Abstract
Background
Higher hospital and surgeon volumes are independently associated with improved mortality following open repair of abdominal aortic aneurysms (AAA) in the era prior to endovascular repair (EVAR). The effects of both surgeon and hospital volume on mortality following EVAR and open repair in the current era are less well defined.
Methods
We studied Medicare beneficiaries who underwent elective AAA repair from 2001–2008. Volume was measured by procedure type over the one-year period preceding each procedure and was further categorized into quintiles (Q) of volume for both surgeon and hospital. Multilevel logistic regression models were used to evaluate the effect of surgeon volume, accounting for hospital volume, on mortality, adjusting for patient demographic and comorbid conditions as well as the analogous effect of hospital volume adjusting for surgeon volume. The multilevel models included random effects for surgeon and hospital to account for the clustering of multiple patients within the same surgeon and within the same hospital.
Results
We studied 122,495 patients who underwent AAA repair (Open: 45,451, EVAR: 77,044). Following EVAR, perioperative mortality did not differ by surgeon volume (Q1 (0–6 EVARs): 1.8%, Q5 (28–151 EVARs): 1.6%, P = 0.29), but decreased with greater hospital volume (Q1 (0–9 EVARs): 1.9%, Q5 (49–198 EVARs): 1.4%, P < .01). Following open repair, perioperative mortality decreased with both higher surgeon volume (Q1 (0–3 open repairs): 6.4%, Q5 (14–62 open repairs): 3.8%, P < .01) and hospital volume (Q1 (0–5 open repairs): 6.3%, Q5 (14–62 open repairs): 3.8%, P < .01). After adjustment for other predictors, surgeon volume was not associated with perioperative mortality after EVAR (OR:0.9, 95% CI:0.7–1.1); however, there was an association between hospital volume and higher perioperative mortality (Q1(OR: 1.5, 95% CI:1.2–1.9), Q2 (OR:1.3, 95% CI:1.02–1.6), and Q3 (OR:1.2, 95% CI:1.01–1.5) as compared to Q5). Following open repair, higher surgeon volume was also associated with lower mortality (Q1 (OR:1.5, 95% CI:1.3–1.8), Q2 (OR:1.3 95% CI:1.1–1.6), and Q3 (OR:1.2, 95% CI:1.1–1.4) compared with Q5). Risk of mortality also was higher for patients treated at lower volume hospitals (Q1 (OR:1.3, 95% CI:1.1–1.5), Q2 (OR:1.3, 95% CI:1.1–1.5), and Q3 (OR:1.2, 95% CI:1.1–1.4) versus Q5).
Conclusion
Following EVAR, hospital volume is minimally associated with perioperative mortality with no such association for surgeon volume. Following open AAA repair, both surgeon and hospital volume are strongly associated with mortality. These findings suggest that open surgery should be concentrated in hospitals and surgeons with high volume.
Introduction
Operative volume has been identified as an important predictor of patient morbidity and mortality following multiple complex surgical procedures.1 Following open repair of abdominal aortic aneurysms (AAA), studies using data from Medicare, the National Inpatient Sample, and international databases have all found improved mortality with increased volume.1–6 More recent studies have attempted to evaluate both surgeon and hospital volumes to better assess the independent impact of each. While some have suggested surgeon volume is the primary driver of improved mortality, others have concluded that both hospital and surgeon volume have important effects on perioperative mortality.1–3, 7 Importantly, previous studies have not evaluated the effect of surgeon and hospital volume on perioperative mortality after open repair in the endovascular era.
Few studies have evaluated the association between volume and mortality following endovascular repair of AAA (EVAR). Three studies assessing hospital volume have identified an inverse relationship between volume and in-hospital mortality.4, 6, 8 Alternatively, in one of the only studies to account for surgeon and hospital volume, neither surgeon nor hospital volume had a significant impact on in-hospital mortality. However, this study was unable to assess 30-day mortality due to limitations of the National Inpatient Sample (NIS).3 Given these gaps in knowledge, this study aims to evaluate the independent effect of both surgeon and hospital volume on perioperative mortality following EVAR and open repair.
Methods
Overview
We used comprehensive data from the Medicare program to identify all cases of aortic aneurysm repair that occurred during the time period 2001–2008. Cases included open and endovascular repair of intact and ruptured abdominal and thoracoabdominal aneurysms. We used these data to calculate institutional and surgeon volume for endovascular and open repair for each year. In order to evaluate the relationship between surgical volume and mortality, we restricted our analyses to cases of elective AAA repair confined to the abdominal aorta in order to compare similar patients across institutions and surgeons.
