10545-006-0278-z. [DOI] [PubMed] [Google Scholar]
- 33.Feinstein J.A., O'Brien K. Acute metabolic decompensation in an adult patient with isovaleric acidemia. South. Med. J. 2003;96:500–503. doi: 10.1097/01.SMJ.0000051141.03668.1D. [DOI] [PubMed] [Google Scholar]
- 34.Saudubray J.M., Sorin M., Depondt E., Herouin C., Charpentier C., Pousset J.L. Isovaleric acidemia. Study and treatment in 3 brothers. Arch. Fr. Pediatr. 1976;33:795–808. [PubMed] [Google Scholar]
- 35.Marriage B., PB A. Nutrition Management of Patients With Inherited Metabolic Disorders. Jones and Barllet; Massachesetts: 2010. Nutrition management of patients with inherited disorders of branched chain amino acid metabolism; pp. 175–236. [Google Scholar]
- 36.Isovaleric Acidemia: Quick Reference Guide. 2014. European Registry and network for introduction type metabolic diseases (E-IMD)http://www.e-imd.org/rc/e-imd/htm/Article/2014/e-imd-20140716-085102-695/src/htm_fullText/en/IVA%20guideline_Quick%20reference%20guide_Ensenauer_201408.pdf (accessed 27.01.17) [Google Scholar]
- 37.Adam S., Almeida M.F., Assoun M., Baruteau J., Bernabei S.M., Bigot S., Champion H., Daly A., Dassy M., Dawson S., Dixon M., Dokoupil K., Dubois S., Dunlop C., Evans S., Eyskens F., Faria A., Favre E., Ferguson C., Goncalves C., Gribben J., Heddrich-Ellerbrok M., Jankowski C., Janssen-Regelink R., Jouault C., Laguerre C., Le Verge S., Link R., Lowry S., Luyten K., Macdonald A., Maritz C., McDowell S., Meyer U., Micciche A., Robert M., Robertson L.V., Rocha J.C., Rohde C., Saruggia I., Sjoqvist E., Stafford J., Terry A., Thom R., Vande Kerckhove K., van Rijn M., van Teeffelen-Heithoff A., Wegberg A., van Wyk K., Vasconcelos C., Vestergaard H., Webster D., White F.J., Wildgoose J., Zweers H. Dietary management of urea cycle disorders: European practice. Mol. Genet. Metab. 2013;110:439–445. doi: 10.1016/j.ymgme.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Loots D.T., Erasmus E., Mienie L.J. Identification of 19 new metabolites induced by abnormal amino acid conjugation in isovaleric acidemia. Clin. Chem. 2005;51:1510–1512. doi: 10.1373/clinchem.2005.048421. [DOI] [PubMed] [Google Scholar]
- 39.Niederhoff H., Lehnert W., Witt I., Richter O., Limberg J. Isovaleric acidemia in a small infant. Monatsschr. Kinderheilkd. 1977;125:466–468. [PubMed] [Google Scholar]
- 40.Kolker S., Valayannopoulos V., Burlina A.B., Sykut-Cegielska J., Wijburg F.A., Teles E.L., Zeman J., Dionisi-Vici C., Baric I., Karall D., Arnoux J.B., Avram P., Baumgartner M.R., Blasco-Alonso J., Boy S.P., Rasmussen M.B., Burgard P., Chabrol B., Chakrapani A., Chapman K., Cortes I.S.E., Couce M.L., de Meirleir L., Dobbelaere D., Furlan F., Gleich F., Gonzalez M.J., Gradowska W., Grunewald S., Honzik T., Horster F., Ioannou H., Jalan A., Haberle J., Haege G., Langereis E., de Lonlay P., Martinelli D., Matsumoto S., Muhlhausen C., Murphy E., de Baulny H.O., Ortez C., Pedron C.C., Pintos-Morell G., Pena-Quintana L., Ramadza D.P., Rodrigues E., Scholl-Burgi S., Sokal E., Summar M.L., Thompson N., Vara R., Pinera I.V., Walter J.H., Williams M., Lund A.M., Garcia-Cazorla A. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 2: the evolving clinical phenotype. J. Inherit. Metab. Dis. 2015;38:1059–1074. doi: 10.1007/s10545-015-9840-x. [DOI] [PubMed] [Google Scholar]
