Summary
Characterizing plant responses to past, present, and future changes in atmospheric [CO2] is critical for understanding and predicting the consequences of global change over evolutionary and ecological timescales. Previous CO2 studies have provided great insights into the effects of rising [CO2] on leaf-level gas exchange, carbohydrate dynamics, and plant growth. However, scaling [CO2] effects across biological levels, especially in field settings, has proved challenging. Moreover, many questions remain about the fundamental molecular mechanisms driving plant responses to [CO2] and other global change factors. Here we discuss three examples of topics in which significant questions in CO2 research remain unresolved: (1) mechanisms of [CO2] effects on plant developmental transitions; (2) implications of rising [CO2] for integrated plant–water dynamics and drought tolerance; and (3) [CO2] effects on symbiotic interactions and eco-evolutionary feedbacks. Addressing these and other key questions in CO2 research will require collaborations across scientific disciplines and new approaches that link molecular mechanisms to complex physiological and biological interactions across spatiotemporal scales.
Keywords: atmospheric carbon dioxide, climate change, eco-evolutionary feedbacks, flowering time, leaf gas exchange, plant hydraulics, plant, microbe interactions
I. Introduction
CO2 studies have comprised an important component of plant biology research for decades, as CO2 is the primary carbon source for photosynthesis and a major driver of climate change. Only 20000 yr ago, atmospheric [CO2] were among the lowest levels that occurred during the evolution of land plants (180–200 ppm). Since then, [CO2] has more than doubled to 401 ppm due to greenhouse gas emissions, levels that plants have not encountered for several million years (Tripati et al., 2009). Consequently, [CO2] may be rising faster than the rate at which some plant species can evolve to function optimally (Lau et al., 2007; but see Ward et al. 2000). In addition, current levels of plasticity for functional traits may be inadequate to maintain optimal functioning in novel future environments (Franks et al., 2014; Anderson & Gezon, 2015).
Over the past several decades, CO2 studies have met with great success in characterizing the effects of elevated [CO2] on leaf-level gas exchange, carbohydrate dynamics, and plant growth (Ainsworth & Long, 2005; Franks et al., 2013). Large-scale studies (particularly free-air CO2 enrichment studies, FACE) have further provided insight into the effects of elevated [CO2] on plant community dynamics and productivity (Norby et al., 2016). Nonetheless, many questions remain about plant responses to rising [CO2], as well as interactions with other changing environmental factors, particularly at the most fundamental mechanistic levels. Specifically, molecular and whole plant responses to [CO2] scale to influence higher order processes; however, such scaling factors are far from resolved and are highly variable across ecosystems. Additionally, increases in [CO2] are strongly coupled with rising temperatures and drought, and the interactive effects of these drivers on plant functioning are unclear at almost every level. The new generation of FACE studies can help address these challenges by facilitating cross-site analysis of plant through ecosystem responses to experimental [CO2] manipulations (Norby et al., 2016). Studies spanning preindustrial through future [CO2] can further provide a baseline for plant functioning before human intervention (Medeiros & Ward, 2013; Becklin et al., 2016). Finally, multigenerational and molecular-level studies can elucidate potential evolutionary responses to [CO2] (Ward et al., 2000; Watson-Lazowski et al., 2016).
Later we discuss three topics in CO2 research with immediate consequences for global carbon cycling, food security, and ecosystem services (Fig. 1). How does [CO2] affect plant developmental transitions? What are the implications of rising [CO2] for plant–water dynamics? And ho w does [CO2] impact feedbacks in plant–microbe symbioses? Incorporating the interactive effects of [CO2] and climate as well as questions of scale will be important for understanding each of these topics. Although this is by no means an exhaustive list, we use these examples to emphasize why CO2 research is more critical than ever, and will remain so long into the future.
Fig. 1.