Surgeon and Hospital Volume
Surgeon and hospital procedure volume were measured within each procedure type over the 365-day period preceding each operation. Surgeon and hospital volume were assessed independently. Because experience in treating ruptured and complex aneurysms contributes to surgeon and hospital experience, the total volume counts included all ruptured, thoracoabdominal, and intact aortic aneurysms. To calculate surgeon volume, the performing physician was identified using the unique physician identification number listed on each patient’s Medicare claim. If 2 surgeons were identified in a single case (EVAR: 53%, Open: 35%), the higher volume was assigned to the case when one surgeon was not identified as an assistant. No physician was identified in 6,103 (4.7%) cases, which were therefore excluded from analysis (EVAR: 2,419, Open: 3,684). All other data were complete.
To simplify interpretation of results, hospital and surgeon volumes were divided into quintiles using cut points that most closely separated the patients into groups of equal size. The quintiles were set using average annual volume across all of the years, which meant that the proportion of cases assigned to each quintile varied across years. We computed volume cut points based on open and endovascular repair separately and at each time point assigned each hospital and surgeon to a volume quintile.
Study Population and Outcomes Assessment
For the assessment of outcomes, we applied several restrictions to the above population in order to create a homogenous sample and thus minimize the influence of confounding by case mix. To do so, we focused on the repair of intact AAAs among patients 67 years or older with a discharge diagnosis of AAA without rupture (ICD-9-CM, code 441.4) who also had a procedure code for open surgical repair – 38.44 (resection of abdominal aorta with replacement), 39.25 (aorto-iliac-femoral bypass), or for endovascular repair - 39.71 (endovascular implantation of graft). We excluded all those with diagnosis codes for AAA rupture (441.3), thoracic aneurysm (441.1, 441.2), thoracoabdominal aortic aneurysm (441.6, 441.7), or aortic dissection (441.0). We also excluded those with procedure codes for repair of the thoracic aorta (38.35, 38.45, 39.73), or visceral/renal bypass (38.46, 39.24, 39.26). We examined outcomes for Medicare cases for whom we had at least two years of claims history prior to the procedure. Only those beneficiaries with continuous Part A and B coverage were included. Beneficiaries enrolled in health maintenance organizations during any portion of the 24 months prior to their procedure were excluded from the analyses due to incomplete data.
The primary outcome measured was perioperative mortality, defined as death within the index hospitalization, including contiguous transfers to other acute care facilities, or within thirty days of the date the procedure was performed. Mortality was assessed using the Medicare Beneficiary Summary File.
Statistical Analysis
Hospital and surgeon volumes for each procedure were first examined over time. We then compared the admission characteristics of the cohorts according to quintiles of hospital or surgeon volume using chi-square tests for categorical variables or t-tests for continuous, normally distributed variables. In these comparisons our focus was on the magnitude of the differences (i.e., the lack of balance) between the groups, not the statistical significance of the difference, as this quantifies the potential for confounding bias due to the admission patient characteristics and thus the extent to which the statistical model is relied upon to perform the suitable adjustments.
We first estimated separate multilevel multivariable logistic regression models with surgeon and hospital volume respectively as the primary predictor of interest to estimate the overall effect of each and then subsequently estimated models with both surgeon and hospital volume as predictors to isolate the independent effect of each controlling for the other. We also tested for interactions between hospital and surgeon volume but found none and so limit the results reported herein to the main effect (i.e., no interaction) model. All models included the year of surgery as well as baseline beneficiary demographic and clinical characteristics obtained from claims during the two-year period prior to, but not including, the index admission. We measured clinical co-morbidities using a version of the Elixhauser algorithm that was adapted to also include diagnoses that occurred only in the outpatient setting.9, 10 The highest quintile was utilized as the reference group. The multilevel models included random effects for surgeon and hospital to account for the fact that multiple patients were treated by the same surgeon and within the same hospital, appropriately inflating standard errors to generalize results to the population of all US surgeons and hospitals. Observed mortality in each quintile was then compared with the expected mortality adjusted for patient demographics under the counterfactual assumption that all procedures occurred in a hospital at the lowest-volume quintile.
All statistical analyses were completed using SAS (version 9.4), and images were created using GraphPad (version 6.0) programing. The Institutional Review Board of Harvard Medical School approved this study and consent was waived due to the retrospective nature of this study.
Results
Surgical volume was measured based on a total of 268,939 patients including 166,759 endovascular repairs and 102,180 open repairs. Outcomes were then assessed among the 122,495 patients who underwent elective repair of an abdominal aortic aneurysm (EVAR: 77,044; Open: 45,451). The number of EVARs and open repairs in each quintile are shown in Table I.
Table I.
