Abstract
Background
Diagnosis of chronic low back pain (CLBP) is traditionally predicated on identifying underlying pathological or anatomical causes, with treatment outcomes modest at best. Alternately, it is suggested that identification of underlying pain mechanisms with treatments targeted towards specific pain phenotypes may yield more success. Differentiation between nociceptive and neuropathic components of CLBP is problematic; evidence suggests that clinicians fail to identify a significant neuropathic component in many CLBP patients. The painDETECT questionnaire (PDQ) was specifically developed to identify occult but significant neuropathic components in individuals thought to have predominantly nociceptive pain.
Methods
Using the PDQ, we classified 50 CLBP patients into two distinct groups; those with predominantly nociceptive pain (Group 1) and those with a significant neuropathic component (Group 2). We characterised these two distinct CLBP sub-groups using a) questionnaire-based behavioural evaluation measuring pain-related function and quality of life, pain intensity and psychological well-being and b) sensory examination, using two-point and tactile threshold discrimination.
Objective
We sought to determine if differences in the pain phenotype of each CLBP sub-group would be reflected in sensory and behavioural group profiles.
Results
We report that Group 1 and Group 2 sub-groups demonstrate unique clinical profiles with significant differences in sensory tactile discrimination thresholds and in a wide range of behavioural domains measuring pain intensity, disability and psychological well-being.
Conclusion
We have demonstrated distinct clinical profiles for CLBP patient sub-groups classified by PDQ. Our results give diagnostic confidence in using the PDQ to characterise two distinct pain phenotypes in a heterogeneous CLBP population.
Keywords: Low back pain, Neuropathic pain, Nociceptive pain, Chronic pain
Highlights
-
•
Identification of underlying CLBP pain phenotypes is challenging.
-
•
We used painDETECT to identify nociceptive and neuropathic CLBP subgroups.
-
•
We demonstrate unique sensory and behavioural clinical profiles for each group.
-
•
More accurate identification of CLBP pain phenotypes may improve treatment outcomes.
1. Introduction
Heterogeneity in the clinical presentation of Chronic Low Back Pain (CLBP) makes diagnosis and treatment challenging. CLBP treatment pathways are traditionally predicated on identifying pathophysiological causes, which are not possible to identify in 90% of patients (Koes et al., 2006). Attention has focused on identifying sub-groups within the heterogeneous CLBP population, in order to more effectively target treatments (Delitto, 2005, Foster et al., 2011, Huijnen et al., 2015, O'Sullivan, 2005, Stanton et al., 2011, Turk, 2005). These subgroups may be variously defined by physiological or psychological determinants. Alternately, it has been suggested that identification of underlying pain mechanisms and treatments targeted towards specific pain phenotypes may yield more successful outcomes (Woolf, 2004). Clinically, this is important, as patients with neuropathic pain (NeuP) demonstrate poorer outcomes and greater comorbidities than patients with nociceptive pain (Smith and Torrance, 2012, Smith et al., 2007 Jensen et al., 2007).
The definition and diagnosis of NeuP and its differentiation from nociceptive pain remains controversial. Current IASP guidelines stipulate that a demonstrable lesion or disease of the somatosensory nervous system is necessary in order to arrive at a definitive neuropathic classification (International Association for the Study of Pain, 2016) For a full up to date description of the issues underlying the diagnosis and definition of neuropathic pain see Finnerup et al. (2016). Using the current IASP guidelines, only a small percentage of CLBP patients can be classified as ‘neuropathic’ yet, in routine clinical practice, many patients with low back pain present with symptoms that are indicative of a significant neuropathic component (i.e spread of pain, paroxysmal pain, dysaesthesia, allodynia). However, these patients may either present with no history or confirmatory evidence of a lesion or disease process, with equivocal examination findings and with pain that is not in a ’neuroanotomically plausible’ distribution. Recent work suggests that clinicians fail to identify significant neuropathic components in a number of people with LBP and that the true incidence of CLBP patients with a significant neuropathic component may be under-diagnosed (Freynhagen et al., 2006). In addition, evidence shows that neuropathic LBP is not restricted to patients that present with a typical radicular presentation (Attal et al., 2011, Forster et al., 2013). Patients with occult neuropathic symptoms may therefore be mis-classified as nociceptive, in spite of a seemingly neuropathic symptom profile, or else idiopathic, which allows no indication of underlying pain mechanisms. Worst of all, an idiopathic classification may hint at a pejorative ‘functional’ label to a patient's symptoms (Cohen et al., 2011).
Importantly, failure to identify patients with occult neuropathic components may lead to sub-optimal treatment regimes. It is proposed that improved ability to identify these patients and target appropriate treatments will result in better outcomes.
The PainDETECT questionnaire (PDQ) was specifically developed to identify neuropathic components in patients with CLBP and to differentiate LBP patients with a significant neuropathic component from LBP patients with predominantly nociceptive, mechanical pain without the need for physical examination or confirmatory diagnostic markers. The PDQ has been shown to have a high sensitivity (85%), specificity, (80%) and positive predictive accuracy (83%) in LBP (Freynhagen et al., 2006). In addition, as clinical tests such as spinal palpation, slump testing and straight leg raising are largely qualitative in nature and therefore suffer from variability and limitations of sensitivity and specificity (Rubinstein and van Tulder, 2008, van der Windt et al., 2010), identification by PDQ was chosen to reduce possible inconsistencies in patient selection criteria and as a means to standardise the selection process.
We therefore chose to use the PDQ to identify two sub-groups within our CLBP population: LBP patients with predominantly nociceptive, mechanical pain (Group 1) and LBP patients with a significant neuropathic component (Group 2).
The primary objective of this study was to characterise these two groups. Our hypothesis stated that the psychophysical clinical profiles of our participants would reflect the clinical phenotypes and underlying pain mechanisms of Group 2 and Group 2 patients, identified using the PDQ. We also hypothesised that, in particular, Group 2 patients would display a more complex profile compared to Group 1 patients. If the profiles of each group were truly different, then diagnostic confidence that the PDQ is a valid tool, able to characterise pain phenotypes in a heterogeneous population, would be strengthened.
2. Methods
2.1. Recruitment
Fifty patients with CLBP were recruited from the same inner city London hospital, together with twenty age and sex-matched controls. All patients consented to clinical profiling by collection of behavioural questionnaire data, sensory examination and subsequent structural and functional neuroimaging (no neuroimaging data will be shown here but will form the basis of a subsequent paper).