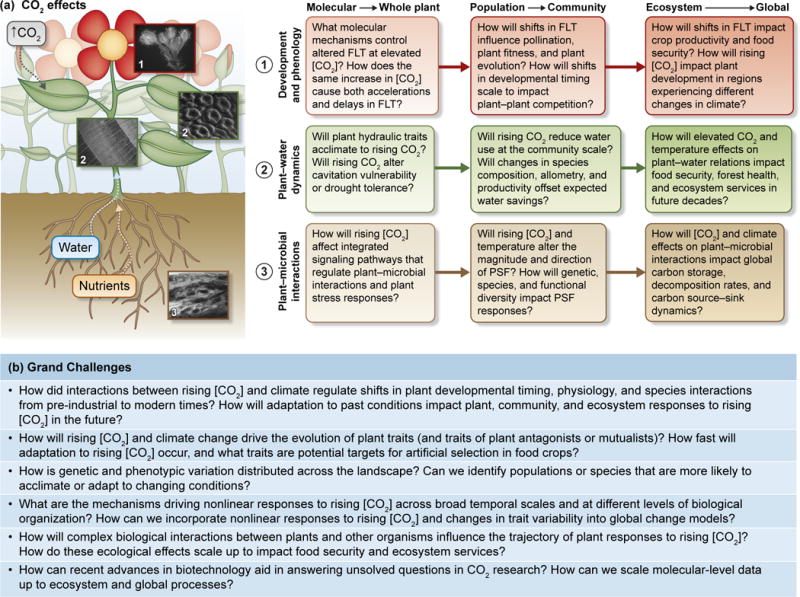
Direct and indirect effects of rising [CO2] on plant development and phenology, plant physiology and water dynamics, and plant interactions with other organisms can have cascading effects on ecological and evolutionary processes across spatial and temporal scales (a). Here we highlight examples of key questions in CO2 research that will require an integrative approach that bridges gaps between molecular biology, plant physiology, ecological and evolutionary biology, and ecosystem science (b). This is by no means an exhaustive list, and not all questions are specifically discussed in the main text. Images show (a1) a developing Arabidopsis flower bud, (a2) stoma (upper image, Juniperus) and xylem (lower image, Picea) structures, and (a3) arbuscular mycorrhizal fungi colonizing herbaceous roots. Images were provided courtesy of R. Atkinson, K. M. Becklin, V. Bui, and S. M. Walker. FLT, flowering time; PSF, plant–soil feedback.
II. CO2 effects on plant development and phenology
Numerous studies show that elevated [CO2] can impact plant developmental processes, particularly flowering time (FLT). However, we are just beginning to characterize the mechanisms driving these developmental shifts and their implications for individual plants, communities, and ecosystems. Shifts in FLT can alter the course of evolution, influence community competition, disrupt plant–pollinator interactions, and affect crop/food production (Bartomeus et al., 2011; Rafferty & Ives, 2012). In a literature survey, 57% of wild species and 62% of crop species exhibited altered FLT when grown at elevated [CO2], with extreme FLT responses ranging from accelerations of 60 d to delays of 16 d depending on the species (Springer & Ward, 2007). The effects of elevated [CO2] on FLT can also vary within species, such as in Arabidopsis thaliana ecotypes and soybean lines, which exhibited both delayed and accelerated FLT at elevated [CO2] (Ward & Kelly, 2004; Bunce & Hilacondo, 2016). Additionally, plants do not always flower at the same size in novel environments, including elevated [CO2] (Springer et al., 2008; Springate & Kover, 2014). This may be due to [CO2] effects on signaling mechanisms that act independently of plant size or growth rate. Thus, rising [CO2] can alter plant size at key life cycle milestones, including flowering (Fig. 2), with possible downstream effects on fitness and carbon cycling.
Fig. 2.
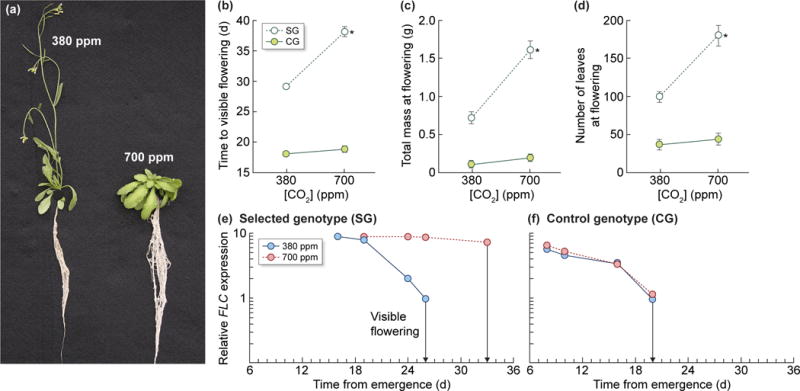
(a) A genotype of Arabidopsis thaliana (SG; see Springer et al., 2008) that is sensitive to elevated [CO2] with respect to flowering time. Both representative plants are of the same genotype and age and were grown at 380 and 700 ppm [CO2]. Note that the elevated [CO2] plant has not yet flowered, although it has accumulated more total biomass and a greater number of leaves. (b) Time to visible flowering, (c) total biomass at flowering, (d) total number of leaves at flowering for the SG Arabidopsis genotype (white circles) and CG (green circles, control genotype) grown at 380 and 700 ppm [CO2]. (e) Real-time RT-PCR expression patterns for FLC in SG and (f) CG Arabidopsis genotypes grown at 380 ppm (solid blue line) and 700 ppm CO2 (dashed red line). Sampling was performed at the same developmental stages for each genotype. Signals were normalized to the expression level at the time of flowering at 380 ppm within the same genotype. The photo in (a) is courtesy of B. Burgert, and (b–f) were reproduced from Springer et al. (2008).
