Abstract
Although p53 is not essential for normal embryonic development, it plays a pivotal role in many biological and pathological processes, including cell fate determination-dependent and independent events and diseases. The expression and activity of p53 largely depend on its two biological inhibitors, MDM2 and MDMX, which have been shown to form a complex in order to tightly control p53 to an undetectable level during early stages of embryonic development. However, more delicate studies using conditional gene-modification mouse models show that MDM2 and MDMX may function separately or synergistically on p53 regulation during later stages of embryonic development and adulthood in a cell and tissue-specific manner. Here, we report the role of the MDM2/MDMX-p53 pathway in pancreatic islet morphogenesis and functional maintenance, using mouse lines with specific deletion of MDM2 or MDMX in pancreatic endocrine progenitor cells. Interestingly, deletion of MDM2 results in defects of embryonic endocrine pancreas development, followed by neonatal hyperglycemia and lethality, by inducing pancreatic progenitor cell apoptosis and inhibiting cell proliferation. However, unlike MDM2-knockout animals, mice lacking MDMX in endocrine progenitor cells develop normally. But, surprisingly, the survival rate of adult MDMX-knockout mice drastically declines compared to control mice, as blockage of neonatal development of endocrine pancreas by inhibition of cell proliferation and subsequent islet dysfunction and hyperglycemia eventually lead to type 1 diabetes-like disease with advanced diabetic nephropathy. As expected, both MDM2 and MDMX deletion-caused pancreatic defects are completely rescued by loss of p53, verifying the crucial role of the MDM2 and/or MDMX in regulating p53 in a spatio-temporal manner during the development, functional maintenance, and related disease progress of endocrine pancreas. Also, our study suggests a possible mouse model of advanced diabetic nephropathy, which is complementary to other established diabetic models and perhaps useful for the development of anti-diabetes therapies.
Keywords: MDM2, MDMX, p53, pancreas development, pancreas function, type 1-diabetes, high blood glucose level, metabolism, glucose tolerance
INTRODUCTION
Pancreatic development is a highly dynamic process of successive steps involving the maturation of endocrine, exocrine, and ductal cells stemmed from identical progenitor cells of the embryonic endoderm. For instance, in mice, the embryonic pancreas arises from one dorsal and two ventral buds at around embryonic day 9 (E9). The two buds develop quickly in response to signals from adjacent non-pancreatic tissues, and the progenitor cells begin to differentiate into different types of pancreatic cells by E9.5. Endocrine progenitor cells then travel into the neighboring mesenchyme to form clusters while exocrine progenitor cells differentiate into acinar cells to form acini, and the dorsal and ventral ducts grow to fuse and establish communication by E16–17, resulting in the formation of highly branched structures of pancreas through further growth and maturation (Cano et al., 2007; O’Dowd and Stocker, 2013). In rodents, pancreatic islets continue developing throughout neonatal life until weaning, which may involve a short peak of developmental apoptosis taking place around two weeks after birth in order to delete some β-cells and replace them with new islets (Hill and Duvillie, 2000; O’Dowd and Stocker, 2013). This apoptosis-mediated step is critical for the normal metabolism in future adult life, as the newly generated cell population is sensitive to glucose for glucose-stimulated insulin secretion (GSIS). A similar apoptotic event of β-cells has also been identified in the late stage of human fetus development in order to obtain the glucose sensitivity of β-cells (Hill and Duvillie, 2000).
Throughout the whole process of pancreatic morphogenesis, all the different stages are tightly regulated by a complex cascade of transcriptional factors that coordinates cell fate determination, cell differentiation, cell growth, and unique cell phenotype maintenance, and most of the factors are expressed and function at multiple levels (Wilson et al., 2003). For example, duodenal homeobox gene 1 (pdx1) encodes the PDX1 transcriptional factor that is induced earliest (~E8.5) in the pre-pancreatic endoderm by the instructive signals from adjacent tissues, with expression becoming limited to endocrine cells at ~E15.5 and maintained in adult insulin-expressing β-cells, functioning as a major regulator of pancreatic development and β-cell differentiation (Cano et al., 2007; Wilson et al., 2003). Another transcriptional factor Pax6 (paired box homeodomain), originally identified during neural development (Glaser et al., 1994), is also essential for endocrine differentiation, cell subtype specification and maintenance, and normal transcription of pancreatic hormone genes including glucagon, insulin, and somatostatin (O’Dowd and Stocker, 2013; Wilson et al., 2003). Ngn3 is expressed in endocrine progenitor cells and essential for the differentiation of the endocrine lineage (Cano et al., 2007; O’Dowd and Stocker, 2013), while PTF1A/p48 is detected in progenitors of all three cell types (endocrine, exocrine, and ductal cells) and becomes restricted to acinar precursor cells by E13.5, playing an exocrine-specific role in mid-pancreatic development (Cano et al., 2007; O’Dowd and Stocker, 2013). Mouse genetic studies indicate that mutations of these genes cause pancreatic agenesis, hypoplasia, and/or diabetic phenotypes (Cano et al., 2007; Hill and Duvillie, 2000; Wilson et al., 2003). Consistently in human beings, genetic mutations of the key transcriptional factors, such as PDX1, PAX6, PTF1A, INS, etc., have been linked to neonatal diabetes, owing to developmental failure of the pancreas, β-cell apoptosis, and/or dysregulation of insulin processing and release (Aguilar-Bryan and Bryan, 2008; Cano et al., 2007; Garin et al., 2010; Hill and Duvillie, 2000; Shield, 2007; Wilson et al., 2003).
To suppress potentially abnormal cell growth and tumorigenesis, p53 serves as the most important transcription factor by monitoring cell cycle arrest, cell death, autophagy and senescence, through transactivation of many cell death and survival related protein-encoding genes, or other transcription-independent mechanisms (Boominathan, 2010; Lane and Levine, 2010) (Leung and Sharp, 2010). But beyond that, p53 has also been linked to other hyperplasia-independent cell events and diseases. In humans, the P72R single nucleotide polymorphism (SNP) of p53 is associated with increased risk of type 2 diabetes (Bitti et al., 2011; Burgdorf et al., 2011; Gloria-Bottini et al., 2011), which is supported by a recent study demonstrating that mice with the R72 variant of p53 develop more severe metabolic syndrome and type 2 diabetic phenotype under High-fat diet (HFD) (Kung et al., 2016). Increased DNA damage and p53 activity have also been established to contribute to glucokinase induced β-cell death in type 2 diabetes (Tornovsky-Babeay et al., 2014). However, surprisingly, deletion of p21, a p53 target involved in cell cycle arrest, exacerbates the development of HFD-induced diabetes, and activation of p21 by Nutlin-3a, a p53 activator by inhibiting p53-MDM2 interaction, protects from diet-induced diabetic phenotype (Mihailidou et al., 2015). In addition, serum anti-p53 antibodies have been detected in some patients with type 1 diabetes (Di Cesare et al., 2001), and p53 was shown to reduce the occurrence of autoimmune diabetes in mice by inhibiting proinflammatory cytokines and STAT-1 (Zheng et al., 2005). These studies suggest that tight control of p53 level and activity is crucial for pancreatic development, function, and pathology.
In most cells under normal conditions, two physiological protein inhibitors of p53, MDM2 and MDMX, are crucially important for restraining p53 expression to an undetectable level and protect cells from p53’s detrimental effects (Chen et al., 1993; Kawai et al., 2007; Kruse and Gu, 2009; Linke et al., 2008; Momand et al., 1992; Oliner et al., 1993; Wade et al., 2010). Both MDM2 and MDMX bind to the transactivation domain of p53 with similar affinities to directly inhibit its transcriptional activity (Shadfan et al., 2012). Furthermore, MDM2 also serves as an E3 ubiquitin ligase and mediates p53 protein degradation (Kruse and Gu, 2009). Although loss of p53 exerts no significantly adverse effects on embryonic development (Donehower et al., 1992; Jacks et al., 1994), both MDM2 and MDMX null mice die in the early stages of embryonic development due to p53 over-activation. It has been indicated that MDM2 forms a complex with MDMX through their C-terminal RING domains to inhibit p53 during early embryogenesis (Huang et al., 2011; Pant et al., 2011). However, during later developmental stages of organogenesis and adult life, MDM2 and MDMX may independently or cooperatively regulate p53 to delicately control cell proliferation, differentiation, and death so that each organ maintains unique shape and function, which is supported by the more severe phenotypes observed with conditional deletion of MDM2 in lens epithelium (Zhang et al., 2014), cardiomyocytes (Xiong et al., 2007), central nervous system (Xiong et al., 2006), smooth muscle cells (Boesten et al., 2006), and erythroid progenitor cells (Maetens et al., 2007), compared to those with loss of MDMX. Considering the participation of cell proliferation, differentiation, and apoptosis in the pancreatic development and the potential involvement of p53 in pancreas-related diseases as described above, we hypothesized that MDM2 and MDMX could also be critical for controlling these p53-dependent events during pancreatic development and diabetes initiation and progression. To address this hypothesis, we crossed mice containing MDM2 or MDMX conditional alleles with mice expressing Cre beginning at E9.5 under the control of the Pax6 enhancer specifically in both pancreatic endocrine progenitor cells and lens epithelial progenitors (LE-Cre) (Ashery-Padan et al., 2000; Ashery-Padan et al., 2004). Interestingly, loss of MDM2 impaired the embryonic development of endocrine pancreas and resulted in neonatal lethality. However, MDMX was not required for islet development at embryonic stage. Instead, MDMX deletion caused type 1 diabetic phenotypes of adult mice as a result of strikingly shrunken islet area, leading to severe diabetic nephropathy and significantly shortened survival in adult mice. These results provide new evidence for the tissue specific difference of the roles MDM2 and MDMX play in organ development, functional maintenance, and diseases. Also, we found that homozygous loss of p53 alleles could completely rescue the defects of pancreas caused by deleting either MDM2 or MDMX, indicating that tight regulation of p53 by both MDM2 and MDMX is crucial for islet development and functional maintenance. In addition, our study provides a promising and stable mouse model for late-stage diabetic nephropathy.
MATERIALS AND METHODS
Antibodies and reagents
Antibodies used in the study include mouse monoclonal anti-MDM2 (SMP14, Santa Cruz, 1:50 for immunostaining), rabbit polyclonal anti-p53 (fl393, Santa Cruz, 1:100 for immunostaining), mouse monoclonal anti-p53 (NCL-p53–505, Novocastra, 1:50 for immunostaining), rabbit polyclonal anti-Insulin (ab63820, Abcam, 1:200 for immunostaining), mouse monoclonal anti-Glucagon (K79Bb10, Sigma, 1:200 for immunostaining), rabbit polyclonal anti-Pdx1 (PRB-278P, Convance, 1:200 for immunostaining), mouse monoclonal anti-Ki67 (BD, 1:100 for immunostaining), and so on. The fluorescein In situ cell death detection kit was purchased from Roche.
