Abstract
Introduction
Azithromycin (AZM) reduces pulmonary inflammation and exacerbations in patients with COPD having emphysema. The antimicrobial effects of AZM on the lower airway microbiome are not known and may contribute to its beneficial effects. Here we tested whether AZM treatment affects the lung microbiome and bacterial metabolites that might contribute to changes in levels of inflammatory cytokines in the airways.
Methods
20 smokers (current or ex-smokers) with emphysema were randomised to receive AZM 250 mg or placebo daily for 8 weeks. Bronchoalveolar lavage (BAL) was performed at baseline and after treatment. Measurements performed in acellular BAL fluid included 16S rRNA gene sequences and quantity; 39 cytokines, chemokines and growth factors and 119 identified metabolites. The response to lipopolysaccharide (LPS) by alveolar macrophages after ex-vivo treatment with AZM or bacterial metabolites was assessed.
Results
Compared with placebo, AZM did not alter bacterial burden but reduced α-diversity, decreasing 11 low abundance taxa, none of which are classical pulmonary pathogens. Compared with placebo, AZM treatment led to reduced in-vivo levels of chemokine (C-X-C) ligand 1 (CXCL1), tumour necrosis factor (TNF)-α, interleukin (IL)-13 and IL-12p40 in BAL, but increased bacterial metabolites including glycolic acid, indol-3-acetate and linoleic acid. Glycolic acid and indol-3-acetate, but not AZM, blunted ex-vivo LPS-induced alveolar macrophage generation of CXCL1, TNF-α, IL-13 and IL-12p40.
Conclusion
AZM treatment altered both lung microbiota and metabolome, affecting anti-inflammatory bacterial metabolites that may contribute to its therapeutic effects.
Trial registration number
Keywords: Bronchoscopy, COPD ÀÜ Mechanisms
Key messages.
What is the key question?
Using culture-independent techniques, can we identify how macrolides reduce inflammation in emphysema?
What is the bottom line?
Azithromycin (AZM) exerts multiple effects on the structure and composition of the lower airway microbiota and increases the levels of several microbial metabolites in the lung that have anti-inflammatory effects.
Why read on?
This data suggest that the beneficial therapeutic effects of AZM may be mediated through altering the lung microbiome interaction with the host immune system and highlight the relevance of understanding the metabolism of the resident microbes as potential targets for immunomodulation.
Introduction
COPD is an inflammatory disorder often caused by cigarette smoking. The COPD clinical course, punctuated by exacerbations, is frequently treated with antibiotics and anti-inflammatory drugs. Chronic treatment with macrolides reduces the frequency of exacerbations in patients; however, the salient mechanisms are undefined.1 2 Although sputum culture shows altered bacterial compositions during exacerbations, the role of bacterial colonisation in COPD progression is uncertain.3 In a recent single-blinded, randomised, placebo-controlled trial with moxifloxacin, doxycycline, azithromycin (AZM) or placebo, the bacterial load of sputum did not change significantly.4 However, description of the lower airway microbiome has been challenging because bacterial burden in the lung is approximately 1 million-fold lower than in the gut and 100-fold lower than the upper airway.5 6 The presence of supraglottic microbes, such Veillonella or Prevotella, in the lower airway commonly occurs5–10 and detection of these bacteria in bronchoalveolar lavage (BAL) fluid is associated with increased inflammation,5 11 supporting the hypothesis that microbiota changes are linked with pulmonary effects. In response to stress, bacterial cells produce anti-inflammatory metabolites, including indole-3-acetate, which may induce biofilms and reduce antibiotic susceptibility.12 13 Bacterial stress responses during antibiotic treatment could therefore impact innate immunity, benefiting the local bacterial community.
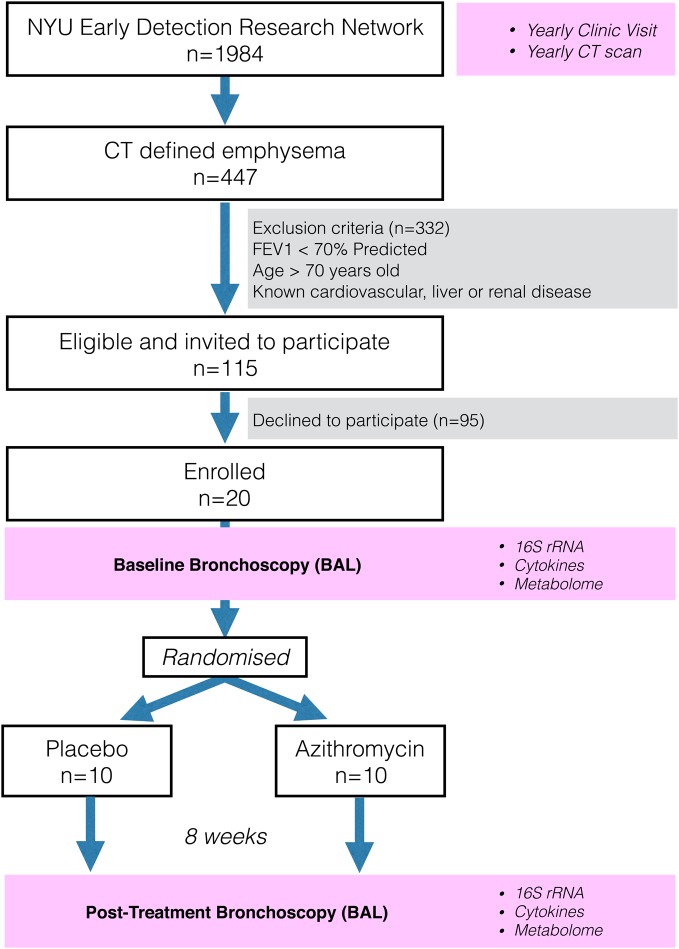
To explore the pulmonary effects of macrolide exposure, we performed a randomised trial comparing AZM treatment with placebo for 8 weeks in 20 subjects with CT-defined emphysema who had not been treated with antibiotics or corticosteroids (figure 1, NCT02557958; http://www.clinicaltrials.gov). Acellular BAL samples were obtained before and after treatment to determine the effects of AZM on microbiota, metabolome and inflammation in the lower airways.
Figure 1.
CONsolidated Standards of Reporting Trials diagram showing the selection of subjects from Early Detection Research Network (EDRN) cohort. Subjects underwent bronchoscopy with bronchoalveolar lavage (BAL) at baseline. After bronchoscopy, subjects were randomised to either placebo or azithromycin for 8 weeks. Post-treatment bronchoscopy was performed at the end of the treatment period. BAL was used to evaluate microbiome (16S), metabolome and inflammation. NYU, New York University.