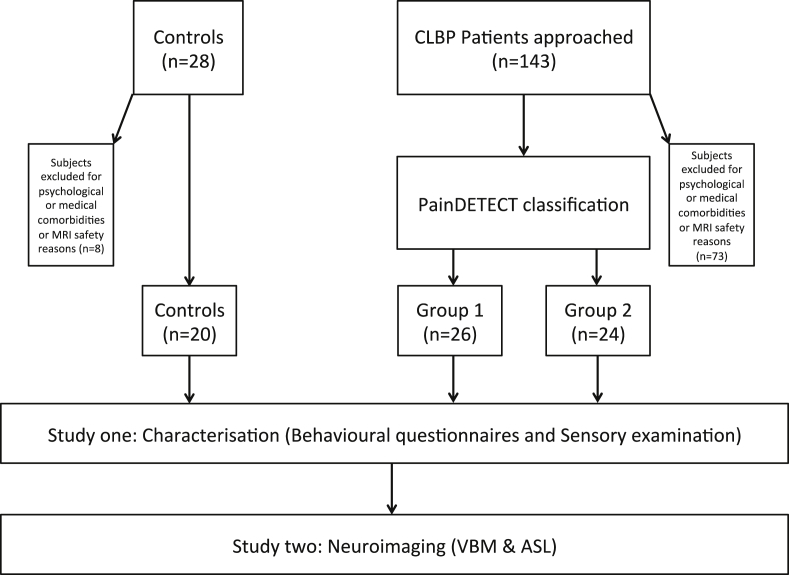
In order to be eligible for inclusion, patients needed to report a history of LBP for at least 12 months and were required to score 3 or above on an 11 point numerical rating scale (NRS) on the day of screening. Subjects were excluded if they complained of chronic or current pain conditions other than LBP or if they were currently experiencing, or had any history of, clinically significant or unstable medical or psychological conditions that would compromise participation in the study. All control subjects, were required to be free of any painful conditions and other significant medical and psychological confounding factors. All subjects were screened for MRI safety. There were no exclusion criteria for pain medication and all subjects continued with their usual medication, which included paracetamol, non-steroidal anti inflammatory medication, neuropathic pain medication (anti-convulsants and anti-depressives) and opiates. Using chi-squared tests for independence (with Yate's continuity correction), no significantly different levels of medication usage across all categories were found for between groups. Further information on the recruitment process is given in Fig. 1. Formal ethical approval for the study was granted by XXXXX Research Ethics Committee (08/H0810/51).
Fig. 1.
Recruitment pathway algorithm. All patients were required to attend for a single visit. All back pain patients were classified by the PainDETECT questionnaire into Group 1 and Group 2 sub-groups. All patients, including controls, underwent sensory examination and psychometric and behavioural assessment (Study One) that preceded structural (VBM: Voxel-based morphometry) and functional neuroimaging (ASL: Arterial spin labeling) (Study Two). Details of Study Two will be reported in a separate paper.
Although all patients were initially selected at random, during the later stages of the recruitment process it was observed that more Group 1 than Group 2 patients (as determined by the PDQ) had been recruited at the first check point. This reflects the demographic incidence of neuropathic pain compared to non-neuropathic pain in clinical populations (Smith and Torrance, 2012, Torrance et al., 2006). It was therefore deemed necessary during the latter stages of recruitment to preferentially select Group 2 patients, as determined by PDQ, in order to balance patient numbers between the LBP groups.
2.2. Clinical and psychometric assessments
The following questionnaires were administered in order to assess pain, disability and psychological status: painDETECT Questionnaire (PDQ) (Freynhagen et al., 2006), numeric rating scale (NRS) for pain, Short Form McGill Pain Questionnaire (SFMPQ) (Melzack, 1987), RAND Medical Outcomes 36-Item Short Form Survey Instrument (SF-36) (Ware and Sherbourne, 1992), Centre for Epidemiologic Studies Depression Scale Questionnaire (CES-D) (Radloff, 1977), State Trait Anxiety Inventory (STAI) (Spielberger, 1983) and the Revised Symptom Checklist 90 Questionnaire (SCL-90-R) (Derogatis and Unger, 2010). Detailed information on each questionnaire is provided in Table 1.
Table 1.
Description of behavioural questionnaires used including questionnaire constructs, domains and details of scoring.
| Name | Construct Intended to measure | Domains | Scoring | Comments |
|---|---|---|---|---|
| Numeric Rating Scale (NRS) | Pain intensity | n/a | 11 point scale, 0–10 | |
| Short Form McGill Pain Questionnaire (SFMPQ) (Melzack, 1987) | Multidimensional measure of pain |
Measures a) sensory and b) affective dimensions of the pain experience. Also includes five-item present pain intensity scale (PPI) to describe overall pain intensity and 10-cm visual analogue scale (VAS). |
Intensity scale from 0 (none) to 4 (severe). | Widely used in adult chronic pain populations, including CLBP (Ruoff et al., 2003) and has modest predictability in discrimination of neuropathic and musculoskeletal pain of spinal cord injury (Putzke et al., 2002). |
| Rand Medical Outcomes 36-Item Short Form Survey Instrument (SF-36) (Ware and Sherbourne, 1992) | General health related quality of life questionnaire. | Measures: 1) limitations in physical activities because of health problems (SF-36 Physical Function); 2) limitations in social activities because of physical or emotional problems (SF-36 Social Function); 3) limitations in usual role activities because of physical health problems (SF-36 Role-Physical); 4) bodily pain (SF-36 Pain); 5) psychological distress and emotional well-being (SF-36 General Mental Health); 6) limitations in usual role activities because of emotional problems (SF-36 Role-Emotional); 7) energy and fatigue (SF-36 Vitality); 8) general health perceptions (SF-36 General Health). | For each of the eight domains an aggregate percentage score is produced. The percentage scores range from 0% (lowest or worst possible level of functioning) to 100% (highest or best possible level of functioning). Two summary scores of physical quality of life (Physical Component Summary; PCS) and psychological well-being and general health perception (Mental Component summary; MCS) can also be obtained by combining physical and mental domains respectively (Ware et al., 1995). PCS and MCS scales are scored to have the same average (50) and standard deviation (10) (norm-based scores). Therefore scores below and above 50 represent above and below average values of physical and mental health and functioning with increasingly low scores represent increasing degrees of psychological distress and disability (Ware et al., 1993). A cut off score of 35 or less on the MCS is able to identify depressive symptoms (as measured by the CES-D) in LBP patients (Walsh et al., 2006). | Widely used measure of health-related quality of life; has been shown to discriminate between subjects with different chronic conditions and between subjects with different severity levels of the same disease (Kosinski et al., 1999) |
| Centre For Epidemiologic Studies Depression Scale Questionnaire (CES-D) (Radloff, 1977) | 20-item questionnaire of symptoms associated with depression | Scores range from 0 to 60, with high scores indicating greater depressive symptoms. It cannot be used to diagnose depression in itself. However, scores of 16 or greater can be used to identify individuals at risk for clinical depression in primary care and 19 or greater in the chronic pain population (Turk and Okifuji, 1994). | ||
| State Trait Anxiety Inventory (STAI) (Spielberger, 1983) | Anxiety | Measures two components of anxiety: Anxiety in the present moment (“state”) and anxiety as a general, ongoing personal characteristic (“trait”). | Twenty questions are each scored on a four point Likert scale; higher scores are associated with higher levels of anxiety. A cut-point of 39–40 is normally used for clinically significant symptoms of a state of anxiety (Julian, 2011, Knight et al., 1983) | |
| Revised Symptom Checklist 90 Questionnaire (SCL-90-R) (Derogatis and Unger, 2010) |
The SCL-90 is designed to assess a broad range of psychological problems and the current psychopathology of subjects along nine symptom constructs. |
Symptom constructs: Somatisation, Obsessive-Compulsive Symptoms, Interpersonal Sensitivity, Depression, Anxiety, Hostility, Phobic-Anxiety, Paranoid Ideation and Psychoticism. Three additional scales have also been developed; the Positive Symptom Total (PST) measuring the total number of self reported symptoms, the Positive Symptom Distress Index (PSDI) measuring intensity of symptoms and the Global Severity Index (GSI), designed to measure overall psychological distress which can be used as a summary of the test. |
90-item questionnaire: Raw scores are calculated by dividing the sum of scores for a domain by the number of items in the domain. The global severity index is computed by summing the scores of the nine domains and additional items and then dividing by the total number of responses. Scores are converted to standard T-scores using the norm group appropriate for the patient. The SCL-90-R scoring manual contains normative data for psychiatric outpatients, psychiatric inpatients, adult non-patients, adolescent non-patients (http://www.pearsonclinical.co.uk) (Schmitz et al., 2000) |
2.3. Sensory testing
Sensory evaluation was carried out using two-point discrimination (2PD) and tactile threshold discrimination (TTD). Participants were positioned comfortably in prone lying with a pillow underneath the stomach to standardise lumbar position. The examiner identified and marked the spine in line with the spinous processes of L1, L3 and L5 bilaterally in line with the inferior angle of the scapula. The same assessor examined all patients in order to reduce the inter-rater variability inherent in these techniques (Catley et al., 2013). Testing was undertaken separately on left and right sides of the back and the order of testing was randomised, as was the order of levels tested.