Mouse lines and ethics statement
The MDM2f/f, MDMXf/f, p53f/f and Le-Cre transgene mouse lines were described previously (Ashery-Padan et al., 2000; Grier et al., 2006; Grier et al., 2002; Jonkers et al., 2001; Marino et al., 2000), and individually maintained with homozygotes or heterozygotes. Briefly, the Le-Cre transgene expression starts at around E9, and the Cre-mediated recombination is evident since E9.5 in most cells of the surface ectoderm, and subsequently in the SE-derived eye structures and pancreatic endocrine precursors (Ashery-Padan et al., 2000). To obtain the mouse lines with MDM2 or MDMX specific deletion in developing pancreas, Le-Cre mice were crossed with either MDM2f/f or MDMXf/f homozygotes, then Le-Cre; MDM2f/+ or Le-Cre; MDMXf/+ heterozygous offsprings were further crossed with MDM2f/f or MDMXf/f homozygotes, respectively. Le-Cre; MDM2f/f and Le-Cre; MDMXf/f mice were compared with MDM2 f/+ and Le-Cre; MDM2 f/+ mice, or MDMX f/+ and Le-Cre; MDMX f/+, respectively. To obtain Le-Cre; MDM2f/f; p53f/f mice, Le-Cre; MDM2f/+ were first crossed with p53f/f mice, then Le-Cre; MDM2 f/+; p53f/+ offsprings were further crossed with MDMX f/+; p53f/+ offspring. To obtain Le-Cre; MDMXf/f; p53f/f mice, Le-Cre; MDMXf/f mice were first crossed with p53f/f mice, then Le-Cre; MDM2 f/+; p53f/+ offsprings were further crossed with MDMX f/+; p53f/+ offsprings. The mice from these breeders were recruited in the experiments as shown in Fig. 7, including MDM2 f/f (or MDMX f/f); p53f/f (control groups), and Le-Cre; MDM2 f/f (or MDMX f/f); p53f/f mice. Their genotypes were determined by PCR analysis of the genomic DNA extracted from their tails. For the embryo collection, noon of the day of vaginal plug observation was considered as E0.5 of embryogenesis. All the animals used in this study were maintained in mixed genetic background.
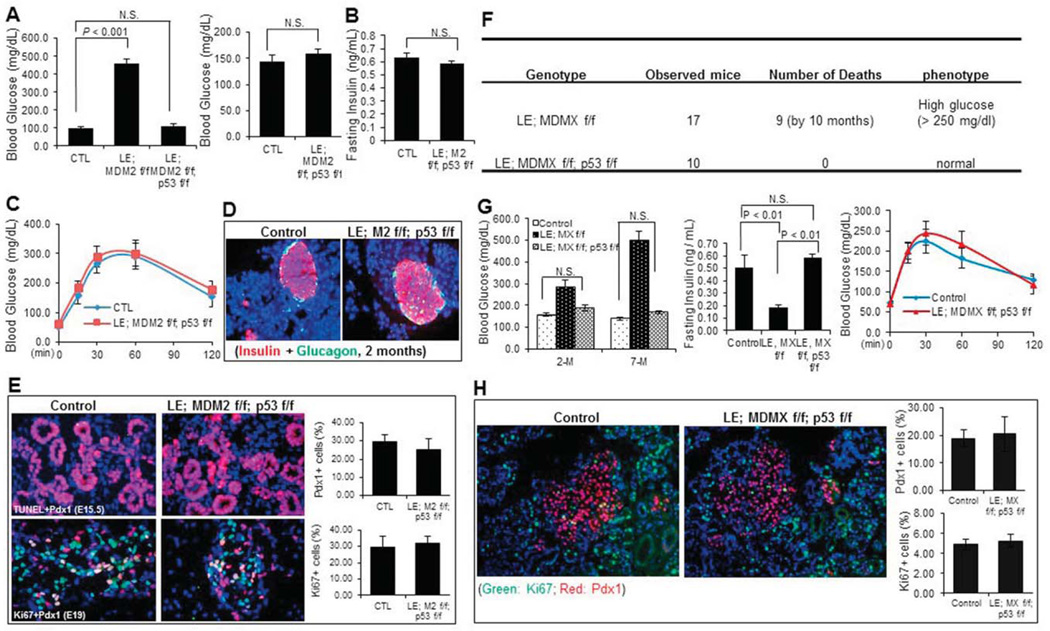
Fig. 7. p53 deletion rescues the pancreatic defects caused by MDM2 or MDMX deletion.
A) Random blood Glucose level of neonatal mice (left, n=10), and 2-month-old adult male mice with indicated genotypes was measured at least 3 times on different day (right, n=9). B) Fasting Insulin level of 2-month-old male MDM2/p53-double knockout and control mice was measured (n=9). C) GTT was performed with 2-month-old male MDM2/p53-double knockout and control mice (n=9). D) Representative images of immunostaining with anti-Insulin and anti-Glucagon antibodies on pancreas cryo-sections of 2-month-old male MDM2/p53-double knockout and control mice. E) Representative images of TUNEL staining (upper), and co-immunostaining with anti-Ki67 and anti-PDX1 antibodies (lower) on pancreas cryo-sections of MDM2/p53 double knockout and control mice at the indicated embryonic stages. The percentage of PDX1 positive cells in pancreas and percentage of Ki67 positive cells in PDX1 positive cell population were calculated, respectively (right panels). F) Loss of p53 completely rescued the decreased survival rate of MDMX-conditional male mice. G) Random blood Glucose level (left panel) of 2-and 7-month-old adult male mice with indicated genotypes was measured at least 3 times on different days (n=8~10 / group). Fasting Insulin level (middle panel) of 7-month-old male mice with indicated genotypes was measured (n=6 / group). GTT (right panel) was performed with 2-month-old male MDMX/p53-double knockout and control mice (n=8~9 / group). H) Representative images of co-immunostaining with anti-Ki67 and anti-PDX1 antibodies on pancreas cryo-sections of MDMX/p53 double knockout and control mice at postnatal day 10. The percentage of PDX1 positive cells in pancreas and percentage of Ki67 positive cells in PDX1 positive cell population were calculated, respectively (right panels).
All animal experiments were conducted in accordance with the National Institutes Health “Guide for the Care and Use of Laboratory Animals” and were approved by the Institutional Animal Care and Use Committee at Tulane University School of Medicine and our animal protocol number is 4257R.
Glucose tolerance test (GTT)
GTT was performed with age and gender-matched adult mice with different genotypes (as indicated in the result section), and at least 8 mice per group were recruited. Briefly, mice for test were transferred to new cages and fasted for 16 hours (overnight). In the following morning the mice were prepared for GTT: they were weighed. The tails were nicked with a fresh razor blade by a horizontal cut of the very ends, and around 35–50 µl of blood per mouse was collected by gently massaging the tail. Then baseline blood glucose level was measured using a glucometer. Then 2 grams/kg body weight of 20% D-glucose (0.2g/ml) was injected into the intraperitoneal cavity. Blood glucose was measured with the glucometer at 15, 30, 60, and 120 minutes after injection. Glucose injections and blood glucose measurement were timed to take approximately the same amount of time per animal to ensure the accuracy of the test. Measurement of fasting immunoreactive insulin levels was done as follows: whole blood samples collected as described above were centrifuged at 2,000×g for 20 minutes, and the serum were transferred to a clean tube. Fasting insulin levels were tested using an ELISA assay kit (Crystal Chem INC, Catalog #90082) with mouse insulin as a standard according to the protocol that comes with the kit.
Insulin tolerance test (ITT)
ITT was performed with 2-month-old MDMX- knockout and control mice, and 9–10 mice per group were recruited. Briefly, food was removed for 6 hr (from 8:00AM to 2:00PM) by putting the mice to be tested in new cages. Insulin was prepared at 0.15 U/ml with PBS in advance. After fasting the mice were weighted and blood glucose of each mouse was determined by glucometer to get the value for 0 time point. Then 0.75 U/kg body weight of Insulin was intraperitoneally injected into the mice, and blood glucose level was measured at 15, 30, 60, and 90 minutes after injection. The data was presented by percent change from fasting glucose.
H&E staining
Mouse embryos were isolated and rinsed with PBS, followed by fixation with 4% PFA overnight, serial dehydration and paraffin embedding. Paraffin blocks were then cut into 8µm-thick sections, and subjected to hematoxylin/eosin staining to determine possible morphological changes. Basically, 8µM-thick tissue sections underwent regular deparaffinization and rehydration, and then were dipped into hematoxylin solution for 3 min and Eosin solution for 45 sec, followed by dehydration in 70%, 95% and 100% ethanol and xylene. Slides were finally covered with permanent mounting medium (Vector Laboratories, CA) and stored at RT, and scanned using a microscope equipped with a digital camera (Zeiss 200) with a 10× objective.
Periodic acid-Schiff (PAS) staining
PAS staining was used for detection of glycogen deposition in kidney tissues of different mice as indicated in the result section. Basically, formalin-fixed, paraffin-embedded kidney tissue sections were deparaffinized, hydrated to water and oxidized in 0.5% periodic acid solution for 5 minutes. After rinsed in distilled water, the sections were placed in Schiff reagent for 15 minutes, followed by being washed in tap water for 5 minutes. Then the sections were counterstained in hematoxylin for 1 minute and washed in running water for 5 minutes. Slides were finally covered with permanent mounting medium (Vector Laboratories, CA) after sequential dehydration in 70%, 95% and 100% ethanol and xylene, and stored at RT. The stained sections were scanned using a microscope equipped with a digital camera (Zeiss 200) with a 20× objective. Glycogen is usually demonstrated as red/purple color.
Immunohistochemistry
Immunohistochemistry was performed with 6µM-thick cryosections. Briefly, mouse embryos were fixed in 4% PFA, put into 30% sucrose overnight, embedded with O.C.T, and cut into 6µm-thick sections. The cryosections were boiled in fresh citrate buffer (10mM Sodium Citrate, 0.05% Tween-20, pH 6.0) using a steamer for 40 min for antigen retrieval. After being blocked with blocking buffer (5% Goat serum, 0.3% TritonX-100 in 1×PBS) for 1h at RT, the sections were incubated with primary antibodies in a humid chamber at 4°C overnight followed by incubation with Alexa 488 or 594-conjugated goat-anti mouse or goat-anti rabbit IgG secondary antibodies (Biorad) at RT for 30 min, then the sections were washed with 1× PBS for 3 times, and covered with anti-fading buffer. For some immunofluorescence staining, the sections were incubated with HRP-conjugated goat-anti mouse or goat-anti rabbit IgG secondary antibody after incubation with a primary antibody, and then subjected to FITC tyramide signal amplification (TSA)-plus or cyanine 3 (Cy3) TSA-plus (PerkinElmer) incubation for 10 min to amplify the signal. Images were obtained under a fluorescence microscope (Zeiss 200) with 10× or 20× objective. Cell proliferation was measured as the ratio of Ki67-positive cells to PDX1-positive endocrine cells by Image J software. Two parameters were used to determine the islet area: The ratio of total islets area (indicated by Insulin positive area) to total pancreas area per section was measured using Image J software. At least 3 sections per mouse were included. Also, the average of islet area in 5 sections of pancreas per mouse that were obtained from the center part of the pancreas, was calculated by Image J software. One-way ANOVA analysis was performed, and four mice per group were recruited to obtain statistical significance.
TUNEL staining
Apoptosis was also determined by in situ TUNEL staining, using the Fluorescein In situ cell death detection kit (Roche) according to manufacturer’s instructions. Briefly, the cryosections were permeabilized in permeabilisation buffer (0.1% TritonX-100, 0.1% sodium citrate) at RT for 5min, followed by incubation with TUNEL reaction mixture at 37 °C for 1h, and mounted with anti-fading buffer after rinsing with PBS. Images were obtained under a fluorescence microscope equipped with a digital camera (Zeiss 200) with a 20× objective.