Methods
Randomised study design and participants
Subjects were enrolled from the Early Detection Research Network (EDRN, 5U01CA086137-13, institutional review board (IRB)# 8896, NCT00301119, PI Rom). All subjects signed informed consent to participate in this study and the research protocol was approved by the New York University (NYU) and Bellevue Hospital Center (New York, New York, USA) IRB# 8896. This cohort has 1984 subjects receiving yearly follow-up including chest CT and 447 have CT-defined emphysema. We enrolled 20 EDRN subjects who fulfilled the following inclusion criteria: diagnosis of emphysema as documented in the report by radiologist and significant smoking history (>20 pack/year). For all subjects, exclusion criteria included FEV1<70% predicted, age >70 years, known cardiovascular, liver or renal disease (due to increased bronchoscopic risk), recent treatment with antibiotics or steroids in the prior 3 months. None of the subjects had received any inhaled medication for at least 1 month. For scoring of emphysema, a 6-point score of CT emphysema was performed and the percentage of lung voxels below −950 HU in the entire lung volume was automatically computed (see online supplementary material for more details).
thoraxjnl-2016-208599supp.pdf (574.2KB, pdf)
Procedures
Research bronchoscopy was performed at baseline using a nasal approach as described.5 Briefly, two bronchoscopes were used, in which the first one was used to obtain a supraglottic sample. Then, the second bronchoscope was passed without suctioning until it was wedged for BAL. For BAL, the right middle lobe and/or lingula were preferred and 150 mL of sterile saline was used for the lavage. Background samples were obtained by passing sterile saline through the suctioning channel of bronchoscope prior to bronchoscopy. From the BAL fluid, the total and differential cell counts were obtained after centrifugation. Subjects then were randomised to receive either placebo or AZM 250 mg/daily for 8 weeks. Based on previous data, we considered this treatment period sufficient to observe significant changes in inflammatory mediators.14 Both placebo and AZM were prepared in an opaque capsule by the NYU pharmacy and were given to each subject after the first bronchoscopy. The NYU pharmacy controlled the randomisation scheme to ensure that the study was double-blinded. Each subject completed a pill diary. After 8 weeks of treatment with AZM or placebo, subjects underwent post-treatment research bronchoscopy in which BAL was performed in same lung segments as in the baseline bronchoscopy. BAL fluid aliquots were frozen at −80°C.
Bacterial 16S rRNA gene quantitation and sequencing
DNA was then extracted from background, supraglottic and BAL samples using an ion exchange column (Qiagen). Total bacterial and human DNA levels were determined by quantitative PCR. The BAL samples used in this study were acellular BAL fluid. High-throughput sequencing of bacterial 16S rRNA-encoding gene amplicons encoding the V4 region (150 bp read length, paired-end protocol) was performed on the Illumina MiSeq platform.
The obtained 16S rRNA gene sequences were examined using the Quantitative Insights Into Microbial Ecology package for analysis of community sequence data. To estimate within-sample diversity (α-diversity), we calculated the number of distinct operational taxonomic units (OTUs) at different sequence depths as well as the Shannon's diversity index. PERMANOVA (Adonis) testing was used to compare β-diversity of groups. β-Diversity refers to between-sample similarity based on bacterial composition. For β-diversity (between-sample taxonomic diversity), we used the ade4 package in R to construct Principal Coordinate Analysis based on weighted UniFrac distances. Additionally, Nonmetric Multidimensional Scaling (NMDS) based on Bray-Curtis dissimilarity was used to visualise potential clustering patterns among samples based on the estimated β-diversity and its relationship with dominant taxa. For comparisons of α-diversity, β-diversity or taxonomy between the placebo and AZM groups at baseline, non-parametric (Mann-Whitney) tests were used. Paired statistics (Wilcoxon signed-rank test) were used for pre-treatment and post-treatment comparison of continuous parameters such as changes in αdiversity post-treatment with AZM or placebo. To evaluate changes in β-diversity pre-treatment versus post-treatment, we performed Procrustes analysis of the principal coordinate matrices generated after β-diversity determination on each pair of BAL samples (before and after treatment) for every subject. False discovery rate was used to control for multiple testing. To evaluate differences between groups of 16S data or inferred metagenomes, we used linear discriminant analysis (LDA) effect size (LEfSe) in paired samples (before and after treatment).15 All data are publicly available in Gene Expression Omnibus under accession number GSE74396 (see online supplementary material for more details).
Measurement of metabolites in BAL fluid
For metabolomics analysis, we used 4 mL of BAL samples obtained before and after treatment with AZM or placebo to perform gas chromatography time-of-flight (GC-TOF) mass spectrometry. Identified metabolites were reported if present in at least 50% of the samples per study design group (as defined in the software). Data were mean-centred and divided by the SD of each variable using MetaboAnalyst. For association with discrete factors, we used the Mann-Whitney test (in the case of two categories). Paired statistics (Wilcoxon signed-rank test) were used for pre-treatment and post-treatment comparisons of continuous parameters, such as changes in metabolites with AZM or placebo (see online supplementary material for more details).
Measurement of in vivo cytokines in BAL fluid and alveolar macrophages
In vivo inflammation was assessed by BAL cell count differential and cytokines. Since analytes in the epithelial lining fluid are diluted with sterile saline during BAL, a concentration step was performed via dialysis against Tris 10 mM pH 7.5, EDTA 1 mM and lyophilisation, using albumin as an internal control. For this, the initial volume of acellular BAL fluid was 5 mL. After lyophilisation at −80° C, sample was resuspended in 60 μL of phosphate-buffered saline. This approach yielded measurable concentrations in 30/39 cytokines in a Luminex platform (Human Cytokine Panel I, Millipore) for all samples. The following cytokines levels were below the detectable limit of the assay (standard curve range=3.2–10 000 pg/mL) and are not included in the analyses: interferon (IFN)-α, IFN-γ, tumour necrosis factor (TNF)-β, interleukin (IL)-1β, IL-2, IL-3, IL-4, IL-10 and IL-17. Values obtained in concentrated BAL fluid were extrapolated to represent concentration in BAL by dividing the obtained levels in picogram/millilitre by 83.3 (since 5 mL of BAL fluid was lyophilised and resuspended in 60 μL). Alveolar macrophages from baseline bronchoscopy in 12 subjects were isolated from BAL and cultured (106 cells/mL) for 24 hours in Roswell Park Memorial Institute media with 40 ng of lipopolysaccharide (LPS)/mL (Escherichia coli 0.55:B4 and B5, Sigma-Aldrich, St Louis, Missouri, USA) with or without AZM (10 μg/mL, Pfizer, New York, New York, USA). Since we observed changes in microbial metabolites during treatment with AZM, we tested the ex-vivo anti-inflammatory effects of two of those metabolites (glycolic acid and indole-3-acetate) in macrophages obtained from a similar cohort of smokers. To this end, we isolated alveolar macrophages from an additional eight subjects (two smokers and six ex-smokers) who were enrolled in an NIH-funded protocol (Lung Microbiome and Inflammation in Early COPD, NYU IRB# S14-01546, online supplementary table S1). Alveolar macrophages (106 cells/mL) were cultured for 24 hours with 40 ng LPS/mL or LPS added together with glycolic acid (2 mM, Sigma-Aldrich, St Louis, Missouri, USA) or LPS added together with indole-3-acetate (2 mM, Sigma-Aldrich).16 All ex-vivo conditions were done in duplicate and mean values for the measured cytokines were used.