2.3.1. TTD testing
Semmes-Weinstein monofilaments of varying thickness with corresponding target forces (1.65, {0.008g}; 2.83, {0.07g}; 3.61, {0.4g}; 4.31, {2g}; 4.56, {4g}; 6.65, 300 g) were employed to measure tactile sensory thresholds at the level of L1, L3 and L5 spinous processes. Following a standardised, brief explanation of what the filaments were, each filament was applied perpendicular to the skin with enough force to create a visible bend in the filament. Patients were instructed to “Say ‘TOUCH’ every time you feel the filament on your skin”. A standardised up-down-up protocol for all patients was carried out beginning with a 1.65, 0.008 g filament. Filaments were applied 5 times with a 2 s delay between each repetition. For a positive result, patients had to report that 3 out of 5 applications elicited a response for each filament (Yarnitsky, 1997). Threshold was established by the method of limits, where stimuli were increased stepwise in filament strength until a response was elicited. The filament with a bending pressure immediately below the established threshold filament was then reapplied to confirm the exact threshold (Yarnitsky, 1997).The threshold for each level (L1, L3, L5) was recorded on a standardised body chart.
2.3.2. 2PD testing
2PD threshold testing followed the principles of the ‘down-up-down’ method described by Moberg (1990) and Seltzer and Seltzer (1986). A set of electronic digital callipers (Precision Gold®) with a scale measured in 1 mm gradations was lightly applied until the first blanching of the skin. Testing was undertaken bilaterally at each level, and the order of the side of testing was randomised. Based on normative data for 2PD threshold (Nolan, 1985), testing was commenced with callipers set at 70 mm. The distance between the points was decreased in 5 mm increments until the subject was able to perceive only one point instead of two. Each participant was instructed to say ‘one’ when they felt one point and ‘two’ when they felt two points. This was confirmed by descending 5 mm below this point. An ascending sequence was then applied in 2 mm increments until the patient again reported two points. Testing continued around these initial values using ascending and descending sequences in 1 mm increments until a consistent response was obtained. Catch trials were used to verify that participants were not guessing.
The same examiner conducted the sensory examination and collected the questionnaire data. In order to reduce the risk of bias, the painDETECT and other questionnaire data were not analysed until after all the examination procedures were completed.
2.4. Data analysis
All analyses were performed using SPSS 20 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
2.4.1. Questionnaire data
Pain intensity and discrimination were examined using pain on day of assessment NRS and all domains of the SFMPQ outcome measures. Independent samples t-tests were used to identify differences between Group 1 and Group 2 groups. Pain-related functional status and quality of life were assessed using all domains of the SF-36. Psychological well-being was assessed using all domains of the SCL-90-R, CES-D and STAI. One-way between groups analysis of variance (ANOVA) was used to identify differences between all three groups (controls, Group 1, and Group 2). Planned comparisons were then used to test the primary hypotheses that CLBP patients suffer greater psychological distress and poorer quality of life compared to controls and that Group 2 patients suffer greater psychological distress and poorer quality of life compared to Group 1 patients. Homogeneity of variance across groups was tested for using Levene's test. Post-hoc and t-tests and planned contrast results were adjusted if homogeneity of variance was violated. Due to the large number of comparisons undertaken, post –hoc correction using the Benjamini–Hochberg procedure (Benjamini and Hochberg, 1995) was applied to the subgroup data containing multiple domains order to correct for type-1 errors. To facilitate understanding of the clinical significance of each finding in relation to our CLBP subgroups, we also calculated Cohen's d, a standardised measure of effect size, d being equivalent to the number of standard deviations by which the two groups differ. An effect size of <0.2 is considered ’small’, 0.5 = moderate and >0.8 = large (Cohen, 1988).
2.4.2. Sensory examination data
Mean 2PD and TTD values for all points recorded on the back were calculated for each individual. One-way analysis of variance testing (ANOVA) was used to examine group differences in 2PD and TTD examination scores. Planned comparisons were then used to test the primary hypothesis that 2PD is disrupted in CLBP participants compared to control patients, and also in Group 2 compared to Group 1 patients. The same planned comparisons were applied to the TTD data and Cohen's d effect sizes were also calculated.
3. Results
3.1. Demographics
3.1.1. painDETECT
After recruitment patients were classified into Group 1 and Group 2 subgroups using the PDQ. Patients scoring less than 19 were classified as likely to have nociceptive mechanical low back pain (Group 1, n = 26); patients scoring 19 or more were classified as likely to have a significant neuropathic component to their pain (Group 2 n = 24). This cut-off value is based on the classification established by Freynhagen (Freynhagen et al., 2006). The mean painDETECT score for Group 1 was 9.0 (SD 5.15) and for Group 2 it was 23.9 (SD 4.36).
3.1.2. Age
Patients varied from 21 to 59 years in age (mean = 39 years, SD 9.91). There was no significant difference in age across groups (ANOVA F (2, 65) = 2.4, p = 0.10).
3.1.3. Duration of pain symptoms
Duration of LBP across LBP patients varied from 12 to 360 months. Qualitatively, there was little clinically relevant difference in duration of pain symptoms across the groups. Mean duration of pain symptoms in Group 1 patients was 102 months (median, 54 months), and for Group 2 patients it was 98 months (median, 72 months).
3.2. Clinical and psychometric assessment
3.2.1. Measures of pain
Using Student's t-test, Group 2 patients reported significantly higher examination day pain than Group 1 patients as measured by NRS. Group 2 patients also demonstrated significantly greater SFMPQ VAS pain scores, significantly greater SFMPQ Sensory Pain Descriptor Scale scores and significantly greater SFMPQ Present Pain Intensity scale scores than patients with Group 2. Mean scores and standard deviation values for all groups, as well as values of the student's T-statistic and associated significance values and Cohen's d are described in Table 2. There was no significant difference in McGill Affective Pain Scores between Group 1 and Group 2 groups; however only 35 Scores were completed (Group 1 N = 20, Group 2 N = 15) due to an administrative error (see Table 2).