Reverse transcription and quantitative PCR (RT-qPCR) analyses
Total RNA was extracted from whole pancreatic tissue of 1-week-old control and MDMX-knockout mice using Trizol (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. Total RNAs (2µg) were used as templates for reverse transcription using poly-(T)20 primers and M-MLV reverse transcriptase (Promega, Madison, WI, USA). Quantitative PCR (qPCR) was conducted using SYBR Green Mix according to the manufacturer’s protocol (BioRad, Hercules, CA, USA). The primers for mouse MDMX are: 5’-AGCGGCCGTAAGTTTGCT −3’ and 5’- GCAGTTTTGGCCGCACC −3’. The primers for mouse p21 are: 5’- CCAGCAGAATAAAAGGTGCCACAGG −3’ and 5’-GCATCGCAATCACGGCGCAA −3’.
Statistics
Data were reported as means ± SEM with N being the sample size. Comparisons among different groups were conducted by using One-way ANOVA. Probability values of P<0.05 were considered statistically significant.
RESULTS
Loss of MDM2 in endocrine pancreas causes neonatal lethality
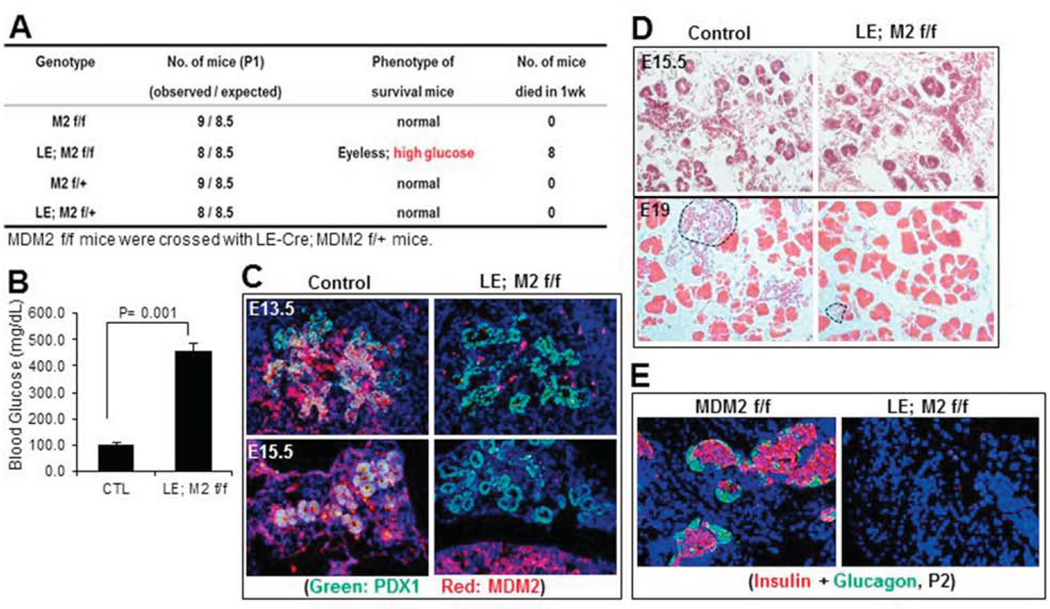
To obtain mice with MDM2 deletion specifically in developing endocrine pancreas, we first crossed Le-Cre; MDM2f/+ with MDM2f/f mice as described in the Methods section, and the genotypes of their offspring were determined by PCR analysis as shown in our previous study (Zhang et al., 2014). Four genetic types of pups including Le-Cre; MDM2f/f, Le-Cre; MDM2f/+, MDM2f/f and MDM2f/+, were obtained with a normal Mendelian ratio as shown in Fig. 1A, indicating that the deletion of MDM2 in developing pancreas does not cause embryonic lethality. However, all the Le-Cre; MDM2 f/f pups were eyeless (Zhang et al., 2014), developed hyperglycemia with random blood glucose level higher than 400 mg/dl (Fig. 1B), and died within one week after birth (Fig. 1A). In this study, we only focused on pancreatic organogenesis, as the lens phenotypes have been reported previously (Zhang et al., 2014). Through fluorescence immunostaining we confirmed the deletion of MDM2 specifically in embryonic pancreatic endocrine by concomitantly expressing LE-Cre (Fig. 1C). We found that deletion of MDM2 floxed alleles by Le-Cre resulted in severe endocrine dysmorphogenesis that could be the reason for neonatal lethality. As shown in Fig. 1D, at ~E15.5, Le-Cre; MDM2 f/f embryos displayed relatively similar structure of pancreas when compared to MDM2f/+, MDM2f/f or Le-Cre; MDM2f/+ mice (control), indicating branching and differentiation of endocrine and exocrine cells from the epithelial cells are normal in MDM2 knockout pancreas. At E19, ductal, acinar, and islet cells were clearly found in control pancreas; however, the islet area was dramatically shrunk in MDM2 deleted pancreas (Fig. 1D), which was further confirmed by immunofluorescence staining of insulin and glucagon showing the both insulin and glucagon positive cells were almost absent at around postnatal day 2 (P2) in Le-Cre; MDM2f/f pancreas, even though the surrounding non-endocrine structure appeared normal (Fig. 1E). Since there was no obvious pancreas defect observed in Le-Cre; MDM2f/+, MDM2f/+ and MDM2f/f (data not shown), we combined the three groups as a control afterward (Fig. 1B). Together, these results indicate that loss of MDM2 during embryogenesis causes irreversible defects of late-stage endocrine development, leading to neonatal diabetic phenotype that further causes neonatal lethality of MDM2-knockout mice.
Fig. 1. Loss of MDM2 in endocrine pancreas causes neonatal lethality due to late-stage embryonic developmental defects of endocrine pancreas.
A) Loss of MDM2 in endocrine pancreas causes neonatal lethality within 1 week after birth. B) Random blood glucose level of neonatal mice with the indicated genotypes was measured at least 3 times on different days (n=6 per group). C) Representative images of immunofluorescence staining with anti-PDX1 (green) and anti-MDM2 (red) antibodies on embryonic pancreas cryo-sections of MDM2-knockout or control mice at embryonic days 13.5 and 15.5 (E13.5 and E15.5). D) Representative images of H&E staining on E15.5 and E19 embryonic pancreas sections of MDM2-conditional knockout or control groups. Dashed areas in lower panel indicate pancreatic islets. E) Representative images of immunofluorescence staining with anti-Insulin and anti-Glucagon antibodies on pancreas cryo-sections of MDM2-knockout or control mice at postnatal day 2 (P2). Red color indicates Insulin and green color indicates Glucagon.
Loss of MDMX in endocrine pancreas significantly decreases the survival rate and develops type 1 diabetic phenotypes in adult mice
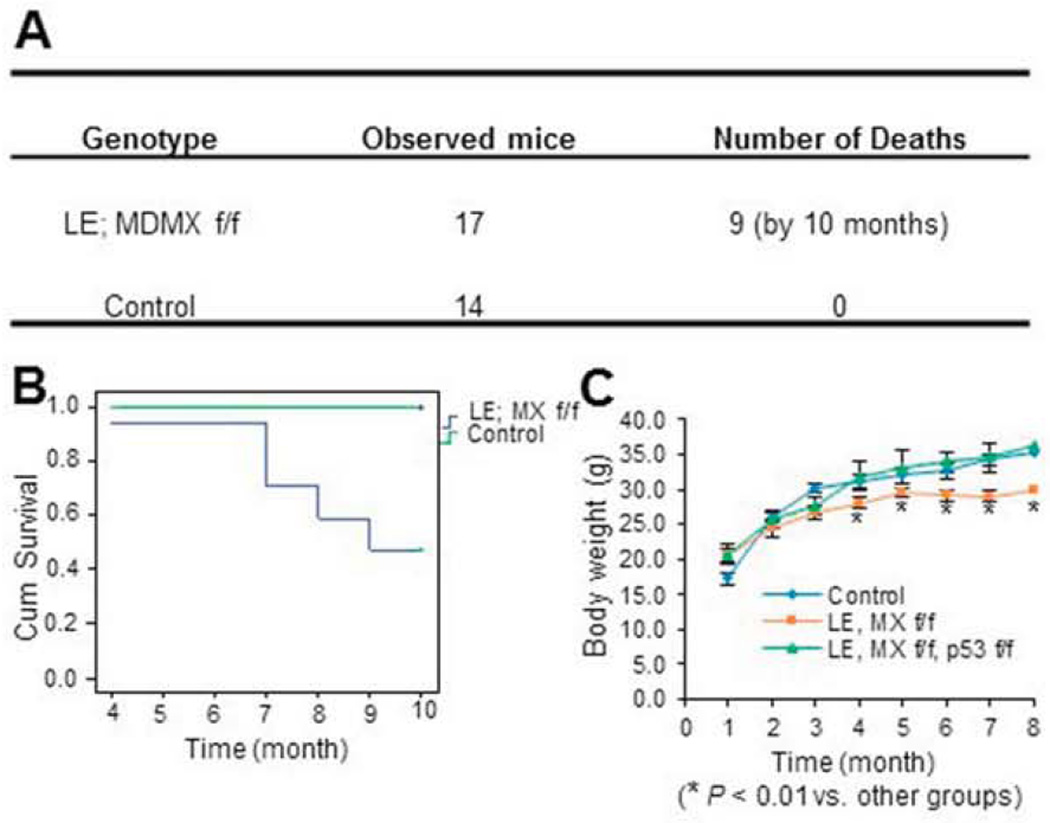
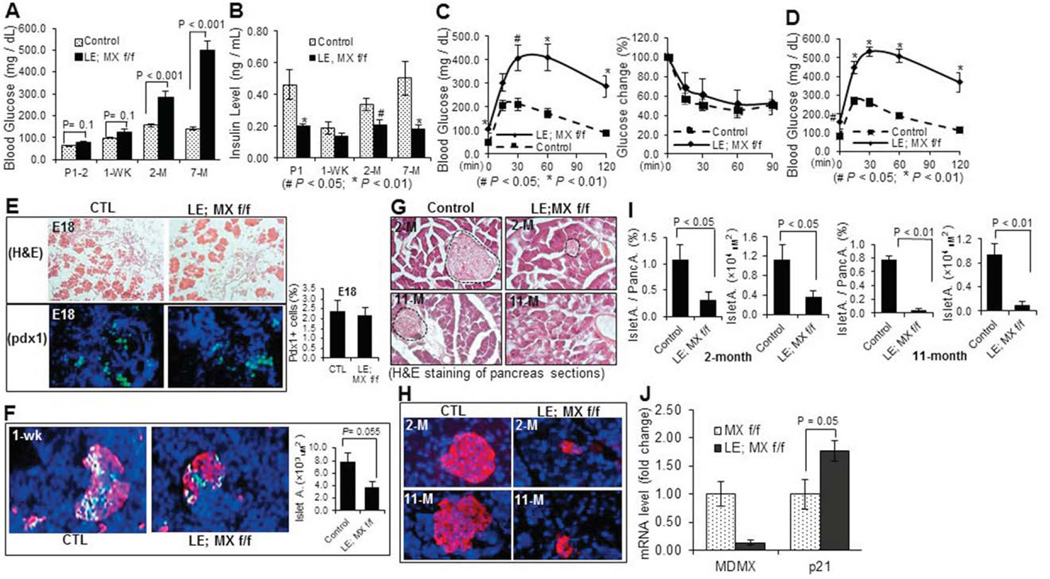
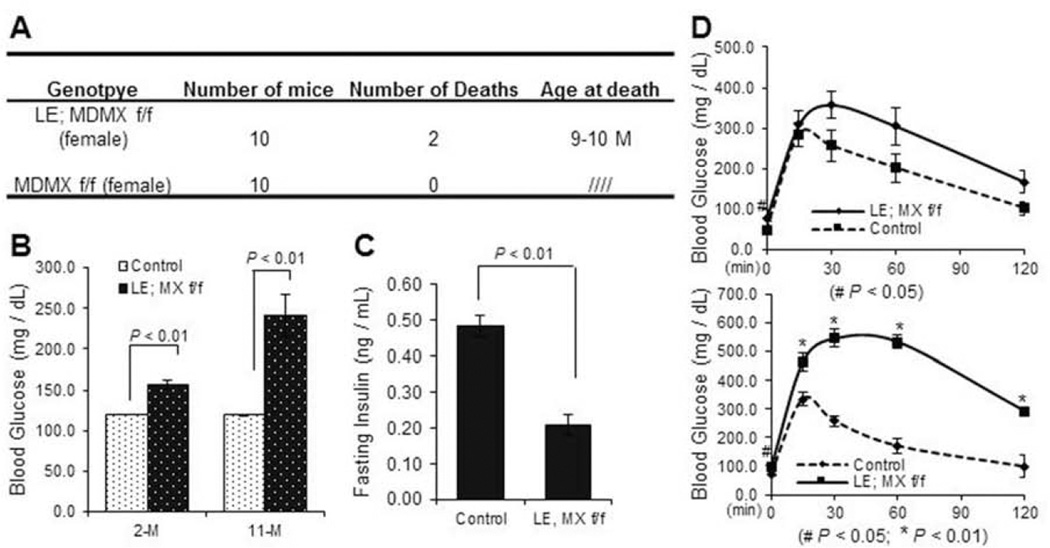
Since previous studies have demonstrated that loss of MDM2 or MDMX causes developmental defects with tissue specificity (Boesten et al., 2006; Grier et al., 2006; Hilliard et al., 2011; Xiong et al., 2007; Xiong et al., 2006), we also tried to determine whether endocrine pancreas would undergo dysmorphogenesis when the MDMX gene is deleted in the developing endocrine by using the same genetic approach as described above. After MDMXf/f mice were crossed with Le-Cre; MDMXf/+ mice, 4 genetic types of pups, including Le-Cre; MDMXf/f, Le-Cre; MDMXf/+, MDMXf/f, and MDMXf/+ were obtained with a normal Mendelian ratio (data not shown), and we pooled the latter three groups of male mice together as a control (Fig. 2A). Herein, all of the MDMX knockout related experiments were performed with male mice unless otherwise noted. The deletion of MDMX in endocrine pancreas was confirmed through RT-qPCR. As shown in Fig. 3J, the mRNA level of 1-week-old whole pancreatic tissue in Le-Cre; MDMX f/f group was significantly reduced to only a seventh of that in MDMX f/f group. The slight, but detectable, expression of MDMX mRNA in the entire knockout pancreas should be attributed to that in the unaffected non-islet tissue. In contrast to the MDM2-knockout mice, all the mice including Le-Cre; MDMXf/f group survived over the pups period and developed regularly. But, surprisingly, the survival rate of Le-Cre; MDMXf/f mice started to decrease at ~4 months, and significantly reduced to below 50% when they reached the age of 10 months (Figs. 2A and B). In addition, the average body weight of MDMX knockout mice was also 20–25% lower than that of the control group by 8-month-old with statistical significance (Fig. 2C). To decipher the underlying reason for the lower body weight and increased mortality of adult MDMX-deleted mice, we further examined the potential pancreatic phenotypes of the mice. We observed that the bedding in the cages housing MDMX-knockout mice needed to be