Previous 365-Day Volume Quintiles for EVAR and Open Repair For AAA from 2001–2008
| EVAR | Open Repair | |||
|---|---|---|---|---|
|
| ||||
| Hospital | Doctor | Hospital | Doctor | |
| Quintile 1 | 0 – 9 | 0 – 6 | 0 – 5 | 0 – 3 |
| Quintile 2 | 10 – 18 | 7 –11 | 6– 10 | 4 – 5 |
| Quintile 3 | 19 – 29 | 12 –17 | 11 – 17 | 6 – 8 |
| Quintile 4 | 30 – 48 | 18 – 27 | 18 – 28 | 9 – 13 |
| Quintile 5 | 49 – 198 | 28 – 151 | 29 – 121 | 14 – 62 |
Demographic Characteristics and Comorbidities
Among patients treated with EVAR, the mean age was 77 years. There was minimal variation in the frequency of comorbidities; however, the number of patients per year and the proportion of each race per quintile changed over time for both surgeon and hospital volume (Table II).
Table II.
Patient Characteristics according to Surgeon and Hospital Volume – EVAR
| Volume of Cases Sample size | Surgeon Volume | Hospital Volume | ||||||
|---|---|---|---|---|---|---|---|---|
| Low Quintile 1 (0–6) | Medium Quintiles 2–4 (7–27) | High Quintile 5 (28–151) | P-Value | Low Quintile 1 (0–9) | Medium Quintiles 2–4 (10–48) | High Quintile 5 (49–198) | P-Value | |
| 15388 (%) | 6324 (%) | 15322 (%) | 14,479 (%) | 47086 (%) | 15,479 (%) | |||
| Year | ||||||||
| 2001 | 13 | 5.4 | 4.2 | <.01 | 15 | 5.3 | 2.9 | <.01 |
| 2002 | 8.7 | 7.8 | 10 | 9.1 | 8.1 | 9.2 | ||
| 2003 | 9.1 | 9.1 | 11 | 10 | 10 | 9.1 | ||
| 2004 | 12 | 11 | 13 | 12 | 12 | 11 | ||
| 2005 | 13 | 15 | 15 | 14 | 14 | 15 | ||
| 2006 | 14 | 16 | 16 | 14 | 16 | 17 | ||
| 2007 | 15 | 17 | 16 | 14 | 17 | 17 | ||
| 2008 | 15 | 18 | 14 | 12 | 18 | 18 | ||
| Age – mean (SD) | 77 (0.05) | 77 (0.03) | 77 (0.05) | 0.47 | 77 (0.05) | 77 (0.03) | 77 (0.05) | 0.03 |
| Male Sex | 83 | 82 | 82 | 0.24 | 83 | 82 | 82 | 0.70 |
| Race | ||||||||
| White | 94 | 96 | 97 | <.01 | 94 | 96 | 96 | <.01 |
| Black | 3.5 | 2.7 | 2.1 | 3.2 | 2.8 | 2.3 | ||
| Hispanic | 0.8 | 0.5 | 0.3 | 0.9 | 0.5 | 0.5 | ||
| Other | 1.7 | 1.2 | 0.7 | 2.0 | 1.1 | 1.0 | ||
| Urgent Admission | 1.4 | 1.5 | 1.6 | 0.24 | 1.4 | 1.5 | 1.6 | 0.21 |
| Prior Aneurysm Repair | 8.9 | 8.7 | 9.3 | 0.15 | 8.6 | 8.8 | 9.2 | 0.28 |
| MI - past 6 months | 10 | 10 | 11 | <.01 | 10 | 10 | 12 | <.01 |
| MI - past 24 months | 14 | 14 | 15 | 0.01 | 15 | 14 | 15 | 0.14 |
| PVD | 19 | 19 | 20 | <.01 | 19 | 19 | 21 | <.01 |
| Stroke | 13 | 14 | 14 | 0.01 | 14 | 14 | 14 | 0.09 |
| Hypertension | 65 | 66 | 66 | 0.12 | 65 | 66 | 67 | <.01 |
| Diabetes | 19 | 19 | 19 | 0.49 | 19 | 19 | 19 | 0.97 |
| COPD | 29 | 29 | 29 | 0.57 | 30 | 29 | 29 | 0.26 |
| Renal Failure | 7.1 | 7.4 | 7.9 | 0.02 | 6.8 | 7.5 | 7.9 | <.01 |
| ESRD | 0.7 | 0.6 | 0.6 | 0.90 | 0.7 | 0.6 | 0.6 | 0.93 |
| History of Cancer | 21 | 20 | 20 | 0.03 | 21 | 20 | 20 | 0.11 |
| Obesity | 2.6 | 2.7 | 2.7 | 0.71 | 2.6 | 2.9 | 2.5 | 0.03 |
MI: Myocardial Infarction, PVD: Peripheral Vascular Disease, COPD: Chronic Obstructive Pulmonary Disease, ESRD: End-Stage Renal Disease, Bold Values: P-Value < .05
Among patients treated with open repair, the mean age was 75 years. Comorbidities were similar across all quintiles with the exception of chronic obstructive pulmonary disease, which differed by hospital quintile only. The number of patients per year, the proportion of each race, and gender per quintile appeared to be associated with both surgeon and hospital volume (Table III).