Table 2.
Pain Scores: Mean scores (Mean) and standard deviation (SD) values of Numeric Rating Scale (NRS) for Pain & Short Form McGill Pain Questionnaire (SFMPQ) for CLBP groups, including values of the student's T-statistic with associated significance values and Cohen's d effect sizes for comparisons between Group 1 and Group 2 patients. No pain data were collected for control subjects, as recruitment required them to be pain-free. Original significance values are described alongside Benjamini–Hochberg post –hoc correction values. All data, except significance values, have been corrected to 2 decimal places. *Statistically significant difference (p < 0.05), ** Statistically significant difference (p < 0.001).
| Questionnaire | Domain | Controls (Mean, SD) | NocLBP (Mean, SD) | NuLBP (Mean, SD) | CLBP & Controls |
Group 1 & Group 2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t value | df | Sig. value | t value | df | Uncorrected Sig. value | B-H corrected Sig. value | Cohen's d | ||||||||
| NRS |
– |
– |
4.62 |
2.06 |
6.88 |
1.7 |
– |
– |
– |
4.21 |
48.00 |
<0.001** |
<0.001** |
1.21 |
|
| SFMPQ | VAS | – | – | 61.77 | 18.89 | 80.68 | 12.75 | – | – | – | 3.99 | 46.00 | <0.001** | <0.001** | 1.18 |
| Sensory Pain Descriptors | – | – | 10.19 | 5.87 | 19.77 | 7.53 | – | – | – | 4.95 | 46.00 | <0.001** | <0.001** | 1.46 | |
| Affective Pain Descriptors | – | – | 3.45 | 2.33 | 4.93 | 2.66 | – | – | – | 1.76 | 33.00 | 0.088 | 0.44 | 0.09 | |
| Present Pain Intensity | – | – | 2.46 | 0.81 | 3.71 | 1.19 | – | – | – | 4.12 | 34.02 | <0.001** | <0.001** | 1.23 | |
3.3. Measures of function and quality of life
Using one-way analysis of variance (ANOVA) with planned comparisons to identify differences across subject groups, CLBP patients experienced significantly poorer physical and mental health related quality of life compared to controls as measured by all domains of the SF-36 (see Table 3). CLBP patients also exhibited significantly greater signs of anxiety (STAI), depression (CES-D) and psychological distress across all domains of the SCL-90-R (see Table 4).
Table 3.
SF-36 Scores measuring health related quality of life: Mean scores (Mean) and standard deviation (SD) values of RAND Medical Outcomes 36-Item Short Form Survey Instrument (SF-36) for all groups, including values of planned comparisons t values with associated significance values and Cohen's d effect sizes for comparisons between CLBP and Controls and Group 1 and Group 2 patients. Original significance values are described alongside Benjamini–Hochberg post –hoc correction values. All data, except significance values, have been corrected to 2 decimal places. *Statistically significant difference (p < 0.05), ** Statistically significant difference (p < 0.001).
| Questionnaire | Domain | Controls (Mean, SD) | NocLBP (Mean, SD) | NuLBP (Mean, SD) | CLBP & Controls |
Group 1 & Group 2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t value | df | Sig. value | t value | df | Uncorrected Sig. value | B-H corrected Sig. value | Cohen's d | ||||||||
| SF-36 | Physical Functioning | 100.00 | 0.00 | 55.38 | 20.63 | 35.43 | 19.42 | 19.07 | 46.81 | <0.001** | 3.49 | 46.81 | 0.001* | 0.002* | 1.02 |
| Role-Physical | 100.00 | 0.00 | 36.54 | 40.76 | 26.09 | 38.05 | 12.20 | 46.85 | <0.001** | 0.93 | 46.85 | 0.358 | 0.397 | 0.27 | |
| Bodily Pain | 98.95 | 3.15 | 41.54 | 19.08 | 24.13 | 16.95 | 24.73 | 53.84 | <0.001** | 3.38 | 47.00 | 0.001* | 0.002* | 0.99 | |
| General Health Perception | 85.53 | 10.39 | 52.88 | 19.86 | 47.61 | 25.67 | 8.65 | 58.88 | <0.001** | 0.80 | 41.28 | 0.43 | 0.428 | 0.23 | |
| PCS | 96.12 | 2.60 | 46.59 | 19.12 | 33.32 | 19.10 | 20.07 | 50.49 | <0.001** | 2.43 | 46.28 | 0.019* | 0.022* | 0.71 | |
| Vitality | 73.95 | 14.20 | 48.08 | 22.45 | 36.30 | 18.48 | 6.15 | 65.00 | <0.001** | 2.15 | 65.00 | 0.035* | 0.04* | 0.63 | |
| Social Functioning | 96.71 | 11.67 | 65.38 | 23.53 | 42.93 | 19.15 | 10.48 | 57.66 | <0.001** | 3.68 | 46.70 | 0.001* | 0.001* | 1.07 | |
| Role-Emotional | 94.74 | 12.49 | 70.51 | 40.36 | 47.83 | 42.43 | 5.40 | 60.94 | <0.001** | 1.91 | 45.60 | 0.062 | 0.07 | 0.56 | |
| General Mental health | 81.68 | 10.27 | 71.23 | 16.93 | 58.96 | 17.94 | 4.83 | 54.19 | <0.001** | 2.45 | 45.48 | 0.018* | 0.022* | 0.71 | |
| MCS | 86.77 | 7.51 | 63.80 | 21.10 | 46.51 | 18.41 | 9.56 | 64.98 | <0.001** | 3.06 | 46.99 | 0.004* | 0.005* | 0.89 | |
Table 4.
Psychometric scores: Mean scores and standard deviation values of Centre for Epidemiologic Studies Depression Scale Questionnaire (CES-D), State Trait Anxiety Inventory (STAI) and the Revised Symptom Checklist 90 Questionnaire (SCL-90-R) for all groups, including values of planned comparisons t values with associated significance values and Cohen's d effect sizes for comparisons between CLBP and Controls and Group 1 and Group 2 patients. Original significance values are described alongside Benjamini–Hochberg post –hoc correction values. All data, except significance values, have been corrected to 2 decimal places. *Statistically significant difference (p < 0.05), ** Statistically significant difference (p < 0.001).