replaced every day due to excessive urination (data not shown). The random glucose levels of MDMX-knockout mice were drastically increased to about 300 mg/dl at 2 months of age, and even reached 500 mg/dl at 7-month old (Fig. 3A). Also, the fasting glucose level of MDMX-knockouts was about 2-fold higher than that of controls (left panel of Fig. 3C, and Fig. 3D), accompanying with a significantly lower serum insulin level in adult knockout mice (only 30–60% of that of control group), as shown in Fig. 3B. From GTT assay (left panel of Fig. 3C, and Fig. 3D), the blood glucose level of the knockout group still stayed at 300–400 mg/dl 2 hr after administration of D-glucose solution at both 2-month and 7-month-old ages, which is 3–4 fold higher than that of the control group, indicating a severely impaired glucose tolerance of MDMX-knockout mice. In addition, the insulin sensitivity of 2-month-old MDMX-knockout mice was normal, as indicated by ITT (right panel of Fig. 3C). We further performed H&E and/or immunofluorescence staining of the E18, 1-week-old and adult pancreas tissue sections (Figs. 3 E-H), and calculated the number of PDX1 positive cells and/or islet area (Figs. 3E, F and I). Although the structure of MDMX-knockout pancreas at E18 was normal with a normal PDX1 positive cell population (Fig. 3E), surprisingly, the average islet area (including both β- and α-cells) of 1-week-old MDMX-deleted pups was merely 50% of that of the control group (Fig. 3F). Both H&E and anti-insulin immunostaining showed that the ratio of total islet area to total pancreas area in MDMX-deleted group is only 25%, and the average islet area in MDMX-deleted group is ~30% of that of the control group at 2-month-old age, which further declined to 5–10% at 11-month age (Figs. 3G–I). Furthermore, we found that approximately half of the MDMX-knockout mice developed overt ascitic fluid with decreased activity by the age of 7–8 months (Suppl. 1A and data not shown), implying possible high glucose-mediated chronic renal damage. To confirm this, we then performed the histological analysis of kidneys. H&E staining on the kidney sections showed the pathological changes of global glomerulosclerosis, including glomerular hypertrophy, increased mesangial intercapillary matrix, increased thickness of the capillary walls, expanded glomerular capillaries (microaneurisms), decreased number of podocytes, increased thickness of the Bowman’s capsule basement membrane, and increased tubulointerstitial space (tubulointerstitial fibrosis) (Suppl. 1B). PAS staining also presented typical PAS positive nodules with varying sizes distributed in the same glomerulus, namely Kimmelstiel-Wilson nodules, exhibiting spherical shape with a central acellular area and being surrounded by a ring of cells, as shown in Suppl. 1C. We also evaluated the potential pancreatic defects of female MDMX-conditional knockout mice. As shown in Fig. 4, the random Glucose level of MDMX-deleted female mice was about 150 mg/dl at 2-month-old, and increased to about 240 mg/dl at 11-month-old, which is significantly higher than that of the control group (Fig. 4B), along with higher fasting-glucose level in MDMX-deleted female mice (Fig. 4D). Furthermore, MDMX deletion caused significantly lower fasting-insulin level in 11-month-old female mice (Fig. 4C), and slightly impaired glucose tolerance in young adults, which was further deteriorated in older adults (Fig. 4D). These results suggest a relatively milder diabetes phenotype developed in MDMX-knockout female adults compared to that in males. Consistently, only 20% of female adults died from severe diabetic phenotypes (Fig. 4A). Together, these results reveal that loss of MDMX in endocrine pancreas causes severe type 1 diabetic phenotype with diabetic nephropathy developed in the adult mice, rather than arrests the embryonic development of endocrine pancreas, which eventually leads to early adult lethality of some MDMX-knockout mice.
Fig. 2. Loss of MDMX in endocrine pancreas significantly reduces mouse survival rate.
A) Loss of MDMX in endocrine pancreas significantly increased mortality of adult mice. B) Survival curve of MDMX-conditional knockout and control mice by the age of 10 months. 14 ~17 mice per group were recruited. C) Body weight curve of mice with the indicated genotypes by the age of 8 months (n=14–17 per group).
Fig. 3. Loss of MDMX in endocrine pancreas results in type 1 diabetic phenotype in adult mice without causing defects in embryonic pancreas.
A) Random Glucose level of MDMX-deleted and control mice at the indicated ages was measured at least 3 times on different days (n=9~14 / group). B) Random Insulin level of neonatal mice at P2 or fasting insulin level of adult male mice were measured with tail blood, and shown by fold change to a control group (n=5~6 / group). C) GTT (left) and ITT (right) were performed with MDMX- knockout and control mice at the age of 2 months (n=9 or 10 / group). D) GTT was performed with MDMX- knockout and control mice at the age of 7 months (n=9 or 10 / group). E) Representative images of H&E staining (upper) and immunostaining with anti-PDX1 antibody (lower) on E18 pancreas sections. PDX1 positive cells were indicated by green color, and counted under microscope (right penal). F) Representative images of co-immunostaining with anti-Insulin (red) and anti-Glucagon (green) antibodies, on pancreas cryo-sections of MDMX-knockout and control mice at 1-week-old. Average islet area was calculated based on the immunostaining (right panel). G) Representative images of H&E staining and H) immunostaining with anti-Insulin antibody, on pancreas sections of MDMX-knockout and control mice at the age of 2 and 7 months. Dashed areas in G indicate pancreatic islets. I) Ratio of islet area to total pancreas area, and average islet area of adult mice were calculated based on the immunostaining of Insulin as shown in H). J) The mRNA level of MDMX and p21 was measured by RT-qPCR in 1-week-old pancreas (n=3 / group).
Fig. 4. Loss of MDMX in endocrine pancreas causes diabetic phenotype in female mice, which appears to be milder than that in males.
A) Table showing that loss of MDMX in endocrine pancreas causes increased mortality in adult females by the age of 10 months. B) Random Glucose level of MDMX-deleted and control female mice was measured at the ages of 2- and 11-months at least 3 times on different days (n=7~10 / group). C) Fasting insulin level of 11-month-old female mice were measured, and indicated by fold change to a control group (n=5~6 / group). D) GTT was performed with MDMX- knockout and control female mice at the age of 2 and 11 months (n=11 / group).