Results
There were no differences in demographic and clinical characteristics or extent of emphysema between subjects in the AZM and placebo groups (table 1). As per design, all subjects were current or ex-smokers (current smokers were 4/10 for the placebo and 2/10 for the AZM group, p=ns) with similar pack/year. Similarly, there were no differences in spirometric values. Emphysema score showed no difference between groups. Despite the presence of varying degree of emphysema in all subjects, four subjects in the placebo group and two in the AZM group met Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria for COPD.17 There were no significant differences in cell count differential between the placebo and AZM group.
Table 1.
Demographic, pulmonary function and BAL cell differential
| Placebo | Azithromycin | p Value | |
|---|---|---|---|
| n=10 | n=10 | ||
| Age (years) | 60.7 (53.1–66.1) | 65.7 (62.1–68.2) | 0.09 |
| Male (%) | 6 (60) | 7 (70) | 0.64 |
| Caucasian (%) | 9 (90) | 10 (100) | 0.31 |
| BMI | 27.7 (26.3–29.9) | 26.7 (25.0–32.2) | 0.63 |
| Current smoker (%) | 4 (40) | 2 (20) | 0.33 |
| Pack-years | 46.5 (32.2–53.2) | 35.5 (22.5–48.5) | 0.31 |
| PFT | |||
| FVC* | 98.2 (89.0–110.3) | 99.7 (85.8–105.4) | 0.68 |
| FEV1* | 90.4 (81.2–96.0) | 90.5 (81.5–100.9) | 0.14 |
| FEV1/FVC | 66.9 (61.8–79.0) | 72.7 (67.4–74.3) | 0.31 |
| FVC post-BD* | 96.8 (90.1–105.0) | 98.0 (84.2–104.6) | 0.71 |
| FEV1 post-BD* | 93.9 (85.5–104.1) | 96.0 (84.9–109.0) | 0.55 |
| FEV1/FVC post-BD | 70.8 (65.5–84.2) | 73.0 (70.6–78.4) | 0.55 |
| COPD GOLD criteria | |||
| GOLD 1 | 3 | 2 | 0.61 |
| GOLD 2 | 1 | 0 | 0.31 |
| GOLD criteria not met | 6 | 8 | 0.33 |
| Imaging | |||
| Emphysema score9 11 | 0.83 (0.66–1.25) | 1.08 (0.41–1.66) | 0.91 |
| % lung voxels <−950 HU | 21 (16–23) | 17 (12–25) | 0.65 |
| Centrilobular predominant (%) | 9 (90) | 7 (70) | 0.26 |
| BAL cells (%) | |||
| Macrophages | 90.8 (88.2–92.4) | 89.8 (85.0–95.3) | 0.91 |
| Lymphocytes | 6.5 (4.1–9.4) | 4.3 (3.3–10.7) | 0.52 |
| Neutrophils | 1.8 (1.1–2.9) | 1.6 (1.2–4.6) | 0.68 |
| Eosinophils | 0.0 (0.0–0.3) | 0.1 (0.0–1.0) | 0.73 |
| PneumotypeSPT (%) | 4 (40) | 4 (40) | 1.00 |
Data are presented as percentage or median (IQR).
p Values based on Mann-Whitney U test or χ2 test (continuous or categorical variables, respectively).
*Percentage predicted.
ATS, American Thoracic Society; BAL, bronchoalveolar lavage; BD, bronchodilator (albuterol); BMI, body mass index; ERS, European Respiratory Society; GOLD, Global Initiative for Chronic Obstructive Lung Disease; PFT, pulmonary function testing according to standard ATS/ERS guidelines; PneumotypeSPT, pneumotype for supraglottic predominant taxa.
After 8 weeks, subjects returned for their second bronchoscopy. Two subjects in the AZM group and one in the placebo group experienced a self-limited episode of diarrhoea during the study period. There were no other new symptoms reported by any subject and compliance with treatment based on pill diary was 97% and 98% treatment/days for the placebo group and AZM group, respectively (p=ns). Cell count and differential did not change significantly between baseline and post-treatment in either group (data not shown).