| Questionnaire | Domain | Controls (Mean, SD) | NocLBP (Mean, SD) | NuLBP (Mean, SD) | CLBP & Controls |
Group 1 & Group 2 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t value | df | Sig. value | t value | df | Uncorrected Sig. value | B-H corrected Sig. value | Cohen's d | ||||||||
| SCL-90-R |
Somatisation | 42.68 | 9.10 | 62.69 | 9.12 | 69.58 | 9.38 | 9.45 | 66.00 | <0.001** | 2.65 | 66 | 0.01* | 0.015* | 0.76 |
| Obsessive-Compulsive | 50.00 | 10.29 | 59.77 | 10.45 | 65.21 | 9.97 | 4.52 | 66.00 | <0.001** | 1.88 | 66 | 0.065 | 0.074 | 0.54 | |
| Interpersonal Sensitivity | 46.63 | 6.33 | 53.12 | 12.43 | 61.38 | 12.36 | 4.66 | 60.39 | <0.001** | 2.35 | 47.72 | 0.023* | 0.029* | 0.68 | |
| Depression | 47.32 | 10.61 | 56.92 | 11.29 | 62.48 | 12.91 | 3.92 | 65.00 | <0.001** | 1.66 | 65 | 0.102 | 0.113 | 0.48 | |
| Anxiety | 42.53 | 8.26 | 51.85 | 11.98 | 60.46 | 14.23 | 5.12 | 50.86 | <0.001** | 2.31 | 45.17 | 0.026* | 0.030* | 0.67 | |
| Hostility | 44.79 | 7.42 | 53.27 | 11.33 | 55.38 | 13.86 | 3.85 | 53.63 | <0.001** | 0.59 | 44.54 | 0.561 | 0.609 | 0.17 | |
| Phobic Anxiety | 46.89 | 7.98 | 50.54 | 11.17 | 58.96 | 13.08 | 3.12 | 48.95 | 0.003* | 2.44 | 45.45 | 0.019* | 0.025* | 0.70 | |
| Paranoid Ideation | 46.21 | 7.71 | 49.62 | 10.23 | 54.04 | 15.00 | 2.21 | 50.97 | 0.032* | 1.21 | 40.16 | 0.234 | 0.257 | 0.35 | |
| Psychoticism | 45.63 | 4.55 | 55.85 | 11.38 | 62.25 | 13.39 | 6.55 | 63.15 | <0.001** | 1.82 | 45.35 | 0.076 | 0.085 | 0.53 | |
| Global Severity Index | 43.84 | 11.21 | 58.27 | 9.99 | 65.17 | 10.45 | 6.32 | 66.00 | <0.001** | 2.32 | 66 | 0.023* | 0.028* | 0.67 | |
| Positive Symptom Total | 43.89 | 9.36 | 54.85 | 10.02 | 63.26 | 8.97 | 5.91 | 65.00 | <0.001** | 3.10 | 65 | 0.004* | 0.006* | 0.9 | |
| Positive Symptom Distress Index |
46.58 |
8.58 |
61.31 |
9.95 |
62.42 |
10.48 |
5.79 |
66.00 |
<0.001** |
0.40 |
66 |
0.69 |
0.69 |
0.12 |
|
| STAI |
State | 29.53 | 9.65 | 36.42 | 8.02 | 43.83 | 14.34 | 3.83 | 37.88 | <0.001** | 2.23 | 35.49 | 0.032* | 0.064 | 0.65 |
| Trait |
32.80 |
8.08 |
42.31 |
10.13 |
46.63 |
11.18 |
4.42 |
67.00 |
<0.001** |
1.53 |
67 |
0.131 |
0.261 |
0.44 |
|
| CES-D | 4.55 | 5.69 | 11.42 | 8.37 | 23.33 | 10.67 | 6.88 | 55.70 | <0.001** | 4.37 | 43.60 | <0.001** | <0.001** | 1.26 | |
Group 2 patients experienced significantly poorer physical and mental health related quality of life compared to Group 1 patients as measured by the following domains of the SF-36: Physical functioning, Bodily pain, Physical component summary, Vitality, Social functioning, General mental health and the Mental component summary (see Table 3).
Compared to Group 1 patients, Group 2 patients also exhibited greater signs of depression (CES-D) and psychological distress across the following SCL-90-R psychological domains: Somatisation, Interpersonal sensitivity, Anxiety, Phobic anxiety, Global severity index and Positive symptom total (see Table 4).
3.4. Sensory examination
Using ANOVA with planned comparisons, TTD examination scores revealed significantly increased sensory thresholds to tactile stimulation (t (62) = 2.23, p = 0.029) indicating loss of tactile sensitivity and significantly increased thresholds to two-point discrimination in CLBP patients compared to controls (t (62) = −3.30, p = 0.002). In addition, we observed that TTD examination scores revealed significantly increased sensory thresholds to tactile stimulation in Group 2 compared to Group 1 patients (t (62) = −2.84, p = 0.006). However, there were no significant differences between Group 2 and Group 1 groups in 2PD examination scores (t (62) = −0.06, p = 0.973). Mean scores and standard deviation values for groups, as well as values of the Student's T-statistic and associated significance values are shown in Table 5.
Table 5.
Sensory Examination Scores: Mean scores (Mean) and standard deviation (SD) values of two-point discrimination (2PD) and tactile threshold discrimination (TTD) scores. 2PD scores are in centimetres. Tactile threshold discrimination (TTD) values are means of tactile threshold sensitivity values corresponding to filament thickness. Values of planned comparisons t values with associated significance values and Cohen's d effect sizes for comparisons between CLBP and Controls and Group 1 and Group 2 patients are reported. All data, except significance values have been corrected to 2 decimal places. *Statistically significant difference (p < 0.05).
| Test | Controls (Mean, SD) | MLBP (Mean, SD) | NuLBP (Mean, SD) | CLBP & Controls |
MLBP & NuLBP |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| t value | df | Sig. value | t value | df | Sig. value | Cohen's d | |||||||
| TTD | 2.70 | 0.67 | 2.85 | 0.78 | 3.48 | 0.78 | 2.23 | 62 | 0.029* | 2.84 | 62 | 0.006* | 0.72 |
| 2PD | 4.98 | 0.65 | 6.47 | 1.83 | 6.46 | 1.89 | −3.30 | 61 | 0.002* | 0.03 | 61 | 0.973 | 0.01 |
4. Discussion
The current study revealed significant differences in sensory examination and behavioural and psychometric data in a group of CLBP patients compared to controls and between neuropathic and non-neuropathic CLBP subgroups, as determined by the PDQ. CLBP patients reported significantly poorer physical and mental health related quality of life compared to controls across all SF-36 domains, greater psychological distress in all SCL-90-R domains and also greater signs of anxiety (STAI) and depression (CES-D). CLBP patients also reported significantly increased TTD and 2PD sensory examination thresholds compared to controls.
Group 2 patients reported significantly greater examination day pain using NRS and significantly greater pain (SFMPQ VAS, Sensory Pain Descriptor Scale Present Pain Intensity scale) scores than patients in Group 1. Group 2 patients experienced significantly poorer physical and mental health related quality of life compared to Group 1 patients (SF-36 domains of Physical functioning, Bodily pain, Vitality, Social functioning, General mental health, Mental component summary and Physical component). Group 2 patients also exhibited greater signs of depression (CES-D) and psychological distress across SCL-90-R domains (Somatisation, Interpersonal sensitivity, Anxiety, Phobic anxiety, Global severity index and Positive symptom total) compared to Group 1 patients. TTD examination scores revealed significantly increased sensory thresholds to tactile stimulation in Group 2 compared to Group 1 patients.