Elevated p53 causes cell death and decreases proliferation in embryonic endocrine pancreas lacking MDM2, but inhibits proliferation in postnatal endocrine pancreas lacking MDMX
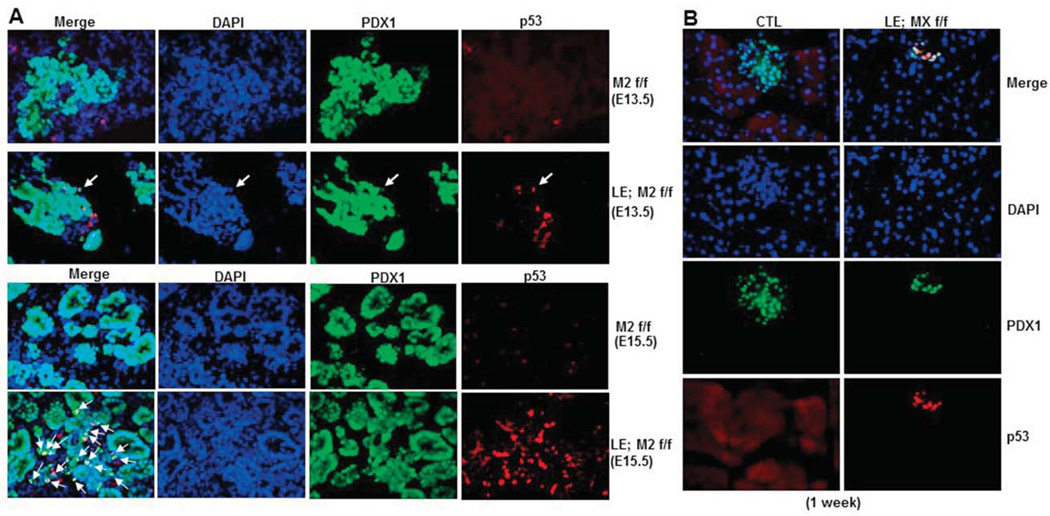
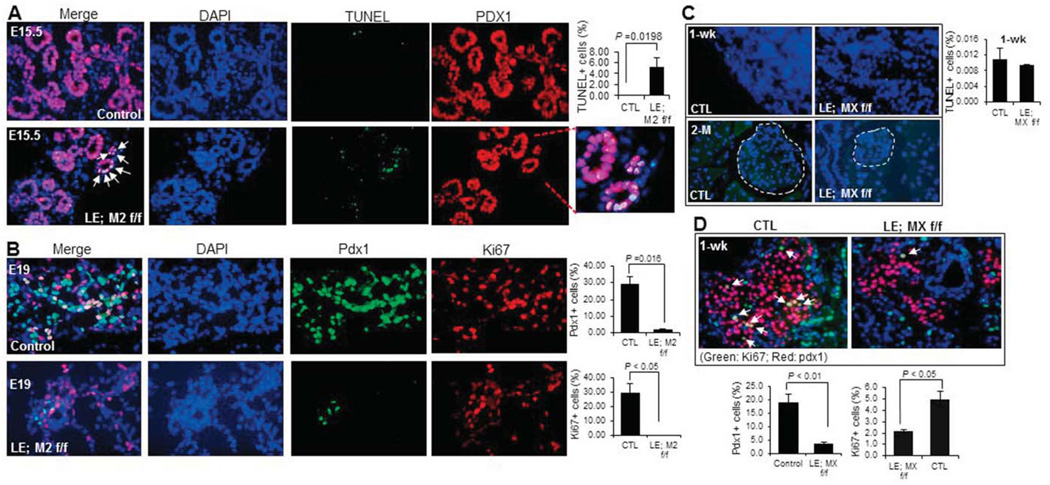
As described above, MDM2 and MDMX are two physiological inhibitors of p53 and work together as a complex to independently or together control p53 level and activity in a spatial-temporally specific manner. Thus, we wanted to determine if p53 is induced in the pancreatic tissue lacking either MDM2 or MDMX. Indeed, the immunofluorescence staining using a p53-specific antibody showed that the p53 level starts to increase at ~E13.5 in the PDX1-positive endocrine progenitor cells of MDM2 lacking mice, and reached a peak in the cells of E15.5 embryos, whereas p53 was undetectable in the control embryos (Fig. 5A). Interestingly, loss of MDMX did not induce p53 protein to a detectable level during embryonic stages (data not shown), but p53 protein expression was significantly enhanced in the PDX1-positive endocrine cells of 1-week-old MDMX-knockout mice (Fig. 5B), which still stayed at a high level in older mice (around 2-month-old) (data not shown). We also validated the activation of p53 in the MDMX-knockout pancreas by measuring the mRNA level of p21, a transcriptional target of p53. As shown in Fig. 3J, the mRNA level of p21 was almost doubled in 1-week-old knockout pancreas when compared to that in control group. To further investigate if p53 activation in MDM2 and MDMX deficient endocrine might lead to any cellular consequences, such as cell death and proliferation inhibition, we then assessed cell apoptosis by a TUNEL assay and cell proliferation rate by anti-Ki67 immunostaining. Indeed, cell apoptosis was remarkably induced to more than 5% in the PDX1-positive endocrine progenitor cells of MDM2-deleted mice at E15.5 (Fig. 6A), when the p53 level was high (Fig. 5A), compared to the control group that did not present any TUNEL-positive endocrine cells (Fig. 6A). Also, the Ki67-positive endocrine progenitor cells of MDM2-deleted mice were startlingly reduced to almost zero, which was still as high as 30% in the control group (Fig. 6B). These results validate that MDM2 deletion causes developmental defects of endocrine pancreas by inducing endocrine progenitor cell apoptosis and inhibiting cell proliferation. By sharp contrast, the number of TUNEL-positive cells was similar in 1-week-old endocrine between the control and MDMX-lacking groups (upper panel of Fig. 6C), and no obvious TUNEL-positive cells was identified in 2-month-old endocrine of both control and MDMX-lacking mice (lower panel of Fig. 6C), even though the p53 expression was detectable in the endocrine of MDMX-lacking mice at the ages of 1 week and 2 months (Fig. 5B). Higher p53 expression in 2-month-old endocrine pancreas of MDMX-knockout mice also exerted no effect on the cell proliferation (data not shown), which is reasonable since endocrine cells in the rodent still undergo developing during neonatal stage, but then become terminally differentiated after weaning. In consistence with the results of Fig. 3F, at 1-week-old, the number of Ki67-positive endocrine cells in MDMX-knockout mice was reduced to less than 40% of the level in control mice (Fig. 6D), which could be due to the increased p53 level and account for the declined number of PDX1-positive cells (Fig. 6D) and shrunken islet area (Fig. 3F) in 1-week-old MDMX-knockout mice, indicating MDMX deletion impedes the postnatal development of pancreas by inhibiting cell proliferation, leading to diabetic phenotypes in adults. Together, the above results demonstrate that p53 induction by MDM2 deletion occurs much earlier and is stronger than that by MDMX deletion in endocrine pancreas, which probably accounts for the distinct phenotypes observed in the mice with endocrine-specific deletion of MDM2 or MDMX.
Fig. 5. p53 expression is drastically induced in embryonic MDM2-deleted endocrine pancreatic progenitors and in endocrine pancreatic cells of adult MDMX-deleted mice. A).
Representative images of co-immunostaining with anti-p53 (red) and anti-PDX1 (green) antibodies on cryo-sections of E13.5 and E15.5 MDM2-knockout and control embryonic pancreas. B) Representative images of co-immunostaining with anti-p53 (red) and anti-PDX1 (green) antibodies on pancreas cryo-sections of 1-week-old MDMX-knockout and control mice.
Fig. 6. Elevated p53 causes cell death and decreases cell proliferation in embryonic endocrine pancreas lacking MDM2, but only inhibits proliferation in postnatal endocrine pancreas lacking MDMX.
A) Representative images of TUNEL staining (green) co-stained with anti-PDX1 antibody (red) on cryo-sections of E15.5 MDM2-knockout and control embryonic pancreas. Arrows indicate TUNEL positive cells. The percentage of TUNEL positive cells in PDX1 positive cell population was calculated (right). B) Representative images of co-immunostaining with anti-PDX1 (red) and anti-Ki67 (green) antibodies on cryo-sections of E19 MDM2-knockout and control embryonic pancreas. The percentage of PDX1 positive cells in pancreas and percentage of Ki67 positive cells in PDX1 positive cell population were calculated, respectively (right panels). C) Representative images of TUNEL staining on pancreas cryosections of 1-week- (upper) and 2-month-old (lower) MDMX-knockout and control mice. Dashed areas in lower panels indicate the pancreatic islets. The percentage of TUNEL positive cells in 1-weeek-old pancreas was calculated (right). D) Representative images of co-immunostaining with anti-PDX1 (red) and anti-Ki67 (green) antibodies on pancreas cryo-sections of 1-week-old MDMX-deleted and control mice. Arrows indicate PDX1 and Ki67 double-positive cells. The percentage of PDX1 positive cells in pancreas and percentage of Ki67 positive cells in PDX1 positive cell population were calculated, respectively (bottom panels).
Loss of p53 rescues mouse endocrine developmental defects and adult diabetes phenotypes caused by conditional MDM2 and MDMX deletion, respectively
As the most important target of MDM2 and MDMX, p53 activation nearly takes all the responsibility for the defects caused by MDM2 or MDMX deletion (Kruse and Gu, 2009; Wade et al., 2010). Since p53 was activated in the MDM2-deleted embryonic or MDMX-deleted postnatal pancreatic endocrine cells (Fig. 5), we next investigated if p53 activation might also be the cause for the developmental defects of pancreas and diabetic phenotypes in the MDM2- and MDMX-deleted mice, by introducing p53 floxed alleles into MDM2 or MDMX conditional knockout mice and deleting p53 gene by Le-Cre. The genotype of p53 floxed allele was identified by PCR as shown in our previous study (Zhang et al., 2014). As expected, the defects of endocrine pancreas caused by knocking out either MDM2 or MDMX were rescued by TP53 deletion. First, all the MDM2/p53-double knockout mice survived through the neonatal phase and developed without any obvious abnormalities (data not shown). Further data (Fig. 7A–D) showed that the random glucose level of both MDM2/p53 double knockout neonates and adults declined to the normal level, and the fasting-insulin level of 2-month-old MDM2/p53-double knockout mice was similar to that of the control group. Also, the adult MDM2/p53-double knockouts presented normal glucose tolerance. Co-immunostaining with anti-Insulin and anti-Glucagon on pancreatic tissue sections demonstrated normal size of islets with regular β-cell and α-cell distribution and secretion function in 2-month-old MDM2/p53-double knockout mice. We further examined the cell apoptosis and cell proliferation, and found that the endocrine progenitor cell apoptosis in MDM2-null mice at E15.5 is completely suppressed, and the endocrine progenitor cell proliferation at E19 restored to a normal level, by loss of p53 (Fig. 7E), leading to a normal PDX1 -positive cell population at E19 in MDM2/p53-double knockout mice (Fig. 7E). Likewise, no MDMX/p53-double conditional knockout mice were found dead by 10 months (Fig. 7F), and the body weight of these double knockout mice was comparable to that of control mice (Fig. 2C). Both random (left panel of Fig. 7G) and fasting glucose levels (right panel of Fig. 7G), and fasting insulin level (middle panel of Fig. 7G) in adult MDMX/p53-double knockout mice were completely rescued to the normal level. Also, GTT displayed substantially overlapping curve of glucose level over 2hr after D-Glucose administration in 2-month-old MDMX/p53-double knockout and control groups (right panel of Fig. 7G), indicating that type 1 diabetic phenotype due to the postnatal endocrine development abnormalities caused by MDMX deletion is rescued by loss of p53. Furthermore, immunostaining confirmed that the endocrine cell proliferation of MDMX/p53-double knockout mice during postnatal period was recovered to the level of control mice (Fig. 7H). In conclusion, these results demonstrate that the increased p53 level and activity, leading to induction of cell death and/or inhibition of proliferation, mainly accounts for the endocrine developmental abnormalities or adult diabetic phenotypes in either MDM2 or MDMX conditional null mice.