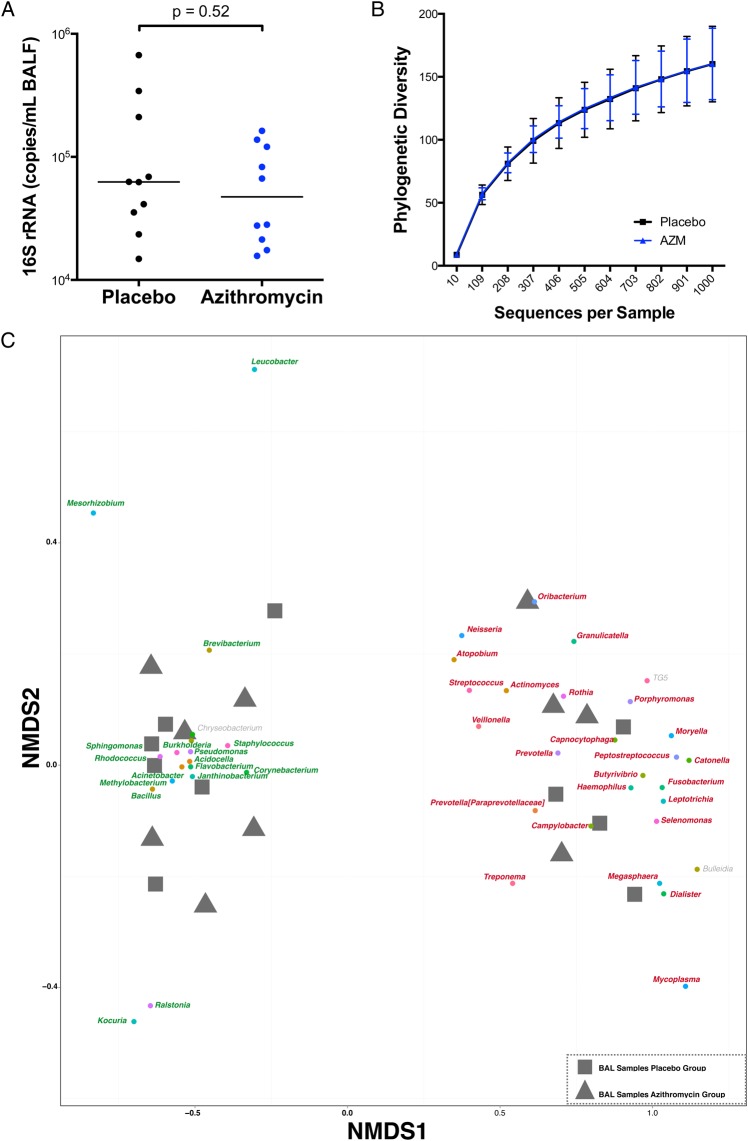
At baseline, the acellular BAL fluids from the placebo and AZM groups had similar bacterial loads (62.5(32.3–243.7) vs 47.3(20.3–125.0)×103 16S rRNA copies/mL respectively, p=ns) (figure 2A). For comparison, the bacterial load present in the suctioning channel of bronchoscope prior to bronchoscopy tended to be lower (27.9(11.4–61.4)×103 16S rRNA copies/mL, p=ns). There were no significant differences in α-diversity (within-sample diversity) as estimated by the number of unique OTUs rarified by sequence depth (figure 2B). Evaluation of microbial similarity between background and supraglottic samples was performed using NMDS based on Bray-Curtis dissimilarity to visualise clustering patterns among samples and its relationship with dominant taxa. Online supplementary figure S1 shows that background samples are clearly distinct from supraglottic samples and are used to define upper airway taxa and background taxa. We then analysed ecological similarity between acellular BAL samples using a similar NMDS approach. Consistent with the two distinct pneumotypes previously defined,5 the acellular BAL samples again showed two groups at baseline, in which the clusters were mainly driven by enrichment with upper airway taxa (defined as pneumotypeSPT for supraglottic predominant taxa) or background taxa (defined as pneumotypeBPT for background predominant taxa) (see figure 2C and online supplementary figure 2). Consistent with prior data, samples allocated to pneumotypeSPT had higher bacterial load than samples allocated to pneumotypeBPT (141.7(72.3–309.5) vs 31.7 (18.4–30.9)×103 16S rRNA copies/mL, p=0.002).5 Importantly, the placebo and AZM groups had similar numbers of BAL samples enriched with upper airway (pneumotypeSPT) or background taxa (pneumotypeBPT). To evaluate the relative contribution of the background microbiota to the BAL samples, we used SourceTracker, where background microbiota was used as the source. This analysis showed that a similar proportion of background characteristic taxa was found in BAL samples of both groups (64(34–87) vs 55(44–73)% for the placebo and AZM groups, respectively), indicating that there were no differences in contamination with environmental microbiome (microbiome present in the bronchoscope prior to bronchoscopy). At baseline, levels of metabolites, cytokine/chemokine/growth factors and cell differentials in BAL were also similar in the placebo and AZM groups (see table 1, online supplementary tables 2 and 3).
Figure 2.
Graphs showing baseline microbiome. (A) Total bacterial 16S rRNA gene was measured by real-time quantitative PCR of 16S rRNA using universal primers. (B) Rarefaction curves of phylogenetic α-diversity showed no significant differences between the placebo and azithromycin (AZM) group at baseline. (C) β-Diversity of baseline bronchoalveolar lavage (BAL) samples was distributed in two distinct clusters. Similar to online supplementary figure S1, Nonmetric Multidimensional Scaling (NMDS) analysis was performed where grey square samples represent samples from subjects assigned to the placebo group and grey triangle samples represent samples from subjects assigned to the AZM group. Similar to online supplementary figure S1, samples clustered in two distinct groups driven by taxa characteristic for background (pneumotypeBPT), represented in green, or by taxa characteristic for supraglottic (pneumotypeSPT), represented in red. Equal number of samples from placebo and AZM groups was present in both clusters (six in pneumotypeBPT and four in pneumotypeSPT from each group). BALF, BAL fluid.
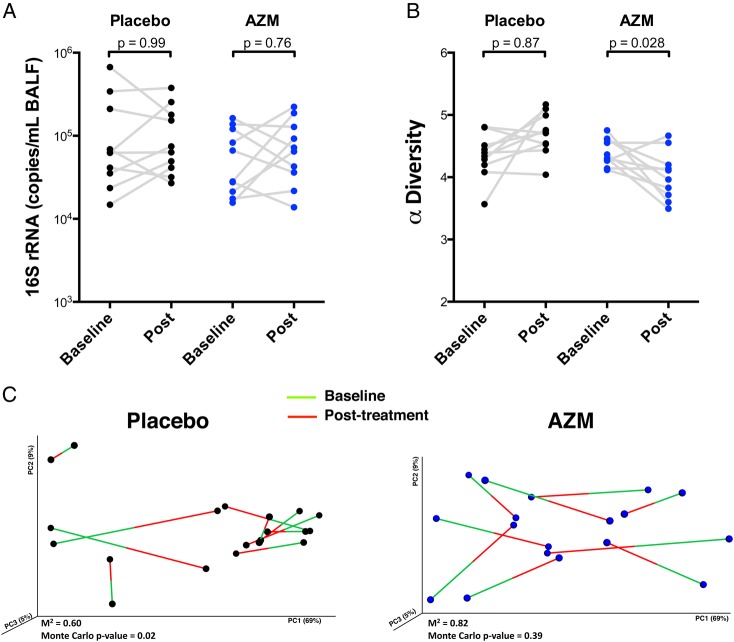
Compared with placebo, AZM did not affect the lower airway bacterial burden (figure 3A) but reduced α-diversity as estimated by Shannon's diversity index (figure 3B). To evaluate whether AZM affected community structure (βdiversity or between-sample diversity), we used Procrustes analysis to assess similarity of UniFrac distances of paired baseline and post-treatment samples (figure 3C). This analysis evaluated how resilient (degree of disturbance of the microbial composition over time) the lower airway microbiome was during treatment with placebo or AZM. Using Monte Carlo simulations, pre-treatment and post-treatment microbiomes in the placebo group were significantly related to one another (Monte Carlo p value=0.02; M2=0.606), indicating stability of lower airway microbial communities over time. However, for the AZM group, baseline β-diversity was not similar to post-AZM β-diversity (Monte Carlo p value=0.39; M2=0.819), indicating that AZM impacted the microbial community composition.