The following discussion will focus on the clinical implications of these data in relation to the assessment of underlying pain phenotypes in CLBP, centering on discussions surrounding the definition of neuropathic pain and in particular, the use of screening questionnaires as aids to diagnosis.
4.1. Re-thinking classification of underlying pain mechanisms in CLBP
In this study, we have described CLBP patients with PDQ scores above 18 as having ‘a significant neuropathic component’ to their LBP as recommended by Freynhagen et al. (2006). This does not necessarily imply that these patients present with well-defined radicular symptoms and an identifiable nerve root lesion. Evidence shows that many patients with a diagnosis of non-specific low back pain have a significant neuropathic component to their pain (Freynhagen et al., 2006) and patients within our Group 2 cohort may fail to conform to the current IASP definition of neuropathic pain, which explicitly states that without evidence of a confirmatory lesion or disease process, signs or symptoms alone cannot justify the use of the term ‘neuropathic’. However, we would suggest that these patients present with a significant neuropathic component to their pain, irrespective of any lesion or pathological status. Our sensory examination findings support the idea that symptoms in the Group 2 group are unlikely to be mediated by a peripheral nerve neuritis or neuropathy as a predictable pattern of sensory loss was not observed. Studies on the sequelae of a mixed peripheral nerve compression show an order of events governed by nerve diameter size starting with large diameter Aδ fibres and the largest Aβ fibres (Nygaard and Mellgren, 1998, Rydevik et al., 1984, Yamashita et al., 2002). Damage to these fibres results in a selective loss of vibration sense, proprioception and 2PD with resulting alterations in sensory testing thresholds. Increasing nerve compression affects the smaller diameter Aβ fibres transducing light touch, resulting in increases to tactile thresholds. The Group 2 patients in our study demonstrated increased tactile thresholds compared to Group 1 patients while 2PD thresholds were unaffected, making a diagnosis of a compression neuropathy unlikely.
The current findings therefore suggest possible supraspinal rather than peripheral mechanisms. The emergent pattern might suggest unwelcome pathology of the CNS, for instance multiple sclerosis, perhaps affecting transmission of sensory input via the dorsal columns. However, this was extremely unlikely to be the cause in our group of patients who, notwithstanding a diagnosis of CLBP, were otherwise healthy and not suffering from any other significant medical or psychological conditions. In addition, all brain scans were screened by a radiologist for signs of abnormal pathology. We propose, therefore, that a more likely explanation is that these findings most likely reflect neuroplastic alterations to representational areas in the somatosensory cortex that are consistent with work showing changes in representational fields associated with alterations in 2PD thresholds (Flor, 1995, Flor et al., 1995, Juottonen et al., 2002, Lotze et al., 2001, Maihofner et al., 2003, Maihofner et al., 2005, Pleger et al., 2004). Supraspinal alterations in grey matter have been demonstrated in both nociceptive and non-nociceptive back pain (Apkarian et al., 2004). Although evidence shows that 2PD is associated with supraspinal neuroplasticity, 2PD was not able to discriminate between back pain subgroups in this study.
Interpretation of our data reflects the ongoing debate within the field as to the definition of NuP. Neuropathic Pain is a clinical description, not a diagnosis (Jensen et al., 2011). Our work lends support to the recent recommendation to reconfigure the ‘nociceptive-neuropathic dichotomy’ that characterises assessment of underlying pathophysiological mechanisms in chronic pain (Kosek et al., 2016). The authors suggest a 3rd mechanistic descriptor for chronic pain states that are neither nociceptive (as no evidence for actual or threatened tissue damage exists), nor neuropathic (as there is no evidence of a lesion or disease state) but are characterised by altered nociception. In particular, we suggest that our data support the view that maladaptive functioning of the nervous system, which was reflected in the term ‘dysfunction’, dropped from the IASP definition in 2011 (Jensen et al., 2011), may underlie the altered nociception resulting in chronic neuropathic pain-like signs and symptoms in CLBP. We believe that the current IASP classification criteria for neuropathic LBP is too restrictive and may fail to identify patients whose symptoms do not fit within current categories of nociceptive and neuropathic. We therefore support the notion of a 3rd descriptor to classify the underlying pain mechanisms of patients who present with symptoms suggestive of alterations in nociceptive function.
4.2. Sub-groups of CLBP patients selected by painDETECT reflect different pain phenotypes
We hypothesised that patients with CLBP would exhibit poorer quality of life, greater psychological distress and also exhibit different sensory examination profiles compared to controls. In addition, we hypothesised that CLBP patients identified by PDQ as having a significant neuropathic component to their pain (irrespective of lesion or disease status) would complain of greater pain, poorer quality of life, greater psychological distress and also exhibit different sensory examination profiles compared to Group 1 patients. Our data supported these hypotheses; group profiles reflect pain phenotypes identified by the PDQ. However, it may be suggested that group differences may be determined by other factors. For instance, psychological profiles and pain intensity differed in each group significantly. However, scores on the PDQ are not determined by any sort of psychological enquiry, nor are there any questions that assess pain intensity, so CLBP categorisation by PDQ is independent of these parameters. We believe, therefore, that these group differences reflect underlying pain mechanisms identified by the PDQ and are consistent with the concept of neuropathic signs and symptoms in LBP being a consequence of maladaptive plasticity of the nervous system and not solely a consequence of a lesion or disease process. In fact, it may be argued that maladaptive central nervous system neuroplasticity may be considered a disease process in its own right (Henry et al., 2011, May, 2008, Tracey and Bushnell, 2009).
5. Conclusion
People with neuropathic pain report significantly higher pain and disability scores, reduced quality of life and higher psychological co-morbidities compared to non-neuropathic pain patients (Freynhagen et al., 2006, Jensen et al., 2007, Smith and Torrance, 2012, Smith et al., 2007). Recent work indicates that current clinical examination methods and characterisation paradigms may fail to detect neuropathic components in many patients with LBP with both axial and distal presentations (Freynhagen et al., 2006). The issues raised from this body of work are central to debates on the definition, diagnosis and categorisation of neuropathic pain that have taken place between members of the International Association for the Study of Pain (IASP) over the last 15 years.
The primary aim of this study was to improve characterisation and clinical profiling of patients with CLBP. These results not only show significant differences in the clinical profiles of CLBP patients compared to controls, but importantly show that CLBP patients with a significant neuropathic component (Group 2) determined by PDQ, differ in clinical profile to patients with predominantly nociceptive, mechanical symptoms (Group 1). These data demonstrate that there are distinct differences in the clinical profiles of patients whose diagnosis of neuropathic LBP rests on assessment of symptom profiles, rather than by evidence of a lesion or disease process and whose symptoms may be caused by an underlying maladaptive plasticity of the nervous system. These data support the use of the PDQ as a quick and easy-to-use tool to aid to diagnosis in a clinical setting when used as an adjunct to expert clinical examination.
Diagnostic uncertainty is coupled with poor clinical outcomes for treatment (Torrance et al., 2006). A failure to identify patients with occult neuropathic symptoms may lead to inappropriate targeted treatments directed towards somatic tissue, resulting in unnecessary ongoing pain, disability and suffering (Gore et al., 2007). There is therefore an urgent need to develop more effective assessment strategies to identify and better differentiate neuropathic from mechanical low back pain. Ultimately, improved diagnostic efficiency and more accurate differentiation of mechanical and neuropathic components in LBP may lead to appropriately targeted treatment strategies and therefore improved outcomes. An important direction for further research will be to explore stratified treatment pathways for each group.