Table III.
Patient Characteristics according to Surgeon and Hospital Volume – Open Repair
| Volume of Cases Sample size | Surgeon Volume | Hospital Volume | ||||||
|---|---|---|---|---|---|---|---|---|
| Low Quintile 1 (0–3) | Medium Quintile 2–4 (4–13) | High Quintile 5 (14–62) | P-Value | Low Quintile 1 (0–5) | Medium Quintile 2–4 (6–28) | High Quintile 5 (29–121) | P-Value | |
| 10,387 (%) | 26,156 (%) | 8,458 (%) | 8,919 (%) | 27,789 (%) | 8,743 (%) | |||
| Year | ||||||||
| 2001 | 32 | 16 | 12 | <.01 | 35 | 17 | 11 | <.01 |
| 2002 | 11 | 16 | 25 | 10 | 15 | 26 | ||
| 2003 | 10 | 16 | 19 | 11 | 15 | 18 | ||
| 2004 | 11 | 15 | 16 | 11 | 15 | 16 | ||
| 2005 | 10 | 13 | 12 | 10 | 13 | 12 | ||
| 2006 | 9.3 | 10 | 8.4 | 8.8 | 10 | 9.3 | ||
| 2007 | 9.0 | 8.1 | 4.5 | 8.0 | 8.5 | 4.7 | ||
| 2008 | 7.5 | 6.5 | 2.9 | 7.2 | 6.6 | 3.0 | ||
| Age – mean (SD) | 75 (0.05) | 75 (0.03) | 75 (0.06) | <.01 | 75 (0.05) | 75 (0.03) | 75 (0.06) | 0.88 |
| Male Sex | 73 | 74 | 75 | <.01 | 73 | 74 | 75 | 0.02 |
| Race | ||||||||
| White | 94 | 95 | 97 | <.01 | 93 | 95 | 97 | <.01 |
| Black | 3.8 | 2.8 | 2.1 | 4.0 | 2.8 | 2.2 | ||
| Hispanic | 0.9 | 0.5 | 0.4 | 1.0 | 0.5 | 0.2 | ||
| Other | 1.6 | 1.3 | 0.7 | 1.7 | 1.3 | 0.6 | ||
| Urgent Admission | 1.5 | 1.6 | 2.0 | <.01 | 1.6 | 1.6 | 1.8 | 0.23 |
| Prior Aneurysm Repair | 7.0 | 7.7 | 8.1 | 0.01 | 7.1 | 8.1 | 7.9 | 0.15 |
| MI - past 6 months | 8.0 | 8.0 | 8.2 | 0.86 | 7.8 | 7.7 | 8.0 | 0.58 |
| MI - past 24 months | 10 | 11 | 10 | 0.64 | 10 | 11 | 11 | 0.84 |
| PVD | 20 | 20 | 20 | 0.50 | 21 | 20 | 20 | 0.42 |
| Stroke | 14 | 14 | 14 | 0.63 | 14 | 14 | 14 | 0.99 |
| Hypertension | 62 | 62 | 62 | 0.37 | 62 | 62 | 61 | 0.41 |
| Diabetes | 15 | 15 | 15 | 0.88 | 15 | 15 | 14 | 0.08 |
| COPD | 27 | 28 | 26 | 0.09 | 28 | 28 | 25 | <.01 |
| Renal Failure | 5.0 | 5.1 | 4.9 | 0.68 | 5.0 | 5.2 | 4.7 | 0.17 |
| ESRD | 0.3 | 0.3 | 0.2 | 0.26 | 0.3 | 0.3 | 0.2 | 0.61 |
| History of Cancer | 16 | 15 | 15 | 0.89 | 15 | 15 | 16 | 0.99 |
| Obesity | 1.6 | 1.7 | 1.9 | 0.28 | 1.7 | 1.8 | 1.7 | 0.89 |
MI: Myocardial Infarction, PVD: Peripheral Vascular Disease, COPD: Chronic Obstructive Pulmonary Disease, ESRD: End-Stage Renal Disease, Bold Values: P-Value < .05