- 41.Grunert S.C., Wendel U., Lindner M., Leichsenring M., Schwab K.O., Vockley J., Lehnert W., Ensenauer R. Clinical and neurocognitive outcome in symptomatic isovaleric acidemia. Orphanet J. Rare Dis. 2012;7:9. doi: 10.1186/1750-1172-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolker S., Garcia-Cazorla A., Valayannopoulos V., Lund A.M., Burlina A.B., Sykut-Cegielska J., Wijburg F.A., Teles E.L., Zeman J., Dionisi-Vici C., Baric I., Karall D., Augoustides-Savvopoulou P., Aksglaede L., Arnoux J.B., Avram P., Baumgartner M.R., Blasco-Alonso J., Chabrol B., Chakrapani A., Chapman K., EC I.S., Couce M.L., de Meirleir L., Dobbelaere D., Dvorakova V., Furlan F., Gleich F., Gradowska W., Grunewald S., Jalan A., Haberle J., Haege G., Lachmann R., Laemmle A., Langereis E., de Lonlay P., Martinelli D., Matsumoto S., Muhlhausen C., de Baulny H.O., Ortez C., Pena-Quintana L., Ramadza D.P., Rodrigues E., Scholl-Burgi S., Sokal E., Staufner C., Summar M.L., Thompson N., Vara R., Pinera I.V., Walter J.H., Williams M., Burgard P. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J. Inherit. Metab. Dis. 2015;38:1041–1057. doi: 10.1007/s10545-015-9839-3. [DOI] [PubMed] [Google Scholar]
- 43.Anthony T.G., McDaniel B.J., Byerley R.L., McGrath B.C., Cavener D.R., McNurlan M.A., Wek R.C. Preservation of liver protein synthesis during dietary leucine deprivation occurs at the expense of skeletal muscle mass in mice deleted for eIF2 kinase GCN2. J. Biol. Chem. 2004;279:36553–36561. doi: 10.1074/jbc.M404559200. [DOI] [PubMed] [Google Scholar]
- 44.Lynch C.J., Hutson S.M., Patson B.J., Vaval A., Vary T.C. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am. J. Physiol. Endocrinol. Metab. 2002;283:E824–E835. doi: 10.1152/ajpendo.00085.2002. [DOI] [PubMed] [Google Scholar]
- 45.Jonker R., Engelen M.P., Deutz N.E. Role of specific dietary amino acids in clinical conditions. Br. J. Nutr. 2012;108(Suppl. 2):S139–S148. doi: 10.1017/S0007114512002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harris R.A., Joshi M., Jeoung N.H. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem. Biophys. Res. Commun. 2004;313:391–396. doi: 10.1016/j.bbrc.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Cheng Y., Meng Q., Wang C., Li H., Huang Z., Chen S., Xiao F., Guo F. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes. 2010;59:17–25. doi: 10.2337/db09-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu X., Krasnow S.M., Roth-Carter Q.R., Levasseur P.R., Braun T.P., Grossberg A.J., Marks D.L. Hypothalamic signaling in anorexia induced by indispensable amino acid deficiency. Am. J. Physiol. Endocrinol. Metab. 2012;303:E1446–E1458. doi: 10.1152/ajpendo.00427.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul A.A., Southgate D.A.T., Russell J. HMSO; London: 1980. First Supplement to McCance and Widdowson's the Composition of Foods. [Google Scholar]
- 50.Vatanavicharn N., Liammongkolkul S., Sakamoto O., Sathienkijkanchai A., Wasant P. Phenotypic and mutation spectrums of Thai patients with isovaleric acidemia. Pediatr. Int. 2011;53:990–994. doi: 10.1111/j.1442-200X.2011.03488.x. [DOI] [PubMed] [Google Scholar]
- 51.Millington D.S., Roe C.R., Maltby D.A., Inoue F. Endogenous catabolism is the major source of toxic metabolites in isovaleric acidemia. J. Pediatr. 1987;110:56–60. doi: 10.1016/s0022-3476(87)80288-3. [DOI] [PubMed] [Google Scholar]
- 52.Ensenauer R., Vockley J., Willard J.M., Huey J.C., Sass J.O., Edland S.D., Burton B.K., Berry S.A., Santer R., Grunert S., Koch H.G., Marquardt I., Rinaldo P., Hahn S., Matern D. A common mutation is associated with a mild, potentially asymptomatic phenotype in patients with isovaleric acidemia diagnosed by newborn screening. Am. J. Hum. Genet. 2004;75:1136–1142. doi: 10.1086/426318. [DOI] [PMC free article] [PubMed] [Google Scholar]