Statement of all funding sources
The principal author received a Clinical Academic Training PhD fellowship grant (CDRF-10-054) from the National Institute for Health Research. Dr. Howard and Professor Williams were supported by the Medical Research Council and the National Institute of Health Research Biomedical Research Centre for Mental Health at the South London and Maudsley NHS trust.
Conflicts of interest disclosure
There were no conflicts of interest identified.
References
- Apkarian A.V., Sosa Y., Sonty S., Levy R.M., Harden R.N., Parrish T.B., Gitelman D.R. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J. Neurosci. Official J. Soc. Neurosci. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal N., Perrot S., Fermanian J., Bouhassira D. The neuropathic components of chronic low back pain: a prospective multicenter study using the DN4 Questionnaire. J. Pain. 2011;12:1080–1087. doi: 10.1016/j.jpain.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 1995:289–300. [Google Scholar]
- Catley M.J., Tabor A., Wand B.M., Moseley G.L. Assessing tactile acuity in rheumatology and musculoskeletal medicine-How reliable are two-point discrimination tests at the neck, hand, back and foot? Rheumatol. (United Kingdom) 2013;52:1454–1461. doi: 10.1093/rheumatology/ket140. [DOI] [PubMed] [Google Scholar]
- Cohen J. second ed. 1988. Statistical Power Analysis for the Behavioral Sciences. edn (Routledge) [Google Scholar]
- Cohen M., Quintner J., Buchanan D., Nielsen M., Guy L. Stigmatization of patients with chronic pain: the extinction of empathy. Pain Med. 2011;12:1637–1643. doi: 10.1111/j.1526-4637.2011.01264.x. [DOI] [PubMed] [Google Scholar]
- Delitto A. Research in low back pain: time to stop seeking the elusive “Magic Bullet”. Phys. Ther. 2005;85:206–208. [PubMed] [Google Scholar]
- Derogatis L.R., Unger R. The Corsini Encyclopedia of Psychology. John Wiley & Sons, Inc.; 2010. Symptom checklist-90-revised. [Google Scholar]
- Finnerup N.B., Haroutounian S., Kamerman P., Baron R., Bennett D.L., Bouhassira D., Cruccu G., Freeman R., Hansson P., Nurmikko T. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157:1599–1606. doi: 10.1097/j.pain.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H. 1995. Phantom-limb Pain as a Perceptual Correlate of Cortical Reorganization Following Arm Amputation. [DOI] [PubMed] [Google Scholar]
- Flor H., Elbert T., Knecht S., Wienbruch C., Pantev C., Birbaumer N., Larbig W., Taub E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- Forster M., Mahn F., Gockel U., Brosz M., Freynhagen R., Tolle T.R., Baron R. Axial low back pain: one painful area–many perceptions and mechanisms. PloS one. 2013;8:e68273. doi: 10.1371/journal.pone.0068273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster N.E., Hill J.C., Hay E.M. Subgrouping patients with low back pain in primary care: are we getting any better at it? Man. Ther. 2011;16:3–8. doi: 10.1016/j.math.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Freynhagen R., Baron R., Gockel U., Tolle T.R. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr. Med. Res. Opin. 2006;22:1911–1920. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- Gore M., Dukes E., Rowbotham D.J., Tai K.S., Leslie D. Clinical characteristics and pain management among patients with painful peripheral neuropathic disorders in general practice settings. Eur. J. pain. 2007;11:652–664. doi: 10.1016/j.ejpain.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Henry D.E., Chiodo A.E., Yang W. Central nervous system reorganization in a variety of chronic pain states: a review. PM R J. Inj. Funct. Rehabilitation. 2011;3:1116–1125. doi: 10.1016/j.pmrj.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Huijnen I.P.J., Rusu A.C., Scholich S., Meloto C.B., Diatchenko L. Subgrouping of low back pain patients for targeting treatments: evidence from genetic, psychological, and activity-related behavioral approaches. Clin. J. Pain. 2015;31:123–132. doi: 10.1097/AJP.0000000000000100. [DOI] [PubMed] [Google Scholar]
- International Association for the Study of Pain . IASP taxonomy. In: Merskey H., Bogduk N., editors. Classification of Chronic Pain. IASP Press; Seattle: 2016. [Google Scholar]
- Jensen M.P., Chodroff M.J., Dworkin R.H. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68:1178–1182. doi: 10.1212/01.wnl.0000259085.61898.9e. [DOI] [PubMed] [Google Scholar]
- Jensen T.S., Baron R., Haanpaa M., Kalso E., Loeser J.D., Rice A.S., Treede R.D. A new definition of neuropathic pain. Pain. 2011;152:2204–2205. doi: 10.1016/j.pain.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Julian L.J. Measures of anxiety. Arthritis Care Res. Hob. 2011;63 doi: 10.1002/acr.20561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juottonen K., Gockel M., Silen T., Hurri H., Hari R., Forss N. Altered central sensorimotor processing in patients with complex regional pain syndrome. Pain. 2002;98:315–323. doi: 10.1016/S0304-3959(02)00119-7. [DOI] [PubMed] [Google Scholar]
- Knight R.G., Waal-Manning H.J., Spears G.F. Some norms and reliability data for the state–trait anxiety inventory and the zung self-rating depression scale. Br. J. Clin. Psychol. 1983;22(Pt 4):245–249. doi: 10.1111/j.2044-8260.1983.tb00610.x. [DOI] [PubMed] [Google Scholar]
- Koes B.W., van Tulder M.W., Thomas S. Diagnosis and treatment of low back pain. BMJ. 2006;332:1430–1434. doi: 10.1136/bmj.332.7555.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosek E., Cohen M., Baron R., Gebhart G.F., Mico J.A., Rice A.S., Rief W., Sluka A.K. Do we need a third mechanistic descriptor for chronic pain states? Pain. 2016;157:1382–1386. doi: 10.1097/j.pain.0000000000000507. [DOI] [PubMed] [Google Scholar]
- Kosinski M., Keller S.D., Ware J.E., Jr., Hatoum H.T., Kong S.X. The SF-36 health survey as a generic outcome measure in clinical trials of patients with osteoarthritis and rheumatoid arthritis: relative validity of scales in relation to clinical measures of arthritis severity. Med. Care. 1999;37 doi: 10.1097/00005650-199905001-00003. Ms23–39. [DOI] [PubMed] [Google Scholar]
- Lotze M., Flor H., Grodd W., Larbig W., Birbaumer N. Phantom movements and pain - an MRI study in upper limb amputees. Brain. 2001;124:2268–2277. doi: 10.1093/brain/124.11.2268. [DOI] [PubMed] [Google Scholar]