Effect of Volume on Mortality
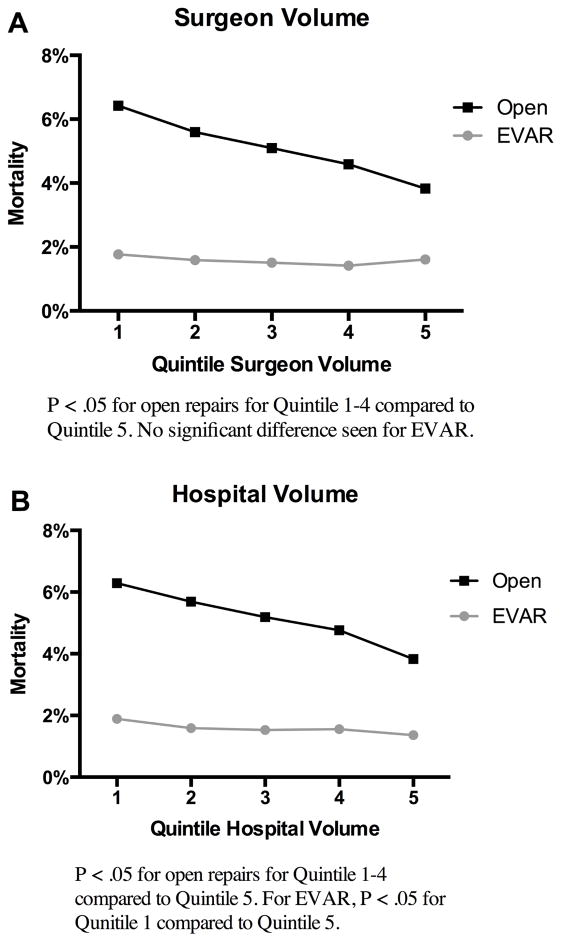
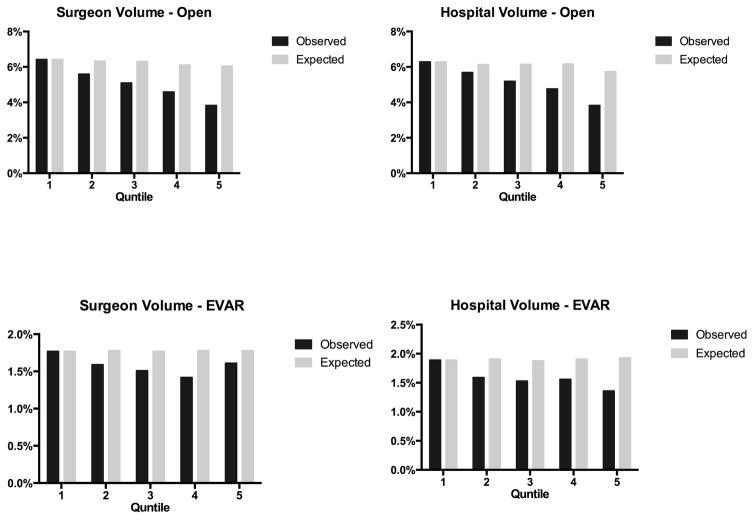
Following EVAR, surgeon volumes was not associated with unadjusted perioperative mortality (Q1: 1.8%, Q5: 1.6%, P = 0.29), but decreased with greater hospital volume (Q1: 1.9%, Q5: 1.4%, P < .01) (Table IV). In patients undergoing open repair, surgeon volume (Q1: 6.4%, Q2: 5.6%, Q3: 5.1%, Q4: 4.6%, Q5: 3.8%) and hospital volume (Q1: 6.3%, Q2: 5.7%, Q3: 5.2%, Q4: 4.8%, Q5: 3.8%) (Figure 1) had approximately linear associations with perioperative mortality. Predicted mortality following EVAR and open repair was similar for both surgeon and hospital volume (Figure 2).
Table IV.
Unadjusted Perioperative Mortality Rates
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | |
|---|---|---|---|---|---|
| EVAR | |||||
| Surgeon Volume | 1.8% | 1.6% | 1.5% | 1.4% | 1.6% |
| Hospital Volume | 1.9% | 1.6% | 1.5% | 1.6% | 1.4% |
| Open | |||||
| Surgeon Volume | 6.4% | 5.6% | 5.1% | 4.6% | 3.8% |
| Hospital Volume | 6.3% | 5.7% | 5.2% | 4.8% | 3.8% |
Figure 1.
Unadjusted Perioperative All-Cause Mortality Rates A: Surgeon Volume B: Hospital Volume
Figure 2.
Actual and Predicted Mortality by Hospital and Surgeon Volume for Endovascular and Open AAA Repair
In patients undergoing EVAR, surgeon volume was not associated with perioperative mortality after adjustment for patient comorbidities and hospital volume. However, when hospital volume was assessed, increased perioperative mortality was noted in lower volume hospitals (quintile 1 (Odds Ratio (OR): 1.5, 95% Confidence Interval (CI): 1.2–1.9), 2 (OR: 1.3, 95% CI: 1.0–1.6), and 3 (OR: 1.3, 95% CI: 1.0–1.6), as compared to quintile 5). In patients undergoing open repair, surgeon volume was strongly associated with mortality (quintiles 1 (OR: 1.5, 95% CI: 1.3–1.7), 2 (OR: 1.3, 95% CI: 1.1–1.6), and 3 (OR: 1.2, 95% CI: 1.0–1.4) versus quintile 5). Hospital volume also was associated with perioperative mortality (quintile 1 (OR 1.3, 95% CI: 1.1–1.5), and 2 (OR 1.2, 95% CI: 1.1–1.5), as compared with quintile 5 (Table V)).