DISCUSSION
Mouse genetic studies have validated that MDM2 and MDMX have to form a hetero-complex to strictly control p53 during early embryogenesis (Huang et al., 2011; Pant et al., 2011). By binding to MDM2 through their RING domains, MDMX can prevent MDM2 auto-ubiquitination and convert monoubiquitination of p53 by MDM2 to polyubiquitination, leading to p53 degradation (Wang and Jiang, 2012). Also, both MDM2 and MDMX bind to p53 with similar affinity to block p53’s transcriptional activity (Shadfan et al., 2012). However, the hetero-complex of MDM2 and MDMX seems not to be essential during later stages of development and adulthood when they may independently or synergistically, not simply forming a complex, regulate p53 level, as suggested by several studies showing diverse phenotypes by conditional deletion of MDM2 or MDMX in different cells and organs (Boesten et al., 2006; Maetens et al., 2007; Xiong et al., 2007; Xiong et al., 2006). Our recent study also highlighted the differences in terms of the regulation of MDM2 or MDMX on p53 during organogenesis by demonstrating that MDM2-deletion causes lens developmental defects that took place earlier and were more severe than in MDMX-deleted lens (Zhang et al., 2014). Consistently, in the current study, we found that specific loss of MDM2 in pancreatic endocrine progenitor cells induces cell apoptosis and inhibits cell proliferation, severely delaying the embryonic development of islets and leading to neonatal lethality. In contrast, loss of MDMX in these cells exerted no significant effect on embryonic development of pancreas, with all the MDMX-lacking mice surviving to adulthood, but impeded the neonatal development and maturity of islets by inhibiting the proliferation of endocrine cells in postnatal pancreas, resulting in overt severe type 1 diabetic phenotypes, including drastically increased blood glucose level (hyperglycemia) and decreased insulin level (insulinopaenia), rigorously impaired glucose tolerance, significantly increased urination (polyuria), and weight loss, which eventually led to serious diabetic nephropathy and death. Notably, all the pancreatic abnormalities caused by either MDM2 or MDMX deletion were rescued by concomitant loss of p53. These findings corroborate that MDM2, rather than MDMX, is essential to control p53 at a minimal level during embryonic development of pancreatic islets, whereas MDMX becomes important for p53 regulation in terminally differentiated pancreatic endocrine cells, demonstrating the independent role of MDM2 and MDMX in p53 regulation during pancreatic morphogenesis and maturity, and implying the significance of the MDM2/MDMX-p53 pathway in the initiation and progress of type 1 diabetes, which is based on the absolutely reduced amount of β-cells and insulin production. Our results are also consistent with a study published recently, demonstrating that deletion of p53 ameliorates STZ-induced type 1 diabetes in mice through Parkin-mediated mitophagy (Hoshino et al., 2014).
In light of the aforementioned studies including ours, it is puzzling why the complex of MDM2/MDMX is critical for early embryogenesis, but less so for later embryogenesis and adult organs. One explanation would be that the MDM2/MDMX complex is dynamic, less stable, and highly regulated, and similarly the MDM2/MDMX/p53 pathway is a developing, evolutionary, acquired, adaptive and dynamic system during embryogenesis and organ function maintenance. At the early stage of embryogenesis, a period with simple environment, the major function of MDM2/MDMX is to restrain p53 at the minimal level so that cells can rapidly proliferate or sometimes decease in number (biological apoptosis) to ensure normal organogenesis, so MDM2 and MDMX form a complex to fully control p53 level during this early period. Once embryogenesis enters mid to late stage when all the progenitor cells have been roughly differentiated and determined even though tissues are still developmentally immature, MDM2 and MDMX start to function independently to meet different requirements of diverse progenitor cells on p53 regulation, with a more dominant role of MDM2 in such a relatively unpretentious environment (as suggested by the current study, our previous study on lens development (Zhang et al., 2014) and the other study on cardiac development (Xiong et al., 2007)). After birth, some functional organs and cells, such as hematopoietic cells, still have proliferative capability, some develop into terminally differentiated cells, such as cardiomyocytes, neurocytes and pancreatic endocrine cells, and some become regenerable cells, such as hepatocytes. All the different types of cells with different proliferative capacities require multiple machineries or signaling pathways to balance cell proliferation, death, and survival, in which p53 plays the most important role, and maintain normal organ function, particularly when encountering external and internal stresses. So along with the process of development and maturity, MDM2 and MDMX gradually acquire the capacity to precisely keep p53 at various levels, in an independent and/or synergistic manner (as suggested by the current study and previous studies (Xiong et al., 2007; Xiong et al., 2006; Zhang et al., 2014)), to give different cells more options to determine their fates under different stresses: irreversible death when stress is insurmountable, or survival following cell cycle arrest and DNA repair when stress is mild. Indeed, numerous studies have shown that different physiological and pathological stress signals can activate p53 by inactivating either MDM2 or MDMX via different molecules, such as ribosomal proteins on MDM2 (Antoniali et al., 2014; Lim et al., 2013; Nalabothula et al., 2010) or AMPK on MDMX (He et al., 2014), as further discussed below.
As mentioned above, clinical and mouse studies have linked the P72R SNP of p53 to type 2 diabetes, with more severe type 2 diabetic phenotypes developed in R72 variant carrying mice that presented higher transactivation of Tnf and Npc1l1 by p53 (Bitti et al., 2011; Burgdorf et al., 2011; Gloria-Bottini et al., 2011; Kung et al., 2016). p53 has been shown to reduce the occurrence of mouse autoimmune diabetes (Zheng et al., 2005) and to transactivate Ins2 gene to induce insulin generation in pancreatic α-cells (Fomina-Yadlin et al., 2012). In addition, a small molecule Nutlin-3a can prevent mice from diet-induced diabetes by specifically disrupting p53-MDM2 interaction and activating p53 and its target p21 (Mihailidou et al., 2015). These previous studies implicate the important role of p53 in diabetes and other metabolism-related diseases. However, the exact mechanism by which p53 is regulated during the progress of these disorders still remains to be deciphered. Our current study substantiates the pivotal management of p53 by MDM2 and/or MDMX during pancreatic development and diabetes progression. At least two pathways have been identified by our previous studies and other groups, through which the interaction between p53 and MDM2 or MDMX can be blocked. On one hand, when cells undergo some stresses, such as DNA damage and metabolic stress (for example glucose deprivation), MDMX can be phosphorylated by ChK1/2, AMPK, etc., thereby enhancing the binding of 14-3-3 to phosphorylated MDMX (He et al., 2014; Jin et al., 2006; Wang et al., 2009). On the other hand, excessive ribosomal proteins, such as L5 and L11, produced under ribosomal stress, can bind to MDM2 and destroy MDM2-p53 interaction (Dai and Lu, 2004; Sun et al., 2007; Zhang and Lu, 2009; Zhang et al., 2003). Both of these pathways eventually lead to the release of p53 from MDMX or MDM2, and subsequent activation of p53. Considering the involvement of proinflammatory cytokines and ER stress, two events that have been correlated to ribosomal biogenesis dysfunction and AMPK activity (Allagnat et al., 2013; Asahara et al., 2009; Brighenti et al., 2014; Ren et al., 2016; Yao et al., 2013), in both the trigger of β-cell autoimmunity during type 1 diabetes initiation (Padgett et al., 2013; Tersey et al., 2012) and the progression of type 2 diabetes (Asahara et al., 2009; Donath and Shoelson, 2011; Wellen and Hotamisligil, 2005), it is inferable that the above-mentioned AMPK-MDMX-p53 and ribosomal stress-MDM2-p53 pathways may participate in pancreas related disorders, such as diabetes mellitus. It would be quite enticing to address these hypotheses in the near future.
Diabetic nephropathy (DN) is a secondary complication of both type 1 and type 2 diabetes and the most common cause of end-stage renal disease in the western world (Chawla et al., 2010; Saran et al., 2015), which is pathologically characterized by glomerular hypertrophy, increased mesangial intercapillary matrix, progressive thickening of the glomerular capillary walls, Kimmelstiel- Wilson nodules distributed in the glomeruli that are the “virtually” pathognomonic of DN, and global glomerulosclerosis at the advanced stage (Betz and Conway, 2014; Soler et al., 2012). A number of animal models have been developed to provide valuable information for studying the pathogenesis, progression, involved signaling pathways, and potential therapeutic approaches of DN (Betz and Conway, 2014, 2016; Breyer et al., 2005; Kaur et al., 2014; Soler et al., 2012). However, most of the current animal models can only recapitulate the early stages of DN (Soler et al., 2012), and lack severe human-like histopathological features of advanced DN, such as nodules in the glomerular tuft and glomerulosclerosis (Breyer et al., 2005; Fujita et al., 2012; Soler et al., 2012), which has largely delayed the progress of research on DN. Here, by specifically deleting the MDMX gene in the pancreatic endocrine cells, we clearly observed the progressive phenotypes of type 1 diabetes, and more intriguingly and importantly, we established that the MDMX-lacking mice with diabetic phenotypes can survive 7–9 months, with gradually progressing DN that finally advanced to the end-stage displaying typical nodular glomerulosclerosis (Suppl. 1). We believe that the MDMX-conditional knockout mouse line may serve as a reliable and promising new model for studying advanced DN and developing new therapeutic strategies. The MDMX gene modification of this mouse line specifically occurs in pancreatic endocrine cells, so the resulting diabetes and subsequent advanced DN essentially initiate from the dysfunction of β-cells, which is similar to human type 1 diabetes, and excludes some artificial and unidentified influence from other organs, such as the non-natural changes in kidney eNOS-knockout mouse line may have due to the systemic eNOS deficiency (Takahashi and Harris, 2014), the direct and non-specific kidney toxicity STZ may cause (Betz and Conway, 2016; Breyer et al., 2005), and atypical features of DN as found in NONcNZO10/LtJ strain (Soler et al., 2012). Furthermore, unlike other models, such as STZ-treated mice that easily die from hypoglycemia or ketoacidosis (Kaur et al., 2014), this is a relatively stable, simple, and economical mouse line to be handled, requiring no special treatment. The reason why the blood glucose level of the MDMX-knockout mouse line was stably kept at 300–600 mg/dl (Fig. 3A and Fig. 6G) may be partially attributed to the concomitant knockout of MDMX in α-cells (Fig. 3F and data not shown), which could moderately compromise the hyperglycemia induced by β-cell death. In addition, it is noteworthy that female mice were more resistant to developing type 1 diabetes by MDMX deletion than the males, which aligns well with the gender differences observed in humans. More intensive analysis of the MDMX-conditional deletion-caused diabetes and end-stage DN is necessary to further evaluate the applicability of this mouse line for studying DN.
Obesity, metabolic syndrome, and type 2 diabetes are risk factors for cancer progression, such as breast cancer, hepatocarcinoma, colon cancer, etc (Shikata et al., 2013). This has been attributed to increased circulating insulin and insulin-like growth factors (Shikata et al., 2013), enhanced serum level and local production of estrogens in adipose tissue (Cleary and Grossmann, 2009), endocrine/paracrine/autocrine-influence of adipokines (Vona-Davis and Rose, 2007), chronic systemic low-grade inflammation (Doyle et al., 2012), hypercholesterolemia (Nelson et al., 2013), and other factors. Studies have also identified hyperglycemia as an independent risk factor for some human cancers (Ryu et al., 2014), which may be associated with the activation of Neuregulin-1 gene (Park et al., 2012). However, the exact mechanisms by which hyperglycemia promotes tumorigenesis are still largely unknown. It is difficult to independently determine the direct effects of hyperglycemia by using the models of metabolic syndrome or type 2 diabetes, as there might be other promiscuous factors. As described above, the currently established MDMX-conditional knockout mouse line with hyperglycemia and type 1 diabetes can also serve as an ideal model in this field, considering that the p53 expression is only enhanced in islet cells of the mice.