- Maihofner C., Forster C., Birklein F., Neundorfer B., Handwerker H.O. Brain processing during mechanical hyperalgesia in complex regional pain syndrome: a functional MRI study. Pain. 2005;114:93–103. doi: 10.1016/j.pain.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Maihofner C., Handwerker H.O., Neundorfer B., Birklein F. Patterns of cortical reorganization in complex regional pain syndrome. Neurology. 2003;61:1707–1715. doi: 10.1212/01.wnl.0000098939.02752.8e. [DOI] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill pain questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Moberg E. Two-point discrimination test. A valuable part of hand surgical rehabilitation, e.g. in tetraplegia. Scand. J. Rehabil. Med. 1990;22:127–134. [PubMed] [Google Scholar]
- Nolan M.F. Quantitative measure of cutaneous sensation. Two-point discrimination values for the face and trunk. Phys. Ther. 1985;65:181–185. doi: 10.1093/ptj/65.2.181. ISSN: 0031-9023 (Print), 0031-9023. [DOI] [PubMed] [Google Scholar]
- Nygaard O.P., Mellgren S.I. The function of sensory nerve fibers in lumbar radiculopathy. Use of quantitative sensory testing in the exploration of different populations of nerve fibers and dermatomes. Spine. 1998;23 doi: 10.1097/00007632-199802010-00012. 348–352; discussion 353. [DOI] [PubMed] [Google Scholar]
- O'Sullivan P. Diagnosis and classification of chronic low back pain disorders: maladaptive movement and motor control impairments as underlying mechanism. Man. Ther. 2005;10:242–255. doi: 10.1016/j.math.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Pleger B., Tegenthoff M., Schwenkreis P., Janssen F., Ragert P., Dinse H.R., Volker B., Zenz M., Maier C. Mean sustained pain levels are linked to hemispherical side-to-side differences of primary somatosensory cortex in the complex regional pain syndrome I. Exp. Brain Res. Exp. Hirnforschung Exp. Cerebrale. 2004;155:115–119. doi: 10.1007/s00221-003-1738-4. [DOI] [PubMed] [Google Scholar]
- Putzke J.D., Richards J.S., Hicken B.L., DeVivo M.J. Interference due to pain following spinal cord injury: important predictors and impact on quality of life. Pain. 2002;100:231–242. doi: 10.1016/S0304-3959(02)00069-6. [DOI] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Rubinstein S.M., van Tulder M. A best-evidence review of diagnostic procedures for neck and low-back pain. Best. Pract. Res. Clin. Rheumatol. 2008;22:471–482. doi: 10.1016/j.berh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Ruoff G.E., Rosenthal N., Jordan D., Karim R., Kamin M. Tramadol/acetaminophen combination tablets for the treatment of chronic lower back pain: a multicenter, randomized, double-blind, placebo-controlled outpatient study. Clin. Ther. 2003;25:1123–1141. doi: 10.1016/s0149-2918(03)80071-1. [DOI] [PubMed] [Google Scholar]
- Rydevik B., Brown M.D., Lundborg G. Pathoanatomy and pathophysiology of nerve root compression. Spine. 1984;9:7–15. doi: 10.1097/00007632-198401000-00004. [DOI] [PubMed] [Google Scholar]
- Schmitz N., Hartkamp N., Franke G.H. Assessing clinically significant change: application to the SCL-90-R. Psychol. Rep. 2000;86:263–274. doi: 10.2466/pr0.2000.86.1.263. [DOI] [PubMed] [Google Scholar]
- Seltzer S.F., Seltzer J.L. Tactual sensitivity of chronic pain patients to non-painful stimuli. Pain. 1986;27:291–295. doi: 10.1016/0304-3959(86)90156-9. [DOI] [PubMed] [Google Scholar]
- Smith B.H., Torrance N. Epidemiology of neuropathic pain and its impact on quality of life. Curr. pain headache Rep. 2012;16:191–198. doi: 10.1007/s11916-012-0256-0. [DOI] [PubMed] [Google Scholar]
- Smith B.H., Torrance N., Bennett M.I., Lee A.J. Health and quality of life associated with chronic pain of predominantly neuropathic origin in the community. Clin. J. pain. 2007;23:143–149. doi: 10.1097/01.ajp.0000210956.31997.89. [DOI] [PubMed] [Google Scholar]
- Spielberger C.D. Consulting Psychologists Press; Palo Alto, CA: 1983. Manual for the State-trait Anxiety Inventory. [Google Scholar]
- Stanton T.R., Fritz J.M., Hancock M.J., Latimer J., Maher C.G., Wand B.M., Parent E.C. Evaluation of a treatment-based classification algorithm for low back pain: a cross-sectional study. Phys. Ther. 2011;91:496–509. doi: 10.2522/ptj.20100272. [DOI] [PubMed] [Google Scholar]
- Torrance N., Smith B.H., Bennett M.I., Lee A.J. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J. Pain. 2006;7:281–289. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Tracey I., Bushnell M.C. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J. Pain. 2009;10:1113–1120. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Turk D.C. The potential of treatment matching for subgroups of patients with chronic pain: lumping versus splitting. Clin. J. Pain. 2005;21 doi: 10.1097/00002508-200501000-00006. 44-55; discussion 69–72. [DOI] [PubMed] [Google Scholar]
- Turk D.C., Okifuji A. Detecting depression in chronic pain patients: adequacy of self-reports. Behav. Res. Ther. 1994;32:9–16. doi: 10.1016/0005-7967(94)90078-7. [DOI] [PubMed] [Google Scholar]
- van der Windt D.A., Simons E., Riphagen, Ammendolia C., Verhagen A.P., Laslett M., Deville W., Deyo R.A., Bouter L.M., de Vet H.C., Aertgeerts B. 2010. Physical Examination for Lumbar Radiculopathy Due to Disc Herniation in Patients with Low-back Pain. The Cochrane database of systematic reviews, Cd007431. [DOI] [PubMed] [Google Scholar]
- Walsh T.L., Homa K., Hanscom B., Lurie J., Sepulveda M.G., Abdu W. Screening for depressive symptoms in patients with chronic spinal pain using the SF-36 Health Survey. Spine J. Official J. North Am. Spine Soc. 2006;6:316–320. doi: 10.1016/j.spinee.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Ware J.E., Jr., Kosinski M., Bayliss M.S., McHorney C.A., Rogers W.H., Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the medical outcomes study. Med. Care. 1995;33:AS264–AS279. [PubMed] [Google Scholar]
- Ware J.E., Jr., Sherbourne C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. care. 1992;30:473–483. [PubMed] [Google Scholar]
- Ware J.E., Snow K.K., Kosinski M. New England Medical Center, The Health Institute; Boston, MA: 1993. SF-36® Health Survey Manual and Interpretation Guide. [Google Scholar]
- Woolf C.J. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann. Intern Med. 2004;140:441–451. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- Yamashita T., Kanaya K., Sekine M., Takebayashi T., Kawaguchi S., Katahira G. A quantitative analysis of sensory function in lumbar radiculopathy using current perception threshold testing. Spine. 2002;27:1567–1570. doi: 10.1097/00007632-200207150-00016. [DOI] [PubMed] [Google Scholar]
- Yarnitsky D. Quantitative sensory testing. Muscle Nerve. 1997;20:198–204. doi: 10.1002/(sici)1097-4598(199702)20:2<198::aid-mus10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]