Although molecular work in this area is still developing, elevated [CO2] has been shown to affect the expression of flowering genes and regulatory pathways. Specifically, the floral repressor, FLC (FLOWERING LOCUS C), was shown to play a key role in influencing FLT at elevated [CO2] in Arabidopsis. In this case, sustained expression of FLC caused a previously selected genotype to flower much later, with much higher total biomass, and many more leaves at elevated vs current [CO2] (Fig. 2) (Springer et al., 2008). Changes in [CO2] have also been shown to regulate the expression of micro RNAs involved in plant development. The miR156 flowering pathway controls the vegetative juvenile-to-adult phase transition and is involved in floral initiation in Arabidopsis (Wang et al., 2009; Wu et al., 2009). A doubling in [CO2] reduced the expression of miR156/157, leading to a 7 d acceleration in FLT (May et al., 2013). Despite these advancements, it is still unclear which upstream processes influence flowering gene expression and micro RNAs during growth at elevated [CO2].
One hypothesis linking elevated [CO2] to altered developmental timing proposes that sugars act as signaling molecules to influence flowering gene expression. Through this mechanism, plant carbohydrate status is sensed in leaves via the metabolite trehalose-6-phosphate (T6P) (Wahl et al., 2013; Figueroa & Lunn, 2016), and FLOWERING LOCUS T (FT) is activated when carbohydrate levels become adequate to support reproduction. FT is then translocated to the meristem where floral induction occurs. Elevated [CO2] generally increases the sugar status of leaves, and altered sugar sensing via T6P may be one possible mechanism for how elevated [CO2] influences FLT (Springer & Ward, 2007; Coneva et al., 2012). Studies in which elevated [CO2] or additions of exogenous sucrose were associated with delays in FLT support this potential mechanism (Posner, 1971). Despite the importance of understanding the mechanisms driving [CO2] effects on plant development and phenology, research that relates sugar-sensing mechanisms to elevated [CO2] is lacking. Further studies exploring molecular drivers of FLT in model and non-model systems will aid in accurately predicting how phenological responses to global change will manifest across spatiotemporal scales.
III. CO2 effects on plant–water dynamics
While we can predict general patterns of how [CO2] alters stomatal conductance, photosynthesis and transpiration in many systems (Ainsworth & Long, 2005), the strength of these effects (Haworth et al., 2016) and major patterns in CO2-responsiveness across plant groups are still being debated (Brodribb & McAdam, 2011; Franks & Britton-Harper, 2016). Thus, despite decades of CO2 research, surprises remain in how rising [CO2] affects plant-water dynamics (Franks et al., 2013). Many of these questions may be better explained by improving our understanding of how plant hydraulics will respond to a range of [CO2], especially in conjunction with increased drought and warming.
When leaves are exposed to elevated [CO2], photosynthesis is stimulated, but stomatal conductance and transpiration decline, increasing leaf water use efficiency. It is commonly assumed that plants grown at elevated [CO2] will therefore use less water and better withstand drought stress; however, this is often not the case (Vaz et al., 2012; Perry et al., 2013; Duan et al., 2014). Elevated [CO2] can increase transpiration or reduce drought tolerance by increasing canopy leaf area (McCarthy et al., 2006; Warren et al., 2011), stimulating nocturnal stomatal conductance (Zeppel et al., 2012), reducing rooting depth (Duursma et al., 2011), and increasing leaf temperatures via lower latent heat loss (Warren et al., 2011). Even when elevated [CO2] promotes soil water savings at larger spatial scales, changes in plant community composition can increase total leaf area index and transpirational potential (Fay et al., 2012), which may leave ecosystems equally vulnerable to drought under current and future [CO2]. Without a greater understanding of how common these responses are under elevated [CO2], we cannot make robust predictions of how rising [CO2] will alter drought tolerance, or properly capture CO2 effects on ecosystem water use efficiency (De Kauwe et al., 2013).