In summary, this study for the first time establishes the spatio-temporal role of MDM2 and MDMX in the endocrine pancreatic development. We substantiate that loss of MDM2 results in the embryonic developmental defects of endocrine pancreas, followed by neonatal hyperglycemia and lethality, by enhancing pancreatic progenitor apoptosis and decreasing cell proliferation. On the contrary, loss of MDMX exerts no remarkable effect on the embryonic development of endocrine pancreas, but blocks the neonatal development of endocrine pancreas by inhibiting cell proliferation, and causes the islet dysfunction, subsequent hyperglycemia and type 1 diabetes with advanced diabetic nephropathy, leading to increased mortality of adults. As expected, either MDM2 or MDMX deletion-caused defects can be rescued by loss of p53, validating the spatio-temporal regulation of p53 by MDM2 and MDMX during endocrine pancreas development and function maintenance. Attractively, our study also provides a promising mouse model for studying the advanced diabetic nephropathy and hyperglycemia-related tumorigenesis, which will largely benefit the development of new-type therapies on these diseases.
Supplementary Material
Acknowledgments
We thank Drs. Xin Zhang and Gigi Lozano for offering Le-cre mice and MDM2/MDMX mice with conditional alleles, respectively, Hongju Wu for active discussion, and Daniel Nguyen for proofreading. Hua Lu and Shelya X Zeng were supported in part by NIH-NCI grants R01 CA095441, R01CA172468, R01CA127724, R21CA190775, and R21CA201889.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Y.W.Z., and H.L. designed the experiments; Y.W.Z. conducted all of the studies; S.X.Z supervised animal work and management; Q.H. helped Y.W.Z. in GTT assays; Y.W.Z., S.X.Z., and H.L. analyzed the data; Y.W.Z., and H.L. composed the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
REFERENCES
- Aguilar-Bryan L, Bryan J. Neonatal diabetes mellitus. Endocrine reviews. 2008;29:265–291. doi: 10.1210/er.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allagnat F, Klee P, Cardozo AK, Meda P, Haefliger JA. Connexin36 contributes to INS-1E cells survival through modulation of cytokine-induced oxidative stress, ER stress and AMPK activity. Cell death and differentiation. 2013;20:1742–1752. doi: 10.1038/cdd.2013.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniali G, Lirussi L, Poletto M, Tell G. Emerging roles of the nucleolus in regulating the DNA damage response: the noncanonical DNA repair enzyme APE1/Ref-1 as a paradigmatical example. Antioxid Redox Signal. 2014;20:621–639. doi: 10.1089/ars.2013.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahara S, Matsuda T, Kido Y, Kasuga M. Increased ribosomal biogenesis induces pancreatic beta cell failure in mice model of type 2 diabetes. Biochemical and biophysical research communications. 2009;381:367–371. doi: 10.1016/j.bbrc.2009.02.047. [DOI] [PubMed] [Google Scholar]
- Ashery-Padan R, Marquardt T, Zhou X, Gruss P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000;14:2701–2711. doi: 10.1101/gad.184000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashery-Padan R, Zhou X, Marquardt T, Herrera P, Toube L, Berry A, Gruss P. Conditional inactivation of Pax6 in the pancreas causes early onset of diabetes. Dev Biol. 2004;269:479–488. doi: 10.1016/j.ydbio.2004.01.040. [DOI] [PubMed] [Google Scholar]
- Betz B, Conway BR. Recent advances in animal models of diabetic nephropathy. Nephron. Experimental nephrology. 2014;126:191–195. doi: 10.1159/000363300. [DOI] [PubMed] [Google Scholar]
- Betz B, Conway BR. An Update on the Use of Animal Models in Diabetic Nephropathy Research. Current diabetes reports. 2016;16:18. doi: 10.1007/s11892-015-0706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitti ML, Saccucci P, Capasso F, Piccinini S, Angelini F, Rapini N, Porcari M, Arcano S, Petrelli A, Del Duca E, Bottini E, Gloria-Bottini F. Genotypes of p53 codon 72 correlate with age at onset of type 1 diabetes in a sex-specific manner. Journal of pediatric endocrinology & metabolism : JPEM. 2011;24:437–439. doi: 10.1515/jpem.2011.058. [DOI] [PubMed] [Google Scholar]
- Boesten LS, Zadelaar SM, De Clercq S, Francoz S, van Nieuwkoop A, Biessen EA, Hofmann F, Feil S, Feil R, Jochemsen AG, Zurcher C, Havekes LM, van Vlijmen BJ, Marine JC. Mdm2, but not Mdm4, protects terminally differentiated smooth muscle cells from p53-mediated caspase-3-independent cell death. Cell death and differentiation. 2006;13:2089–2098. doi: 10.1038/sj.cdd.4401973. [DOI] [PubMed] [Google Scholar]
- Boominathan L. The guardians of the genome (p53, TA-p73, and TA-p63) are regulators of tumor suppressor miRNAs network. Cancer metastasis reviews. 2010;29:613–639. doi: 10.1007/s10555-010-9257-9. [DOI] [PubMed] [Google Scholar]
- Breyer MD, Bottinger E, Brosius FC, 3rd, Coffman TM, Harris RC, Heilig CW, Sharma K. Mouse models of diabetic nephropathy. Journal of the American Society of Nephrology : JASN. 2005;16:27–45. doi: 10.1681/ASN.2004080648. [DOI] [PubMed] [Google Scholar]
- Brighenti E, Calabrese C, Liguori G, Giannone FA, Trere D, Montanaro L, Derenzini M. Interleukin 6 downregulates p53 expression and activity by stimulating ribosome biogenesis: a new pathway connecting inflammation to cancer. Oncogene. 2014;33:4396–4406. doi: 10.1038/onc.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf KS, Grarup N, Justesen JM, Harder MN, Witte DR, Jorgensen T, Sandbaek A, Lauritzen T, Madsbad S, Hansen T, Pedersen O. Studies of the association of Arg72Pro of tumor suppressor protein p53 with type 2 diabetes in a combined analysis of 55,521 Europeans. PloS one. 2011;6:e15813. doi: 10.1371/journal.pone.0015813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano DA, Hebrok M, Zenker M. Pancreatic development and disease. Gastroenterology. 2007;132:745–762. doi: 10.1053/j.gastro.2006.12.054. [DOI] [PubMed] [Google Scholar]
- Chawla T, Sharma D, Singh A. Role of the renin angiotensin system in diabetic nephropathy. World journal of diabetes. 2010;1:141–145. doi: 10.4239/wjd.v1.i5.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Marechal V, Levine AJ. Mapping of the p53 and mdm-2 interaction domains. Molecular and cellular biology. 1993;13:4107–4114. doi: 10.1128/mcb.13.7.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–2542. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai MS, Lu H. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J Biol Chem. 2004;279:44475–44482. doi: 10.1074/jbc.M403722200. [DOI] [PubMed] [Google Scholar]
- Di Cesare E, Previti M, Lombardo F, Di Benedetto A, Mazzu N, Romano G, De Luca F, Lasco A, Cucinotta D. Serum anti-p53 autoantibodies in patients with type 1 diabetes. Annals of clinical and laboratory science. 2001;31:253–258. [PubMed] [Google Scholar]
- Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nature reviews. Immunology. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Doyle SL, Donohoe CL, Lysaght J, Reynolds JV. Visceral obesity, metabolic syndrome, insulin resistance and cancer. The Proceedings of the Nutrition Society. 2012;71:181–189. doi: 10.1017/S002966511100320X. [DOI] [PubMed] [Google Scholar]
- Fomina-Yadlin D, Kubicek S, Vetere A, He KH, Schreiber SL, Wagner BK. GW8510 increases insulin expression in pancreatic alpha cells through activation of p53 transcriptional activity. PloS one. 2012;7:e28808. doi: 10.1371/journal.pone.0028808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Yoh K, Shimohata H, Morito N, Ojima M, Okamura M, Takahashi S, Yamagata K. A novel diabetes mellitus mouse model, MAFA-deficient and beta cell-specific MAFK-overexpressing hybrid transgenic mice, developed severe diabetic nephropathy and improved with TCV-116 (candesartan cilexetil) treatment. Experimental animals / Japanese Association for Laboratory Animal Science. 2012;61:49–57. doi: 10.1538/expanim.61.49. [DOI] [PubMed] [Google Scholar]
- Garin I, Edghill EL, Akerman I, Rubio-Cabezas O, Rica I, Locke JM, Maestro MA, Alshaikh A, Bundak R, del Castillo G, Deeb A, Deiss D, Fernandez JM, Godbole K, Hussain K, O’Connell M, Klupa T, Kolouskova S, Mohsin F, Perlman K, Sumnik Z, Rial JM, Ugarte E, Vasanthi T, Johnstone K, Flanagan SE, Martinez R, Castano C, Patch AM, Fernandez-Rebollo E, Raile K, Morgan N, Harries LW, Castano L, Ellard S, Ferrer J, Perez de Nanclares G, Hattersley AT. Recessive mutations in the INS gene result in neonatal diabetes through reduced insulin biosynthesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3105–3110. doi: 10.1073/pnas.0910533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Ton CC, Mueller R, Petzl-Erler ML, Oliver C, Nevin NC, Housman DE, Maas RL. Absence of PAX6 gene mutations in Gillespie syndrome (partial aniridia, cerebellar ataxia, and mental retardation) Genomics. 1994;19:145–148. doi: 10.1006/geno.1994.1024. [DOI] [PubMed] [Google Scholar]
- Gloria-Bottini F, Banci M, Saccucci P, Magrini A, Bottini E. Is there a role of p53 codon 72 polymorphism in the susceptibility to type 2 diabetes in overweight subjects? A study in patients with cardiovascular diseases. Diabetes research and clinical practice. 2011;91:e64–e67. doi: 10.1016/j.diabres.2010.11.031. [DOI] [PubMed] [Google Scholar]
- Grier JD, Xiong S, Elizondo-Fraire AC, Parant JM, Lozano G. Tissue-specific differences of p53 inhibition by Mdm2 and Mdm4. Mol Cell Biol. 2006;26:192–198. doi: 10.1128/MCB.26.1.192-198.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier JD, Yan W, Lozano G. Conditional allele of mdm2 which encodes a p53 inhibitor. Genesis. 2002;32:145–147. doi: 10.1002/gene.10066. [DOI] [PubMed] [Google Scholar]
- He G, Zhang YW, Lee JH, Zeng SX, Wang YV, Luo Z, Dong XC, Viollet B, Wahl GM, Lu H. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Molecular and cellular biology. 2014;34:148–157. doi: 10.1128/MCB.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DJ, Duvillie B. Pancreatic development and adult diabetes. Pediatric research. 2011;48:269–274. doi: 10.1203/00006450-200009000-00002. [DOI] [PubMed] [Google Scholar]