Table V.
Adjusted Odds Ratios for Perioperative Mortality By Volume Quintile with High Volume (Quintile 5 as Reference)
| Surgeon Volume | Surgeon Volume Adjusted for Hospital Volume | Hospital Volume | Hospital Volume Adjusted for Surgeon Volume | |
|---|---|---|---|---|
| Odds Ratio (95% CI) | Odds Ratio (95% CI) | |||
| Quintile 1 | ||||
| EVAR | 1.1 (0.9–1.3) | 0.9 (0.7–1.1) | 1.4 (1.2–1.7)a | 1.5 (1.2–1.9)a |
| Open | 1.6 (1.4–1.9)a | 1.5 (1.3–1.7)a | 1.5 (1.3–1.8)a | 1.3 (1.1–1.5)a |
| Quintile 2 | ||||
| EVAR | 1.0 (0.8–1.2) | 0.9 (0.7–1.1) | 1.2 (1.0–1.4) | 1.3 (1.0–1.6)a |
| Open | 1.4 (1.2–1.7)a | 1.3 (1.1–1.6)a | 1.4 (1.2–1.7)a | 1.2 (1.1–1.5)a |
| Quintile 3 | ||||
| EVAR | 0.9 (0.8–1.1) | 0.9 (0.7–1.1) | 1.2 (1.0–1.4) | 1.3 (1.0–1.6)a |
| Open | 1.3 (1.1–1.5)a | 1.2 (1.0–1.4)a | 1.3 (1.1–1.5)a | 1.2 (1.0–1.4) |
| Quintile 4 | ||||
| EVAR | 0.9 (0.7–1.1) | 0.8 (0.7–1.0) | 1.2 (1.0–1.4) | 1.2 (1.0–1.5) |
| Open | 1.2 (1.0–1.4)a | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.1 (1.0–1.3) |
Statistically significant effect, CI: Confidence Interval
Discussion
We used comprehensive data from the Medicare program to evaluate the relationship between surgeon volume, hospital volume, and perioperative outcomes following elective AAA repair, and identified several notable results. First, following EVAR, high hospital volume, but not surgeon volume, is associated with lower perioperative mortality. Additionally, following open repair, both higher surgeon and hospital volumes are highly associated with decreasing perioperative mortality and these effects appear to be mostly independent.
In the current healthcare climate focused on quality improvement, organizations such as Leapfrog and the Agency for Health Care Quality (AHRQ) are including hospital volume in their recommendations for quality improvement.11, 12 Moreover, several prominent hospitals, including the University of Michigan, Johns Hopkins, and Dartmouth-Hitchcock, recently announced plans to prohibit low volume surgeons from performing several procedures including complex AAA repairs.13, 14 Previously, Leapfrog guidelines recommended repair of AAA be performed at centers completing 50 or more repairs annually; however, more recently, Leapfrog’s standards for high risk procedures recommend AAA repair be performed at institutions with in-hospital survival of 97.3% or greater.11 These standards are derived from outcomes at the highest quartile of care for all AAA repairs and are likely reflective of the dramatic shift to EVAR, which has a significantly lower mortality rate compared to open repair.11, 15 In the current study, all EVARs met Leapfrog’s current objective; however, even the highest volume institutions did not achieve these rates following open repair. Importantly, neither previous nor current Leapfrog standards differentiate between open and endovascular repair despite dramatic differences in complication rates, perioperative mortality, and hospital stay between EVAR and open repair.15 Moreover, in prior work, we found that at least for hospitals, volume of EVAR did not predict outcomes for open repairs and vice versa, suggesting that the relevant experience is for the specific procedure, not AAA repair overall.6
The Society for Vascular Surgery (SVS) guidelines recommend repair of abdominal aortic aneurysms be completed at hospitals with in-hospital mortality rates less than 5% following open repair and less than 3% following EVAR.16 In the current study, only high volume hospitals in the fourth and fifth quintile achieved mortality rates below 5% after open repair despite similar predicted mortality, suggesting that, in general, hospitals who perform fewer than 18 open repairs annually do not achieve current quality benchmarks. Importantly, following EVAR, all hospitals achieved mortality rates less than 3%, so this standard may be too lenient. Nonetheless, the SVS guidelines may be more appropriate to guide repair of AAA given the clear delineation between EVAR and open repair.