Figure 3.
Effects of azithromycin (AZM) on the lower airway microbiome are shown. (A) Bacterial load assessed by quantitative PCR of 16S rRNA gene from samples obtained post-treatment is shown. (B) Comparison of α-diversity (based on Shannon's diversity index where sequences were rarified at 1000 reads per sample) before and after treatment is shown. (C) Change in β-diversity before and after placebo or AZM had been evaluated using Procrustes analysis. Random permutations had been done using Monte Carlo simulation. BALF, bronchoalveolar lavage fluid.
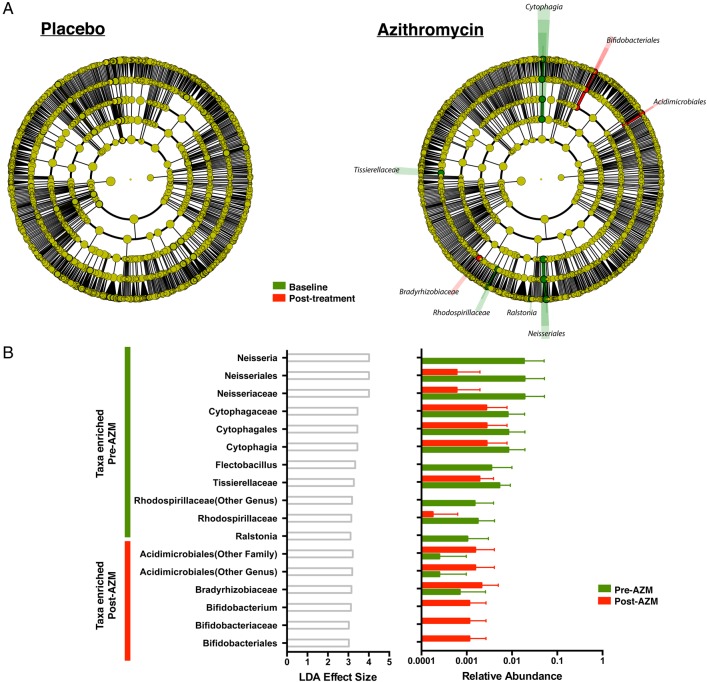
Using LEfSe to define the taxonomic changes in the post-AZM samples, taxa reduced representatives of Tissierellaceae, Cytophaga, Flectobacillus, Neisseria, Ralstonia and Rhodospirillaceae and enriched representatives of Acidimicrobiales, Bifidobacterium and Bradyrhizobiaceae (figure 4A, B). Notably, none of the taxa that shifted due to AZM treatment was highly abundant. For the taxa shown to decrease with AZM treatment, only Neisseria is a known target for macrolides.18 19 However, based on the UniProt database,20 Neisseria, Cytophaga and Flectobacillus have a gene annotated as ‘Macrolide export ATP-binding/permease protein’, while Ralstonia has a gene annotated as Macrolide-efflux protein (RRSL_04706). In contrast, there were no significant taxonomic changes in the placebo group.
Figure 4.
Evaluation of change in taxonomic composition after placebo or azithromycin (AZM) treatment is shown. (A) Linear discriminant analysis (LDA) effect size (LEfSe) is calculated comparing 16S data at baseline and after 8 weeks of placebo/AZM. No taxonomic differences are noted in placebo group. However, there had been several consistent taxonomic changes in the AZM group as evident by differences in colour of cladogram (red increased post-AZM treatment and green decreased post-AZM). (B) LDA effect size of taxa is found to be differentially enriched (LDA>2) pre-AZM and post-AZM treatment and its correspondent relative abundances pre-AZM and post-AZM are plotted as a bar graph.
Since there were taxonomic changes detected in the AZM group, we evaluated whether there were changes in the lower airway microbial genomic potential during placebo and AZM treatment using PICRUSt, a software tool that predicts the functional profile of a bacterial community based on 16S rRNA genes. LEfSe analysis of the inferred metagenome data for paired samples (before and after treatment) showed no significant (LDA effect >2) metagenomic changes over time in the placebo or AZM groups (data not shown).
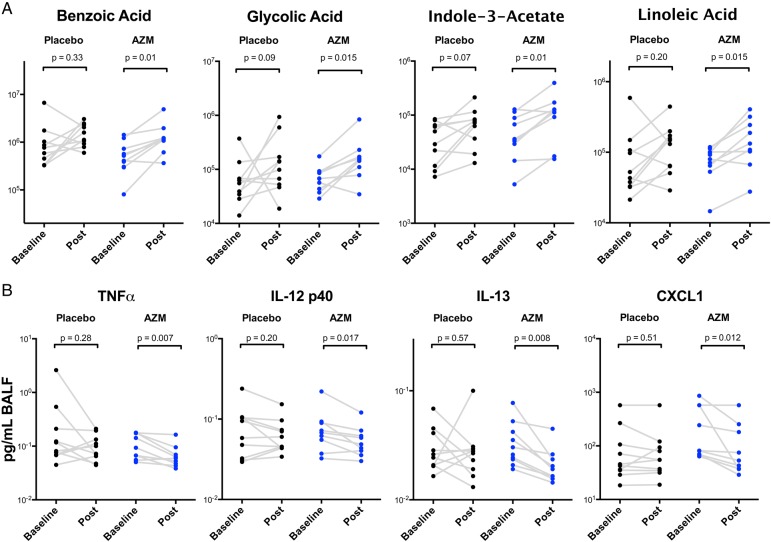
After AZM treatment, of the 119 named metabolites identified by GC-TOF metabolomics in BAL, 14 (11.4%) increased significantly (see online supplementary table S2). Among the metabolites that significantly changed in the AZM group, the highest scores reflected known bacterial metabolites: benzoic acid, indole-3-acetate, glycolic acid and linoleic acid (figure 5A) with a p value <0.02. In contrast, in the placebo group, fewer metabolites changed (8/119) and no metabolites changed with p value <0.02, suggesting that fewer metabolic changes occurred in this group. Importantly, the bacterial metabolites that increased with AZM may reflect responses to stress,11 21–24 supporting the hypothesis that the lower airway microbiome is metabolically active.