- Hilliard S, Aboudehen K, Yao X, El-Dahr SS. Tight regulation of p53 activity by Mdm2 is required for ureteric bud growth and branching. Dev Biol. 2011;353:354–366. doi: 10.1016/j.ydbio.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino A, Ariyoshi M, Okawa Y, Kaimoto S, Uchihashi M, Fukai K, Iwai-Kanai E, Ikeda K, Ueyama T, Ogata T, Matoba S. Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic beta-cell function in diabetes. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3116–3121. doi: 10.1073/pnas.1318951111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Yan Z, Liao X, Li Y, Yang J, Wang ZG, Zuo Y, Kawai H, Shadfan M, Ganapathy S, Yuan ZM. The p53 inhibitors MDM2/MDMX complex is required for control of p53 activity in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12001–12006. doi: 10.1073/pnas.1102309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT, Weinberg RA. Tumor spectrum analysis in p53-mutant mice. Curr Biol. 1994;4:1–7. doi: 10.1016/s0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Jin Y, Dai MS, Lu SZ, Xu Y, Luo Z, Zhao Y, Lu H. 14-3-3gamma binds to MDMX that is phosphorylated by UV-activated Chk1, resulting in p53 activation. EMBO J. 2006;25:1207–1218. doi: 10.1038/sj.emboj.7601010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkers J, Meuwissen R, van der Gulden H, Peterse H, van der Valk M, Berns A. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- Kaur M, Bedi O, Sachdeva S, Reddy BV, Kumar P. Rodent animal models: from mild to advanced stages of diabetic nephropathy. Inflammopharmacology. 2014;22:279–293. doi: 10.1007/s10787-014-0215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H, Lopez-Pajares V, Kim MM, Wiederschain D, Yuan ZM. RING domain-mediated interaction is a requirement for MDM2’s E3 ligase activity. Cancer Res. 2007;67:6026–6030. doi: 10.1158/0008-5472.CAN-07-1313. [DOI] [PubMed] [Google Scholar]
- Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–622. doi: 10.1016/j.cell.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung CP, Leu JI, Basu S, Khaku S, Anokye-Danso F, Liu Q, George DL, Ahima RS, Murphy ME. The P72R Polymorphism of p53 Predisposes to Obesity and Metabolic Dysfunction. Cell reports. 2016;14:2413–2425. doi: 10.1016/j.celrep.2016.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D, Levine A. p53 Research: the past thirty years and the next thirty years. Cold Spring Harb Perspect Biol. 2010;2:a000893. doi: 10.1101/cshperspect.a000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung AK, Sharp PA. MicroRNA functions in stress responses. Mol Cell. 2010;40:205–215. doi: 10.1016/j.molcel.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HC, Xie L, Zhang W, Li R, Chen ZC, Wu GZ, Cui SS, Tan EK, Zeng L. Ribosomal S6 Kinase 2 (RSK2) maintains genomic stability by activating the Atm/p53-dependent DNA damage pathway. PLoS One. 2013;8:e74334. doi: 10.1371/journal.pone.0074334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke K, Mace PD, Smith CA, Vaux DL, Silke J, Day CL. Structure of the MDM2/MDMX RING domain heterodimer reveals dimerization is required for their ubiquitylation in trans. Cell Death Differ. 2008;15:841–848. doi: 10.1038/sj.cdd.4402309. [DOI] [PubMed] [Google Scholar]
- Maetens M, Doumont G, Clercq SD, Francoz S, Froment P, Bellefroid E, Klingmuller U, Lozano G, Marine JC. Distinct roles of Mdm2 and Mdm4 in red cell production. Blood. 2007;109:2630–2633. doi: 10.1182/blood-2006-03-013656. [DOI] [PubMed] [Google Scholar]
- Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- Mihailidou C, Chatzistamou I, Papavassiliou AG, Kiaris H. Regulation of P21 during diabetes-associated stress of the endoplasmic reticulum. Endocrine-related cancer. 2015;22:217–228. doi: 10.1530/ERC-15-0018. [DOI] [PubMed] [Google Scholar]
- Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Nalabothula N, Indig FE, Carrier F. The Nucleolus Takes Control of Protein Trafficking Under Cellular Stress. Mol Cell Pharmacol. 2010;2:203–212. [PMC free article] [PubMed] [Google Scholar]
- Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dowd JF, Stocker CJ. Endocrine pancreatic development: impact of obesity and diet. Front Physiol. 2013;4:170. doi: 10.3389/fphys.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- Padgett LE, Broniowska KA, Hansen PA, Corbett JA, Tse HM. The role of reactive oxygen species and proinflammatory cytokines in type 1 diabetes pathogenesis. Annals of the New York Academy of Sciences. 2013;1281:16–35. doi: 10.1111/j.1749-6632.2012.06826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pant V, Xiong S, Iwakuma T, Quintas-Cardama A, Lozano G. Heterodimerization of Mdm2 and Mdm4 is critical for regulating p53 activity during embryogenesis but dispensable for p53 and Mdm2 stability. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11995–12000. doi: 10.1073/pnas.1102241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Sarode VR, Euhus D, Kittler R, Scherer PE. Neuregulin 1-HER axis as a key mediator of hyperglycemic memory effects in breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:21058–21063. doi: 10.1073/pnas.1214400109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Xu X, Wang Q, Ren SY, Dong M, Zhang Y. Permissive role of AMPK and autophagy in adiponectin deficiency-accentuated myocardial injury and inflammation in endotoxemia. Journal of molecular and cellular cardiology. 2016;93:18–31. doi: 10.1016/j.yjmcc.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Ryu TY, Park J, Scherer PE. Hyperglycemia as a risk factor for cancer progression. Diabetes & metabolism journal. 2014;38:330–336. doi: 10.4093/dmj.2014.38.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saran R, Li Y, Robinson B, Ayanian J, Balkrishnan R, Bragg-Gresham J, Chen JT, Cope E, Gipson D, He K, Herman W, Heung M, Hirth RA, Jacobsen SS, Kalantar-Zadeh K, Kovesdy CP, Leichtman AB, Lu Y, Molnar MZ, Morgenstern H, Nallamothu B, O’Hare AM, Pisoni R, Plattner B, Port FK, Rao P, Rhee CM, Schaubel DE, Selewski DT, Shahinian V, Sim JJ, Song P, Streja E, Kurella Tamura M, Tentori F, Eggers PW, Agodoa LY, Abbott KC. US Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2015;66(Svii):S1–S305. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadfan M, Lopez-Pajares V, Yuan ZM. MDM2 and MDMX: Alone and together in regulation of p53. Translational cancer research. 2012;1:88–89. [PMC free article] [PubMed] [Google Scholar]
- Shield JP. Neonatal diabetes: how research unravelling the genetic puzzle has both widened our understanding of pancreatic development whilst improving children’s quality of life. Hormone research. 2007;67:77–83. doi: 10.1159/000096354. [DOI] [PubMed] [Google Scholar]
- Shikata K, Ninomiya T, Kiyohara Y. Diabetes mellitus and cancer risk: review of the epidemiological evidence. Cancer science. 2013;104:9–14. doi: 10.1111/cas.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler MJ, Riera M, Batlle D. New experimental models of diabetic nephropathy in mice models of type 2 diabetes: efforts to replicate human nephropathy. Experimental diabetes research. 2012;2012:616313. doi: 10.1155/2012/616313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XX, Dai MS, Lu H. 5-fluorouracil activation of p53 involves an MDM2-ribosomal protein interaction. J Biol Chem. 2007;282:8052–8059. doi: 10.1074/jbc.M610621200. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Harris RC. Role of endothelial nitric oxide synthase in diabetic nephropathy: lessons from diabetic eNOS knockout mice. Journal of diabetes research. 2014;2014:590541. doi: 10.1155/2014/590541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tersey SA, Nishiki Y, Templin AT, Cabrera SM, Stull ND, Colvin SC, Evans-Molina C, Rickus JL, Maier B, Mirmira RG. Islet beta-cell endoplasmic reticulum stress precedes the onset of type 1 diabetes in the nonobese diabetic mouse model. Diabetes. 2012;61:818–827. doi: 10.2337/db11-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornovsky-Babeay S, Dadon D, Ziv O, Tzipilevich E, Kadosh T, Schyr-Ben Haroush R, Hija A, Stolovich-Rain M, Furth-Lavi J, Granot Z, Porat S, Philipson LH, Herold KC, Bhatti TR, Stanley C, Ashcroft FM, In’t Veld P, Saada A, Magnuson MA, Glaser B, Dor Y. Type 2 diabetes and congenital hyperinsulinism cause DNA double-strand breaks and p53 activity in beta cells. Cell metabolism. 2014;19:109–121. doi: 10.1016/j.cmet.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Vona-Davis L, Rose DP. Adipokines as endocrine, paracrine, and autocrine factors in breast cancer risk and progression. Endocrine-related cancer. 2007;14:189–206. doi: 10.1677/ERC-06-0068. [DOI] [PubMed] [Google Scholar]
- Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Jiang X. Mdm2 and MdmX partner to regulate p53. FEBS letters. 2012;586:1390–1396. doi: 10.1016/j.febslet.2012.02.049. [DOI] [PubMed] [Google Scholar]
- Wang YV, Leblanc M, Wade M, Jochemsen AG, Wahl GM. Increased radioresistance and accelerated B cell lymphomas in mice with Mdmx mutations that prevent modifications by DNA-damage-activated kinases. Cancer Cell. 2009;16:33–43. doi: 10.1016/j.ccr.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. The Journal of clinical investigation. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Scheel D, German MS. Gene expression cascades in pancreatic development. Mechanisms of development. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- Xiong S, Van Pelt CS, Elizondo-Fraire AC, Fernandez-Garcia B, Lozano G. Loss of Mdm4 results in p53-dependent dilated cardiomyopathy. Circulation. 2007;115:2925–2930. doi: 10.1161/CIRCULATIONAHA.107.689901. [DOI] [PubMed] [Google Scholar]
- Xiong S, Van Pelt CS, Elizondo-Fraire AC, Liu G, Lozano G. Synergistic roles of Mdm2 and Mdm4 for p53 inhibition in central nervous system development. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3226–3231. doi: 10.1073/pnas.0508500103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Bi HE, Sheng Y, Cheng LB, Wendu RL, Wang CH, Cao GF, Jiang Q. Ultraviolet (UV) and hydrogen peroxide activate ceramide-ER stress-AMPK signaling axis to promote retinal pigment epithelium (RPE) cell apoptosis. International journal of molecular sciences. 2013;14:10355–10368. doi: 10.3390/ijms140510355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu H. Signaling to p53: ribosomal proteins find their way. Cancer Cell. 2009;16:369–377. doi: 10.1016/j.ccr.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wolf GW, Bhat K, Jin A, Allio T, Burkhart WA, Xiong Y. Ribosomal protein L11 negatively regulates oncoprotein MDM2 and mediates a p53-dependent ribosomal-stress checkpoint pathway. Mol Cell Biol. 2003;23:8902–8912. doi: 10.1128/MCB.23.23.8902-8912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang X, Lu H. Aberrant activation of p53 due to loss of MDM2 or MDMX causes early lens dysmorphogenesis. Developmental biology. 2014;396:19–30. doi: 10.1016/j.ydbio.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Lamhamedi-Cherradi SE, Wang P, Xu L, Chen YH. Tumor suppressor p53 inhibits autoimmune inflammation and macrophage function. Diabetes. 2005;54:1423–1428. doi: 10.2337/diabetes.54.5.1423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.