Plant hydraulic responses to rising [CO2] have received less attention than leaf-level gas exchange responses despite the importance of water transport traits in determining drought tolerance, carbon assimilation, and growth. Hydraulic conductance often decreases at elevated [CO2] (Tognetti et al., 2005; Domec et al., 2009), but may also increase (Domec et al., 2010) or show no response (Locke et al., 2013), and can vary in an apparently species-specific manner that we cannot fully account for (Domec et al., 2010). Likewise, cavitation resistance can increase at elevated [CO2] in some species (Rico et al., 2013), while vessel implosion strength, a correlate of cavitation resistance, decreases with rising [CO2] in others (Medeiros & Ward, 2013). These findings highlight that we currently lack a generalized framework for predicting how [CO2] rise will alter hydraulic traits. This issue becomes even more problematic when trying to predict integrated plant function at elevated [CO2] in response to water stress. For example, plants grown at low [CO2] maintained their hydraulic conductance and photosynthesis during drought, while these parameters declined in plants grown at elevated [CO2] (Medeiros & Ward, 2013), indicating that rising [CO2] could reduce plant growth and drought tolerance.
The uncertainties in how changes in [CO2] affect plant water balance are exacerbated when we consider that warming, which will increase concurrently with [CO2], usually exacerbates water stress. While leaf-level physiological responses to these drivers suggest that rising [CO2] can offset the negative effects of warming, evidence for this is mixed (Dieleman et al., 2012; Way et al., 2015). Instead, a growing number of studies find that elevated [CO2] does not ameliorate the impact of high temperatures on drought stress, particularly when water is severely limited (Wertin et al., 2012; Zeppel et al., 2012; Duan et al., 2014). Resolving the interactive effects of globally uniform increases in [CO2] and regionally variable increases in temperature and drought will be necessary for scaling global change effects on plant–water dynamics.
IV. CO2 effects on plant–microbe interactions
Changes in plant physiology, development, and growth with rising [CO2] can influence global change responses at higher biological levels through cascading effects on interactions between plants and other organisms (Gilman et al., 2010). Likewise, direct effects of plant mutualists and antagonists on plant traits and fitness can mediate plant acclimation and evolutionary responses to global change (Lau & Lennon, 2012). [CO2] effects on species interactions and subsequent feedbacks between ecological and evolutionary processes may be especially important for plant–microbe symbioses where the functioning and fitness of the interacting organisms are tightly linked (terHorst & Zee, 2016). However, microbial communities, especially those belowground, remain one of the least understood components of terrestrial ecosystems (Bardgett & van der Putten, 2014), which presents a major challenge when modeling [CO2] effects across biological scales (Smithwick et al., 2014).
Mycorrhizal associations, belowground plant-fungus symbioses in which host plants trade carbohydrates for soil nutrients, are one of the best-studied types of plant–microbe interactions. [CO2] effects on mycorrhizal associations have largely been explored in short-term studies from temperate ecosystems in the northern hemisphere, which limits our understanding of mycorrhizal processes at the global scale (Mohan et al., 2014). Additionally, few studies have examined genetic and phenotypic variation in mycorrhizal traits (Johnson et al., 2012), or the interactive effects of multiple global change drivers (Mohan et al., 2014); thus, substantial questions remain regarding mechanisms driving mycorrhizal responses to rising [CO2] (Fig. 1).
Traditionally, mycorrhizal associations are viewed as nutritional mutualisms that are most beneficial to host plants when soil nutrients limit plant growth (Johnson & Graham, 2013). For this reason, rising [CO2] is predicted to promote stronger mycorrhizal mutualisms. However, observations of mycorrhizal responses to rising [CO2] do not always correspond to this theoretical prediction. In fact, competition between host plants and mycorrhizal fungi for increasingly limited nutrients can result in neutral associations, or even reduce plant growth under elevated [CO2] (Kivlin et al., 2013; Becklin et al., 2016).
Recent work calls for a broader view of mycorrhizal associations that incorporates the functional diversity exhibited by mycorrhizal fungi. For example, some fungi stimulate integrated signaling pathways that enhance their host’s ability to tolerate biotic (e.g. herbivore resistance, Pineda et al., 2013) and abiotic stress (e.g. drought tolerance, Worchel et al., 2013). In fact, most phytohormones examined thus far play a role in regulating mycorrhizal associations (Pozo et al., 2015), and common mycorrhizal networks can serve as a conduit for these signals to pass between neighboring plants (Johnson & Gilbert, 2015). How these signals will integrate environmental cues and regulate multidimensional mycorrhizal responses to rising [CO2] in complex natural communities remains unclear.