Figure 5.
Azithromycin (AZM) treatment increased bacterially produced metabolites and decreased inflammatory mediators in the lower airways. (A) Metabolites were measured by gas chromatography-mass spectrometry. Among metabolites shown to change post treatment with AZM (and not placebo), Of the 14 metabolites, 4 were identified as potential microbial metabolites. Comparisons between metabolites levels pre-treatment versus post-treatment had been based on Wilcoxon rank-sum test. (B) Concentrated bronchoalveolar lavage fluid (BALF) was used to measure cytokines with Luminex. Paired comparisons between pre-treatment versus post-treatment had been based on the Wilcoxon rank-sum test. CXCL1, chemokine (C-X-C) ligand 1; IL, interleukin; TNF, tumour necrosis factor.
To assess whether pulmonary inflammation was affected by the treatments, we performed immunoassays on BAL fluid. Levels of TNF-α, IL-12 p40, IL-13 and chemokine (C-X-C) ligand 1 (CXCL1) were significantly reduced by the AZM treatment (figure 5B), but no measured cytokines, chemokines or growth factors changed significantly in the placebo group (see online supplementary table S3). There was no significant change in cell differential in either group (data not shown).
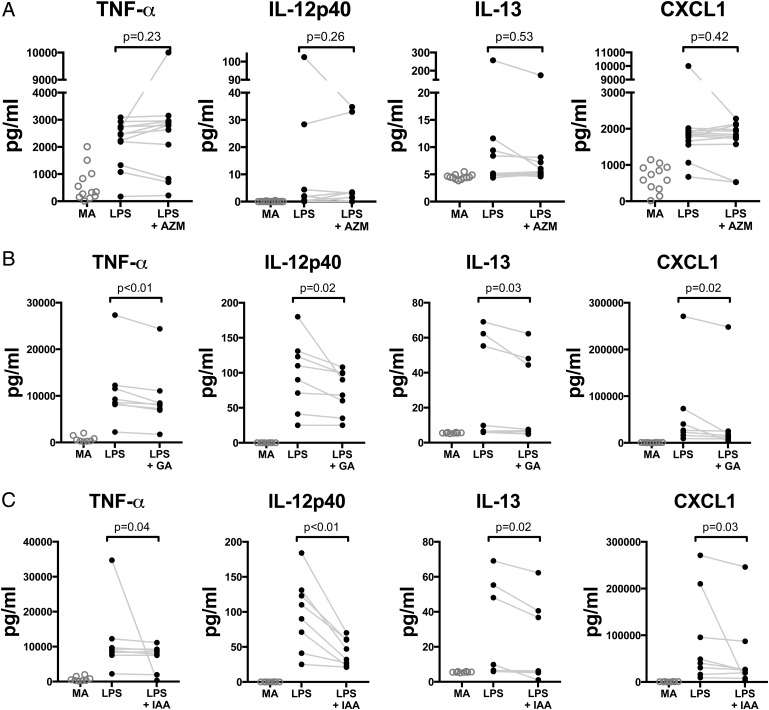
Glycolic acid, a short chain fatty acid, and indole-3-acetate, a tryptophan metabolite, are members of metabolic classes commonly produced by bacteria that may suppress macrophage proinflammatory properties.13 25 Next, we examined whether glycolic acid and indole-3-acetate inhibited production of TNF-α, IL-12 p40, IL-13 and CXCL1 in LPS-stimulated alveolar macrophages from smokers. While AZM did not decrease production of TNF-α, IL-12 p40, IL-13 and CXCL1 during LPS stimulation of macrophages (figure 6A), both glycolic acid and indole-3-acetate blunted ex-vivo production of all four following LPS stimulation (figure 6B, C). These observations in ex-vivo alveolar macrophages suggest that the anti-inflammatory effects seen in the AZM group might be mediated by metabolites, such as glycolic acid and indole-3-acetate, more than through direct effect of AZM on alveolar macrophages.
Figure 6.
Graphs showing evaluation of the effects of azithromycin (AZM), glycolic acid and indole-3-acetate on ex-vivo cytokine production during TLR4 stimulation. (A) Alveolar macrophages obtained during baseline bronchoscopy from 12 enrolled subjects were cultured in media alone (MA), were exposed to lipopolysaccharide (LPS) 40 ng or LPS+AZM (10 μg/mL). Compared with MA, LPS stimulation yielded significant increase in tumour necrosis factor (TNF)-α, interleukin (IL)-12p40, IL-13 and chemokine (C-X-C) ligand 1 (CXCL1) (p value <0.01 for all comparisons). AZM did not significantly decreased the concentration of these cytokines during LPS stimulation. (B and C) Alveolar macrophages from eight subjects from a similar cohort were cultured in MA, LPS 40 ng, LPS+glycolic acid (2 nM) or LPS+indole-3-acetate (2 nM). Compared with MA, LPS stimulation yielded significant increase in TNF-α, IL-12p40, IL-13 and CXCL1 (p value <0.01 for all comparisons). Both glycolic acid and indole-3-acetate significantly decreased the concentration of these cytokines during LPS stimulation. Each pair is an individual's bronchoalveolar lavage alveolar macrophage preparation. Paired comparisons are based on Wilcoxon rank-sum test. TLR4, Toll-like receptor 4.
Discussion
This study demonstrates that AZM affects the lung microbiome in smokers with early emphysema causing a small but consistent reduction of the α-diversity. However, since the overall bacterial load was unchanged, the effects may be considered as substitutional. AZM treatment led to the increase in lower airway concentrations of the bacteria-produced metabolites benzoic acid, glycolic acid, indole-3-acetate and linoleic acid and led to the decrease in levels of TNF-α, IL-12 p40, IL-13 and CXCL1 in the lung. In ex-vivo LPS-stimulated alveolar macrophages, glycolic acid and indole-3-acetate, but not AZM, reduced the levels of TNF-α, IL-12 p40, IL-13 and CXCL1. These in-vivo and ex-vivo findings provide evidence of AZM effects on bacterial populations and their metabolites as factors in suppressing inflammatory changes.