Our study suggests that for EVAR, hospital volume may be a sufficient measure to stratify perioperative mortality risk, and that all hospitals currently meet standards set forth by the SVS and Leapfrog. Our results suggest that only the lowest volume EVAR hospitals (< 9 EVARs) should be avoided, although differences between the lowest and highest volume hospitals were small. Importantly, the volume required is dramatically lower than the 50 cases previously suggested by Leapfrog, which may reflect outdated targets based on open volume. Following open repair, this study suggests that hospital volume alone, used by Leapfrog, is not a sufficient measure for stratifying perioperative mortality risk and that surgeon volume must also be accounted for. These results are supported by the prior work of Dimick et al. who found high hospital volume (>35 cases) and high surgeon volume (>10 cases) to be associated with improved mortality following open AAA repair.2 Similarly, Birkmeyer et al. also found both surgeon and hospital volume to be important predictors of mortality following AAA repair.5
The specific volumes necessary to achieve acceptable perioperative mortality rates are not universally agreed upon, and much of the current data comes from studies analyzing outcomes of open AAA repair prior to the widespread use of EVAR. Historically, Leapfrog recommended a hospital cutoff of 50 AAA repairs. More recently; however, Dartmouth, University of Michigan, and Johns Hopkins released volume thresholds for both hospital and surgeon volume, with cutoffs for complex aortic surgery of 20 operations per hospital and 8 operations per surgeon annually.14 Dimick et al. identified a threshold of 35 cases per hospital and 10 cases per surgeon.2 Finally, in the United Kingdom, arterial surgery was recently centralized to centers performing more than 100 AAA procedures per year.17 In our current study, our results for open repair in particular suggest a fairly linear relationship, which suggests that there is no exact threshold where mortality declines precipitously. Moreover, prior thresholds often have been determined by the arbitrary cutpoints suggested by statistical analysis, rather than clinically meaningful differences in outcomes. Therefore, care should be taken in the interpretation of such results given the significant policy implications associated with imposing volume thresholds.
Regionalization of complex surgery has been proposed as a potential quality improvement strategy to improve the perioperative mortality of low volume centers within the United States. In the United Kingdom these efforts have led to the centralization of arterial surgery and endovascular interventions into high volume vascular arterial centers performing a minimum of 60 AAA repairs and 40 carotid procedures annually.17 However, opponents note such efforts disproportionally displace patients in rural settings, increase the travel costs associated with healthcare, and overburden large institutions which are already frequently over-capacity.17 Nonetheless, early studies among pediatric and adult surgical patients demonstrated success in improving patient outcomes through these efforts with only moderate increases in travel time for most patients.18–20 Given the substantial decrease in open volume that has been seen over the last decade, a regional approach for open repair should be considered. There are several important limitations to this study. First, this study is subject to all of the limitations of the Medicare database including the potential for coding errors, missing data, and data variability. This database also lacks anatomic detail including aneurysm diameter, calcification, and extent. Additionally, some clinical variables could not be assessed, which may impact patient selection; these include smoking status, medications, and pre-operative laboratory values. Additional outcomes of interest including failure to rescue and post-operative complications were not assessed, nor were potential mediators such as nursing staffing ratios, and intensivist staffing, and regional variation. Finally, this study did not evaluate cause of death; however because all mortality was perioperative it should be considered related to AAA repair. This study is, however, strengthened by its large and nationally representative sample size.
Conclusions
Following EVAR, perioperative mortality is not related to surgeon volume; however, mortality significantly decreases at high volume centers. Following open AAA repair, higher surgeon and hospital volume are associated with decreased mortality. These data suggest that AAA repair should be performed at high volume hospitals, and open repair should be performed by high volume surgeons within high volume hospitals.
| Highlight | |
|---|---|
| Significance: | This study provides a fresh look into the impact volume has on the mortality rate of aortic aneurysm repair, with respect to both the surgeon and hospital. |
| Type of Research: | Retrospective analysis of prospectively collected Medicare data of the National Inpatient Sample |
| Take Home Message: | After adjustment for patient comorbidities and hospital volume, surgeon’s volume was strongly associated with mortality after elective open AAA repair. Hospital volume was also significantly associated with mortality |
| Recommendation: | The authors recommend that to decrease perioperative mortality, open AAA repair should be performed by high volume surgeons. They also suggest that open repair maybe best performed in high volume hospitals. |
| Strength of Recommendation: | 1. Strong |
| Level of Evidence: | B. Medium |
Acknowledgments
This work was completed with support from the National Institutes of Health Harvard-Longwood T32 Research Training in Vascular Surgery Grant HL 007734 to Sara L. Zettervall, John C. McCallum, Peter A. Soden, Katie E. Shean, and Sarah E. Deery and was supported by grant 5R01HL105453-03 from the National Heart, Lung, and Blood Institute.