There is growing evidence that within-population variation in mycorrhizal traits can affect mycorrhizal associations and ecosystem processes to the same degree as variation among mycorrhizal species (Johnson et al., 2012). Furthermore, multiple experiments highlight the potential for genetically based variation in mycorrhizal traits to facilitate evolutionary responses to environmental conditions (Johnson et al., 2012; van der Heijden et al., 2015). Thus, it is likely that both inter- and intraspecific variation in mycorrhizal associations will generate feedbacks that can drive plant and community responses to global change (van der Putten et al., 2013; terHorst & Zee, 2016). Characterizing adaptive potential in plant and fungal populations, the strength of selection exerted by rising [CO2] and other global change drivers, and the magnitude of eco-evolutionary feedbacks between mycorrhizal partners will be especially important for scaling mycorrhizal responses to global change.
V. The way forward
While we have had success in understanding some topics in CO2 research, large gaps remain in our knowledge of plant responses to rising [CO2] (Fig. 1). Addressing these challenges will require collaboration across disciplines, experiments that span multiple scales of complexity, and the application of –omics technologies and new molecular tools, such as CRISPR-Cas9 that will allow for genome editing and precise mutations in order to study molecular pathways in ecologically relevant species. Additionally, plants host diverse communities of microorganisms that can affect molecular through ecosystem-level processes. Thus, we need to broaden our view of plants to consider how rising [CO2] impacts the physiology, ecology, and evolution of these symbiotic communities. Finally, understanding how rising [CO2] over recent geological history has affected plant traits will provide insights into the evolutionary pressures that have shaped modern plant species. Using a broader experimental [CO2] gradient will generate more informative response curves (rather than two-point comparisons) of plant and community traits, allowing us to better identify thresholds or nonlinear responses that could surprise us in the future. As we move into an even higher CO2 world, it will be imperative that plant breeding programs account for potential developmental, physiological, and ecological shifts that may occur in response to rising [CO2] when developing future crop varieties and biofuels. Moreover, mechanistic studies of plant responses to rising [CO2] will become even more important for predicting global carbon, water, and nutrient cycling and for determining how ecosystem services may shift in the future.
Acknowledgments
K.M.B., S.M.W. and J.K.W. are supported on NSF IOS #1457236 and a Research Investment Council grant from the University of Kansas. K.M.B. was also supported by a NIH IRACDA Postdoctoral Fellowship, and S.M.W was supported by NSF IGERT and GK-12 Fellowships. D.A.W. is supported by an NSERC Discovery grant, CFI and an Ontario ERA award.
References
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Anderson J, Gezon Z. Plasticity in functional traits in the context of climate change: a case study of the subalpine forb Boechera stricta (Brassicaceae) Global Change Biology. 2015;21:1689–1703. doi: 10.1111/gcb.12770. [DOI] [PubMed] [Google Scholar]
- Bardgett RD, van der Putten WH. Belowground biodiversity and ecosystem functioning. Nature. 2014;515:505–511. doi: 10.1038/nature13855. [DOI] [PubMed] [Google Scholar]
- Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, Winfree R. Climate-associated phenological advances in bee pollinators and bee-pollinated plants. Proceedings of the National Academy of Sciences, USA. 2011;108:20645–20649. doi: 10.1073/pnas.1115559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becklin KM, Mullinix GWR, Ward JK. Host plant physiology and mycorrhizal functionining shift across a glacial through future [CO2] gradient. Plant Physiology. 2016 doi: 10.1104/pp.16.00837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodribb TJ, McAdam SAM. Passive origins of stomatal control in vascular plants. Science. 2011;331:582–585. doi: 10.1126/science.1197985. [DOI] [PubMed] [Google Scholar]