In clinical studies, daily intake of AZM (250 mg) decreased the frequency of COPD exacerbations and improved quality of life1 at tissue concentrations below the minimal inhibitory concentration for common pulmonary pathogens.26 Compared with placebo, 6 months of erythromycin (125 mg thrice daily) reduced total cells, neutrophils and neutrophil elastase in sputum,27 suggesting that macrolides have direct anti-inflammatory effects. However, mechanistic interpretation of the critical events has been limited by inability to comprehensively characterise the microbial community of the lung. Our observations of the impact of AZM on the lower airway microbiota, bacterial metabolites and lower airway cytokines are consistent with its known anti-inflammatory effects. During COPD exacerbations, neutrophils and TNF-α increase, a process associated with loss of lung function. CXCL1, a central chemokine for neutrophil recruitment to the lung, is elevated in both COPD and overt infection28 29 and IL-13 strongly induces CXCL1 in human airway cells.30 However, the lack of AZM effect in our ex-vivo experiments suggests that its anti-inflammatory effects are not mediated through direct interaction with alveolar macrophages. Exposure to antibiotics, including macrolides, are typical stimuli leading to induction of bacterial metabolic genes and metabolites,31 including short chain fatty acids (SCFA) including glycolate,32 found to be elevated in the BALs following AZM. Bacterial SCFA also inhibit cytokine production and inflammation after LPS stimulation of macrophages.16 The increase of indole-3-acetate, a bacterial metabolite, after AZM treatment is induced by oxidative stress.21 33 Erythromycin induction of the tryptophan operon22 23 is consistent with the increased levels of indole-3-acetate post-AZM. That indole-3-acetate and glycolic acid are induced by AZM and inhibit in-vitro production of proinflammatory cytokines in LPS-stimulated alveolar macrophages provides a plausible explanation for some of the anti-inflammatory effects of AZM in the lung.
This investigation is limited by the small sample size and its focus on subjects with relatively mild disease. However, recruitment of this group may help identify early steps in pathogenesis. In our cohort, only few patients met GOLD criteria for COPD and, for the remaining subjects, it is impossible to know who will develop COPD. It will be, therefore, important to also examine the effects of AZM on the microbial environment of the lower airways in a cohort of patients with COPD with more advanced disease and frequent exacerbations, in whom more prolonged treatment with AZM has been shown to exert a beneficial clinical effect.1 A major challenge to study this population is the increased risk of invasive procedures such as bronchoscopy; non-invasive markers would be more desirable. A large proportion of BAL samples were dominated by background taxa, which tend to have low bacterial burden. In these samples, the true composition of the lower airway microbiome is more difficult to establish due to a low signal-to-noise ratio. Studies that include subjects with higher bacterial burden (such in more advanced COPD) are needed to clarify the effects of macrolides on the composition of the microbial community of the lower airways. Of note, a recent placebo-controlled study did not show significant change in bacterial burden in sputum after macrolides,4 similar to the results of this study. Replication is also important because of the limited ability of a small study to control for the effects of multiple comparisons and the modest effect sizes in our assays. This is also important since it is possible that some subject's characteristics might have actually differed between groups but did not reach statistical significance due to the small sample size. Nevertheless, the paucity of changes in the placebo group, the observed changes in multiple platforms and the ex-vivo experimental validation increase confidence in the findings and interpretation. Moreover, our power to detect differences was increased by our longitudinal assessment, using each subject as their own control. In this study, we used acellular BAL fluid, a type of sample that has been used before to describe the lower airway microbiome.5 10 34–36 Other studies have used whole BAL; head-to-head comparison of acellular versus whole BAL showed that in some samples, taxa differed significantly with loss of some Pseudomonas and Escherichia OTUs in the acellular BAL sample.11 While whole BAL fluid may somewhat differ in taxonomic composition compared with acellular BAL samples, the reduction of α-diversity and changes in taxonomic composition before and after AZM, but not in the placebo group, occur in samples that were consistently processed similarly. Thus, the observed differences cannot be explained by differences in technical processing or composition of whole BAL versus acellular BAL sample. Although we provide evidence that some metabolites of possible bacterial origin induced by AZM are anti-inflammatory ex-vivo, we cannot distinguish the antibiotic and anti-inflammatory effects of AZM in these subjects. Neither can we assess the effects of AZM on other microbial communities such as the gut that might contribute to the host immune phenotype. A transcriptome approach would help distinguish microbial from mammalian metabolic effects on measured metabolites. However, technical challenges due to low bacterial concentration further limit the evaluation of microbial genes impacted by AZM in-vivo.
Conclusion
In summary, AZM exerts multiple effects on the structure and composition of the lower airway microbiota. The concomitant increase in several microbial metabolites provides evidence that the lung microbiome is metabolically responsive to the AZM-induced stress. The stressed microbiome releases microbial metabolites with well-defined anti-inflammatory effects, suggesting that AZM's therapeutic benefit includes altering the lung microbiome interaction with the host immune system. Experiments in model systems, required to precisely define the anti-inflammatory effects of microbial metabolic products produced during AZM treatment, may yield novel strategies for COPD.
Footnotes
Contributors: Conception and design: LNS, MJB and MDW. Acquisition of data: LNS, ZG, YL and JPK. Analysis and interpretation of data: LNS, JCC, BGW, JPK, MJB and MDW. Drafting or revising of article: LNS, JCC, WNR, MJB and MDW. Final approval of the manuscript: LNS, JCC, BGW, WRW, ZG, YL, JPK, WNR, MJB and MDW.
Funding: K23 AI102970 (LNS); K24 AI080298 (MDW); CTSI Grant #UL1 TR000038; EDRN 5U01CA086137-13; Diane Belfer Program for Human Microbial Ecology; R01DK090989; U01AI122285-01, UH2 AR57506.
Competing interests: JPK reports a speaker honorarium from Siemens, AG, outside the submitted work; none of the other authors declare any other competing interests.
Ethics approval: New York University Institutional Review Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: All data are publicly available in Gene Expression Omnibus under accession number GSE74396.
This article has an online supplementary data.