Footnotes
Presented at the 2016 Society of Vascular Surgery Vascular Annual Meeting, June 7-11 2016, National Harbor, MD
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimick JB, Cowan JA, Jr, Stanley JC, Henke PK, Pronovost PJ, Upchurch GR., Jr Surgeon specialty and provider volumes are related to outcome of intact abdominal aortic aneurysm repair in the United States. J Vasc Surg. 2003;38(4):739–44. doi: 10.1016/s0741-5214(03)00470-1. [DOI] [PubMed] [Google Scholar]
- 3.McPhee JT, Robinson WP, 3rd, Eslami MH, Arous EJ, Messina LM, Schanzer A. Surgeon case volume, not institution case volume, is the primary determinant of in-hospital mortality after elective open abdominal aortic aneurysm repair. J Vasc Surg. 2011;53(3):591–9. e2. doi: 10.1016/j.jvs.2010.09.063. [DOI] [PubMed] [Google Scholar]
- 4.Holt PJ, Poloniecki JD, Gerrard D, Loftus IM, Thompson MM. Meta-analysis and systematic review of the relationship between volume and outcome in abdominal aortic aneurysm surgery. Br J Surg. 2007;94(4):395–403. doi: 10.1002/bjs.5710. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 6.Landon BE, O’Malley AJ, Giles K, Cotterill P, Schermerhorn ML. Volume-outcome relationships and abdominal aortic aneurysm repair. Circulation. 2010;122(13):1290–7. doi: 10.1161/CIRCULATIONAHA.110.949172. [DOI] [PubMed] [Google Scholar]
- 7.Marlow NE, Barraclough B, Collier NA, Dickinson IC, Fawcett J, Graham JC, et al. Effect of hospital and surgeon volume on patient outcomes following treatment of abdominal aortic aneurysms: a systematic review. Eur J Vasc Endovasc Surg. 2010;40(5):572–9. doi: 10.1016/j.ejvs.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Dimick JB, Upchurch GR., Jr Endovascular technology, hospital volume, and mortality with abdominal aortic aneurysm surgery. J Vasc Surg. 2008;47(6):1150–4. doi: 10.1016/j.jvs.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Baldwin LM, Klabunde CN, Green P, Barlow W, Wright G. In search of the perfect comorbidity measure for use with administrative claims data: does it exist? Med Care. 2006;44(8):745–53. doi: 10.1097/01.mlr.0000223475.70440.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Predicting patient survival of high-risk surgeries. The Leapfrog Group; [May 7, 2016]. Available from: http://www.leapfroggroup.org/sites/default/files/Files/2014LeapfrogReport_HighRiskProceduresFINAL.pdf. [Google Scholar]
- 12.AHRQ Quality Indicators. Agency for Healthcare Research and Quality; [May 7, 2016]. Available from: http://www.qualityindicators.ahrq.gov/Downloads/Modules/IQI/V50/IQI_Brochure.pdf. [PubMed] [Google Scholar]
- 13.Sternberg S. Hospitals move to limit low-volume surgeries: three of nation’s leading hospital systems say they will limit low-voume surgeries. [May 18, 2016];US News & World Report. 2015 Available from: http://www.usnews.com/news/articles/2015/05/19/hospitals-move-to-limit-low-volume-surgeries.
- 14.Urbach DR. Pledging to Eliminate Low-Volume Surgery. N Engl J Med. 2015;373(15):1388–90. doi: 10.1056/NEJMp1508472. [DOI] [PubMed] [Google Scholar]
- 15.Schermerhorn ML, Buck DB, O’Malley AJ, Curran T, McCallum JC, Darling J, et al. Long-Term Outcomes of Abdominal Aortic Aneurysm in the Medicare Population. N Engl J Med. 2015;373(4):328–38. doi: 10.1056/NEJMoa1405778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, et al. The care of patients with an abdominal aortic aneurysm: The Society for Vascular Surgery practice guidelines. J Vasc Surg. 2009;50(4):S2–S49. doi: 10.1016/j.jvs.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Daley J. Invited commentary: quality of care and the volume-outcome relationship--what’s next for surgery? Surgery. 2002;131(1):16–8. doi: 10.1067/msy.2002.120237. [DOI] [PubMed] [Google Scholar]
- 18.Chang RK, Klitzner TS. Can regionalization decrease the number of deaths for children who undergo cardiac surgery? A theoretical analysis. Pediatrics. 2002;109(2):173–81. doi: 10.1542/peds.109.2.173. [DOI] [PubMed] [Google Scholar]
- 19.Grumbach K, Anderson GM, Luft HS, Roos LL, Brook R. Regionalization of cardiac surgery in the United States and Canada. Geographic access, choice, and outcomes. Jama. 1995;274(16):1282–8. [PubMed] [Google Scholar]
- 20.Birkmeyer JD, Siewers AE, Marth NJ, Goodman DC. Regionalization of high-risk surgery and implications for patient travel times. Jama. 2003;290(20):2703–8. doi: 10.1001/jama.290.20.2703. [DOI] [PubMed] [Google Scholar]