- Bunce JA, Hilacondo WC. Responses of flowering time to elevated carbon dioxide among soybean photoperiod isolines. American Journal of Plant Sciences. 2016;7:773–779. [Google Scholar]
- Coneva V, Guevara D, Rothstein SJ, Colasanti J. Transcript and metabolite signature of maize source leaves suggests a link between transitory starch to sucrose balance and the autonomous foral transition. Journal of Experimental Botany. 2012;63:5079–5092. doi: 10.1093/jxb/ers158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Kauwe MG, Medlyn BE, Zaehle S, Walker AP, Dietze MC, Hickler T, Jain AK, Luo Y, Parton WJ, Prentice IC, et al. Forest water use and water use efficiency at elevated CO2: a model–data intercomparison at two contrasting temperate forest FACE sites. Global Change Biology. 2013;19:1759–1779. doi: 10.1111/gcb.12164. [DOI] [PubMed] [Google Scholar]
- Dieleman WIJ, Vicca S, Dijkstra FA, Hagedorn F, Hovenden MJ, Larsen KS, Morgan JA, Volder A, Beier C, Dukes JS, et al. Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Global Change Biology. 2012;18:2681–2693. doi: 10.1111/j.1365-2486.2012.02745.x. [DOI] [PubMed] [Google Scholar]
- Domec J-C, Palmroth S, Ward E, Maier CA, Thérézien M, Oren R. Acclimation of leaf hydraulic conductance and stomatal conductance of Pinus taeda (loblolly pine) to long-term growth in elevated CO2 (free-air CO2 enrichment) and N-fertilization. Plant, Cell & Environment. 2009;32:1500–1512. doi: 10.1111/j.1365-3040.2009.02014.x. [DOI] [PubMed] [Google Scholar]
- Domec J-C, Schäfer K, Oren R, Kim HS, McCarthy HR. Variable conductivity and embolism in roots and branches of four contrasting tree species and their impacts on whole-plant hydraulic performance under future atmospheric CO2 concentration. Tree Physiology. 2010;30:1001–1015. doi: 10.1093/treephys/tpq054. [DOI] [PubMed] [Google Scholar]
- Duan H, Duursma RA, Huang G, Smith RA, Choat B, O’Grady AP, Tissue DT. Elevated [CO2] does not ameliorate the negative effects of elevated temperature on drought-induced mortality in Eucalyptus radiata seedlings. Plant, Cell & Environment. 2014;37:1598–1613. doi: 10.1111/pce.12260. [DOI] [PubMed] [Google Scholar]
- Duursma RA, Barton CVM, Eamus D, Medlyn BE, Ellsworth DS, Forster MA, Tissue DT, Linder S, McMurtrie RE. Rooting depth explains [CO2] × drought interaction in Eucalyptus saligna. Tree Physiology. 2011;31:922–931. doi: 10.1093/treephys/tpr030. [DOI] [PubMed] [Google Scholar]
- Fay PA, Jin VL, Way DA, Potter KN, Gill RA, Jackson RB, Polley WH. Soil-mediated effects of subambient to increased carbon dioxide on grassland productivity. Nature Climate Change. 2012;2:742–746. [Google Scholar]
- Figueroa CM, Lunn JE. A tale of two sugars: trehalose 6-phosphate and sucrose. Plant Physiology. 2016;172:7–27. doi: 10.1104/pp.16.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Adams MA, Amthor JS, Barbour MM, Berry JA, Ellsworth DS, Farquhar GD, Ghannoum O, Lloyd J, McDowell N, et al. Sensitivity of plants to changing atmospheric CO2 concentration: From the geological past to the next century. New Phytologist. 2013;197:1077–1094. doi: 10.1111/nph.12104. [DOI] [PubMed] [Google Scholar]
- Franks PJ, Britton-Harper ZJ. No evidence of general CO2 insensitivity in ferns: one stomatal control mechanism for all land plants? New Phytologist. 2016;211:819–827. doi: 10.1111/nph.14020. [DOI] [PubMed] [Google Scholar]
- Franks SJ, Weber JJ, Aitken SN. Evolutionary and plastic responses to climate change in terrestrial plant populations. Evolutionary Applications. 2014;7:123–139. doi: 10.1111/eva.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Urban MC, Tewksbury J, Gilchrist GW, Holt RD. A framework for community interactions under climate change. Trends in Ecology & Evolution. 2010;25:325–331. doi: 10.1016/j.tree.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Haworth M, Hoshika Y, Killi D. Has the impact of rising CO2 on plants been exaggerated by meta-analysis of free air CO2 enrichment studies? Frontiers in Plant Science. 2016;7:1153. doi: 10.3389/fpls.2016.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heijden MGA, Martin FM, Selosse M-A, Sanders IR. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytologist. 2015;205:1406–1423. doi: 10.1111/nph.13288. [DOI] [PubMed] [Google Scholar]
- Johnson D, Gilbert L. Interplant signalling through hyphal networks. New Phytologist. 2015;205:1448–1453. doi: 10.1111/nph.13115. [DOI] [PubMed] [Google Scholar]