References
- 1.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011;365:689–98. 10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi S, Jones PW, Theron MS, et al. Pulsed moxifloxacin for the prevention of exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Respir Res 2010;11:10 10.1186/1465-9921-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethi S, Evans N, Grant BJ, et al. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med 2002;347:465–71. 10.1056/NEJMoa012561 [DOI] [PubMed] [Google Scholar]
- 4.Brill SE, Law M, El-Emir E, et al. Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: a randomised controlled trial. Thorax 2015;70:930–8. 10.1136/thoraxjnl-2015-207194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal LN, Alekseyenko AV, Clemente JC, et al. Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome 2013;1:19 10.1186/2049-2618-1-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassis CM, Erb-Downward JR, Dickson RP, et al. Analysis of the upper respiratory tract microbiotas as the source of the lung and gastric microbiotas in healthy individuals. mBio 2015;6:e00037 10.1128/mBio.00037-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Charlson ES, Bittinger K, Haas AR, et al. Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 2011;184:957–63. 10.1164/rccm.201104-0655OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris A, Beck JM, Schloss PD, et al. Comparison of the Respiratory Microbiome in Healthy Non-Smokers and Smokers. Am J Respir Crit Care Med 2013;187:1067–75. 10.1164/rccm.201210-1913OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sze MA, Dimitriu PA, Hayashi S, et al. The lung tissue microbiome in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2012;185: 1073–80. 10.1164/rccm.201111-2075OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Segal LN, Clemente JC, Tsay J-CJ, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol 2016;1:16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson RP, Erb-Downward JR, Prescott HC, et al. Cell-associated bacteria in the human lung microbiome. Microbiome 2014;2:28 10.1186/2049-2618-2-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianco C, Imperlini E, Calogero R, et al. Indole-3-acetic acid improves Escherichia coli's defences to stress. Arch Microbiol 2006;185:373–82. 10.1007/s00203-006-0103-y [DOI] [PubMed] [Google Scholar]
- 13.Zelante T, Iannitti RG, Cunha C, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013;39:372–85. 10.1016/j.immuni.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 14.Suzaki H, Asano K, Ohki S, et al. Suppressive activity of a macrolide antibiotic, roxithromycin, on pro-inflammatory cytokine production in vitro and in vivo. Mediators Inflamm 1999;8:199–204. 10.1080/09629359990351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol 2011;12:R60 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang PV, Hao L, Offermanns S, et al. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci USA 2014;111:2247–52. 10.1073/pnas.1322269111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–65. 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 18.Bignell C, Garley J. Azithromycin in the treatment of infection with Neisseria gonorrhoeae. Sex Transm Infect 2010;86:422–6. 10.1136/sti.2010.044586 [DOI] [PubMed] [Google Scholar]
- 19.Furuya R, Nakayama H, Kanayama A, et al. In vitro synergistic effects of double combinations of beta-lactams and azithromycin against clinical isolates of Neisseria gonorrhoeae. J Infect Chemother 2006;12:172–6. 10.1007/s10156-006-0445-z [DOI] [PubMed] [Google Scholar]
- 20.UniProt C. UniProt: a hub for protein information. Nucleic Acids Res 2015;43(Database issue):D204–12. 10.1093/nar/gku989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patten CL, Blakney AJ, Coulson TJ. Activity, distribution and function of indole-3-acetic acid biosynthetic pathways in bacteria. Crit Rev Microbiol 2013;39:395–415. 10.3109/1040841X.2012.716819 [DOI] [PubMed] [Google Scholar]
- 22.Ng WL, Kazmierczak KM, Robertson GT, et al. Transcriptional regulation and signature patterns revealed by microarray analyses of Streptococcus pneumoniae R6 challenged with sublethal concentrations of translation inhibitors. J Bacteriol 2003;185:359–70. 10.1128/JB.185.1.359-370.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia Q, Muraoka WT, Shen Z, et al. Adaptive mechanisms of Campylobacter jejuni to erythromycin treatment. BMC Microbiol 2013;13:133 10.1186/1471-2180-13-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donati AJ, Lee HI, Leveau JH, et al. Effects of indole-3-acetic acid on the transcriptional activities and stress tolerance of Bradyrhizobium japonicum. PLoS ONE 2013;8:e76559 10.1371/journal.pone.0076559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin UH, Lee SO, Sridharan G, et al. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol 2014;85:777–88. 10.1124/mol.113.091165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peckham DG. Macrolide antibiotics and cystic fibrosis. Thorax 2002;57:189–90. 10.1136/thorax.57.3.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He ZY, Ou LM, Zhang JQ, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration 2010;80:445–52. 10.1159/000321374 [DOI] [PubMed] [Google Scholar]
- 28.Sapey E, Stockley JA, Greenwood H, et al. Behavioral and structural differences in migrating peripheral neutrophils from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011;183:1176–86. 10.1164/rccm.201008-1285OC [DOI] [PubMed] [Google Scholar]
- 29.Traves SL, Culpitt SV, Russell RE, et al. Increased levels of the chemokines GROalpha and MCP-1 in sputum samples from patients with COPD. Thorax 2002;57:590–5. 10.1136/thorax.57.7.590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meyer-Hoffert U, Lezcano-Meza D, Bartels J, et al. Th2- and to a lesser extent Th1-type cytokines upregulate the production of both CXC (IL-8 and gro-alpha) and CC (RANTES, eotaxin, eotaxin-2, MCP-3 and MCP-4) chemokines in human airway epithelial cells. Int Arch Allergy Immunol 2003;131:264–71. doi:72138 [DOI] [PubMed] [Google Scholar]
- 31.Dörries K, Schlueter R, Lalk M. Impact of antibiotics with various target sites on the metabolome of Staphylococcus aureus. Antimicrob Agents Chemother 2014;58:7151–63. 10.1128/AAC.03104-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nancucheo I, Johnson DB. Production of glycolic acid by chemolithotrophic iron- and sulfur-oxidizing bacteria and its role in delineating and sustaining acidophilic sulfide mineral-oxidizing consortia. Appl Environ Microbiol 2010;76:461–7. 10.1128/AEM.01832-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gerstle K, Klätschke K, Hahn U, et al. The small RNA RybA regulates key-genes in the biosynthesis of aromatic amino acids under peroxide stress in E. coli. RNA biology 2012;9:458–68. 10.4161/rna.19065 [DOI] [PubMed] [Google Scholar]
- 34.Lozupone C, Cota-Gomez A, Palmer BE, et al. Widespread Colonization of the Lung by Tropheryma whipplei in HIV Infection. Am J Respir Crit Care Med 2013;187:1110–17. 10.1164/rccm.201211-2145OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Twigg HL, Day RB, Gregory RL, et al. Comparison of whole and acellular bronchoalveolar lavage to oral wash microbiomes. Should acellular bronchoalveolar lavage be the standard? Ann Am Thorac Soc 2014;11:S72–3. 10.1513/AnnalsATS.201306-162MG [DOI] [Google Scholar]
- 36.Twigg H, Knox KS, Zhou J, et al. Effect of Advanced HIV Infection on the Respiratory Microbiome. Am J Respir Crit Care Med 2016;194:226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
thoraxjnl-2016-208599supp.pdf (574.2KB, pdf)