- Johnson D, Martin F, Cairney JWG, Anderson IC. The importance of individuals: intraspecific diversity of mycorrhizal plants and fungi in ecosystems. New Phytologist. 2012;194:614–628. doi: 10.1111/j.1469-8137.2012.04087.x. [DOI] [PubMed] [Google Scholar]
- Johnson NC, Graham JH. The continuum concept remains a useful framework for studying mycorrhizal functioning. Plant and Soil. 2013;363:411–419. [Google Scholar]
- Kivlin SN, Emery SM, Rudgers JA. Fungal symbionts alter plant responses to global change. American Journal of Botany. 2013;100:1445–1457. doi: 10.3732/ajb.1200558. [DOI] [PubMed] [Google Scholar]
- Lau JA, Lennon JT. Rapid responses of soil microorganisms improve plant fitness in novel environments. Proceedings of the National Academy of Sciences, USA. 2012;109:14058–14062. doi: 10.1073/pnas.1202319109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JA, Shaw RG, Reich PB, Shaw FH, Tiffin P. Strong ecological but weak evolutionary effects of elevated CO2 on a recombinant inbred population of Arabidopsis thaliana. New Phytologist. 2007;175:351–362. doi: 10.1111/j.1469-8137.2007.02108.x. [DOI] [PubMed] [Google Scholar]
- Locke AM, Sack L, Bernacchi CJ, Ort DR. Soybean leaf hydraulic conductance does not acclimate to growth at elevated [CO2] or temperature in growth chambers or in the field. Annals of Botany. 2013;112:911–918. doi: 10.1093/aob/mct143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May P, Liao W, Wu Y, Shuai B, McCombie WR, Zhang MQ, Liu QA. The effects of carbon dioxide and temperature on microRNA expression in Arabidopsis development. Nature Communications. 2013;4:2145. doi: 10.1038/ncomms3145. [DOI] [PubMed] [Google Scholar]
- McCarthy HR, Oren R, Finzi AC, Johnsen KH. Canopy leaf area constrains [CO2]-induced enhancement of productivity and partitioning among aboveground carbon pools. Proceedings of the National Academy of Sciences, USA. 2006;103:19356–19361. doi: 10.1073/pnas.0609448103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros JS, Ward JK. Increasing atmospheric [CO2] from glacial to future concentrations affects drought tolerance via impacts on leaves, xylem and their integrated function. New Phytologist. 2013;199:738–748. doi: 10.1111/nph.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan JE, Cowden CC, Baas P, Dawadi A, Frankson PT, Helmick K, Hughes E, Khan S, Lang A, Machmuller M, et al. Mycorrhizal fungi mediation of terrestrial ecosystem responses to global change: mini-review. Fungal Ecology. 2014;10:3–19. [Google Scholar]
- Norby RJ, De Kauwe MG, Domingues TF, Duursma RA, Ellsworth DS, Goll DS, Lapola DM, Luus KA, MacKenzie AR, Medlyn BE, et al. Model–data synthesis for the next generation of forest free-air CO2 enrichment (FACE) experiments. New Phytologist. 2016;209:17–28. doi: 10.1111/nph.13593. [DOI] [PubMed] [Google Scholar]
- Perry LG, Shafroth PB, Blumenthal DM, Morgan JA, LeCain DR. Elevated CO2 does not offset greater water stress predicted under climate change for native and exotic riparian plants. New Phytologist. 2013;197:532–543. doi: 10.1111/nph.12030. [DOI] [PubMed] [Google Scholar]
- Pineda A, Dicke M, Pieterse CMJ, Pozo MJ. Beneficial microbes in a changing environment: are they always helping plants to deal with insects? Functional Ecology. 2013;27:574–586. [Google Scholar]
- Posner H. Inhibitory effect of carbohydrate on flowering in Lemna perpusilla. Plant Physiology. 1971;48:361–365. doi: 10.1104/pp.48.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo MJ, López-Ráez JA, Azcón-Aguilar C, García-Garrido JM. Phytohormones as integrators of environmental signals in the regulation of mycorrhizal symbioses. New Phytologist. 2015;205:1431–1436. doi: 10.1111/nph.13252. [DOI] [PubMed] [Google Scholar]
- van der Putten WH, Bardgett RD, Bever JD, Bezemer TM, Casper BB, Fukami T, Kardol P, Klironomos JN, Kulmatiski A, Schweitzer JA, et al. Plant–soil feedbacks: the past, the present and future challenges. Journal of Ecology. 2013;101:265–276. [Google Scholar]
- Rafferty NE, Ives AR. Pollinator effectiveness varies with experimental shifts in flowering time. Ecology. 2012;93:803–814. doi: 10.1890/11-0967.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico C, Pittermann J, Polley HW, Aspinwall MJ, Fay PA. The effect of subambient to elevated atmospheric CO2 concentration on vascular function in Helianthus annuus: implications for plant response to climate change. New Phytologist. 2013;199:956–965. doi: 10.1111/nph.12339. [DOI] [PubMed] [Google Scholar]
- Smithwick EAH, Lucash MS, McCormack ML, Sivandran G. Improving the representation of roots in terrestrial models. Ecological Modelling. 2014;291:193–204. [Google Scholar]
- Springate DA, Kover PX. Plant responses to elevated temperatures: a field study on phenological sensitivity and fitness responses to simulated climate warming. Global Change Biology. 2014;20:456–465. doi: 10.1111/gcb.12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer CJ, Orozco RA, Kelly JK, Ward JK. Elevated CO2 influences the expression of floral-initiation genes in Arabidopsis thaliana. New Phytologist. 2008;178:63–67. doi: 10.1111/j.1469-8137.2008.02387.x. [DOI] [PubMed] [Google Scholar]
- Springer CJ, Ward JK. Flowering time and elevated atmospheric CO2. New Phytologist. 2007;176:243–255. doi: 10.1111/j.1469-8137.2007.02196.x. [DOI] [PubMed] [Google Scholar]
- terHorst CP, Zee PC. Eco-evolutionary dynamics in plant–soil feedbacks. Functional Ecology. 2016;30:1062–1072. [Google Scholar]
- Tognetti R, Raschi A, Longobucco A, Lanini M, Bindi M. Hydraulic properties and water relations of Vitis vinifera L. exposed to elevated CO2 concentrations in a free air CO2 enrichment (FACE) Phyton-Annales Rei Botanicae. 2005;45:243–256. [Google Scholar]
- Tripati AK, Roberts CD, Eagle RA. Coupling of CO2 and ice sheet stability over major climate transitions of the last 20 million years. Science. 2009;326:1394–1397. doi: 10.1126/science.1178296. [DOI] [PubMed] [Google Scholar]
- Vaz M, Cochard H, Gazarini L, Graça J, Chaves MM, Pereira JS. Cork oak (Quercus suber L.) seedlings acclimate to elevated CO2 and water stress: photosynthesis, growth, wood anatomy and hydraulic conductivity. Trees. 2012;26:1145–1157. [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science. 2013;339:704–707. doi: 10.1126/science.1230406. [DOI] [PubMed] [Google Scholar]
- Wang J-W, Czech B, Weigel D. miR156-regulated SPL transcription factors devine an endogenous flowering pathway in Arabidopsis thaliana. Cell. 2009;138:738–749. doi: 10.1016/j.cell.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Ward JK, Antonovics J, Thomas RB, Strain BR. Is atmospheric CO2 a selective agent on model C3 annuals? Oecologia. 2000;123:330–341. doi: 10.1007/s004420051019. [DOI] [PubMed] [Google Scholar]
- Ward JK, Kelly JK. Scaling up evolutionary responses to elevated CO2: lessons from Arabidopsis. Ecology Letters. 2004;7:427–440. [Google Scholar]
- Warren JM, Norby RJ, Wullschleger SD. Elevated CO2 enhances leaf senescence during extreme drought in a temperate forest. Tree Physiology. 2011;31:117–130. doi: 10.1093/treephys/tpr002. [DOI] [PubMed] [Google Scholar]
- Watson-Lazowski A, Lin Y, Miglietta F, Edwards RJ, Chapman MA, Taylor G. Plant adaptation or acclimation to rising CO2? Insight from first multigenerational RNA-Seq transcriptome. Global Change Biology. 2016;22:3760–3773. doi: 10.1111/gcb.13322. [DOI] [PubMed] [Google Scholar]
- Way DA, Oren R, Kroner Y. The space-time continuum: the effects of elevated CO2 and temperature on trees and the importance of scaling. Plant, Cell & Environment. 2015;38:991–1007. doi: 10.1111/pce.12527. [DOI] [PubMed] [Google Scholar]
- Wertin TM, McGuire MA, Teskey RO. Effects of predicted future and current atmospheric temperature and [CO2] and high and low soil moisture on gas exchange and growth of Pinus taeda seedlings at cool and warm sites in the species range. Tree Physiology. 2012;32:847–858. doi: 10.1093/treephys/tps051. [DOI] [PubMed] [Google Scholar]
- Worchel ER, Giauque HE, Kivlin SN. Fungal symbionts alter plant drought response. Microbial Ecology. 2013;65:671–678. doi: 10.1007/s00248-012-0151-6. [DOI] [PubMed] [Google Scholar]
- Wu G, Park MY, Conway SR, Wang J-W, Weigel D, Poethig RS. The sequential action of miR156 and miR172 regulates developmental timing in Arabidopsis. Cell. 2009;138:750–759. doi: 10.1016/j.cell.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeppel MJB, Lewis JD, Chaszar B, Smith RA, Medlyn BE, Huxman TE, Tissue DT. Nocturnal stomatal conductance responses to rising [CO2], temperature and drought. New Phytologist. 2012;193:929–938. doi: 10.1111/j.1469-8137.2011.03993.x. [DOI] [PubMed] [Google Scholar]


