Abstract
The field of Alzheimer disease (AD) prevention has been a culmination of basic science, clinical, and translational research. In the past three years since the new 2011 AD diagnostic guidelines, large-scale collaborative efforts have embarked on new clinical trials with the hope of someday preventing AD. This review will shed light on the historical and scientific contexts in which these trials were based on, as well as discuss potential challenges these trials may face in the coming years. Primary preventive measures, such as lifestyle, multidomain, medication, and supplemental interventions, will be analyzed. Secondary prevention as represented by disease-modifying interventions, such as anti-amyloid therapy and pioglitazone, will also be reviewed. Finally, hypotheses on future directions for AD prevention trials will be proposed.
Introduction
Preventing Alzheimer disease (AD) is a worthy cause for many reasons. The first is the sheer number of those suffering with AD. Over 5 million Americans and 36 million people worldwide have AD dementia [1]. As the sixth leading cause of death in the United States, it is the most common cause of dementia, accounting for 60–80% of all cases. AD dementia occurs in one out of nine people over age 65 and one out of three for those older than 85. It is arguably the costliest disease in the world, amounting to $200 billion annually in the United States alone [2]. Economists predict that preventing or at least delaying the onset of the disease by five years would cut Medicare spending for AD by half [3].
The second reason is that without intervention, the number of individuals with AD will skyrocket in the coming decades. By 2050, the number of people with AD dementia in the United States will be 13.5 million and the costs will increase five-fold [3]. Already, caring for individuals with AD dementia has stretched the fabric of our society and drastically stressed our health care system, leading to overburdened caregivers, lost productivity, and out-of-control costs. Studying potential risk factors for AD and implementing interventions early could lay the tracks for long-term solutions to address this growing chronic problem.
The third reason is that AD currently does not have a cure or even an effective disease-modifying drug. Hence, a diagnosis of AD is akin to a diagnosis of terminal illness, often leading to anxiety and dread for many older persons. The five medications currently approved by the Food and Drug Administration (FDA) for treatment of AD dementia can only address symptoms of AD, but not change its course. Although we have learned much about the pathophysiology of AD in the past three decades, such as the workings of amyloid and tau, understanding the causal mechanisms behind AD pathology is still elusive. AD prevention would hopefully truncate the number of individuals suffering from this disease, thus decreasing the number of years lost, as well as relieving a huge public health burden.
AD prevention is the second major wave of preventive efforts in the field of neurology. The first wave was stroke prevention. Much of what is happening in AD prevention right now happened for stroke prevention two decades ago. The National Stroke Association published the first stroke guidelines in the Journal of the American Medical Association in 1999 [4], and at that time, multiple landmark clinical trials had already established important stroke risk factors. These included hypertension, myocardial infarction, atrial fibrillation, diabetes mellitus, carotid artery stenosis, smoking, alcohol use, low physical activity, and unhealthy diet. Similarly with AD, large prevention trials have only recently started, but multiple longitudinal observational studies have also consistently identified risk factors for AD. Despite the paucity of available data, we were still able to identify some key prevention trials for this review. We identified randomized-controlled trials and observational studies through PubMed, Google Scholar, and the Cochrane Library with search words including but not limited to “Alzheimer,” “prevention,” and “trial.”
The history of AD prevention is relatively short. As a matter of historical perspective, the Institute of Medicine (IOM) published a report on “Reducing Risks for Mental Disorders” twenty years ago, and they featured a chapter on AD [5]. Amyloid had just been discovered a decade before, and mutations in amyloid were just being identified. The discussion of other risk factors revealed the absence of evidence for that time and speaks to the maturation of the AD field in the past two decades. For examples, low education was still not yet an established risk factor, and the report also speculated on whether the age of a person’s mother was a risk factor for AD.
In 2011, President Barack Obama signed into law the National Alzheimer’s Project Act (NAPA) [6], which has invested millions of dollars into AD research and has helped launch multiple large clinical trials to study AD interventions. In comparison to the state of AD research in the 1994 IOM report, NAPA has dramatically fueled AD prevention efforts. Timely and ambitious, the first goal of NAPA is to prevent and effectively treat AD by 2025. The five underlying strategies to prevent AD include building infrastructure for effective research practices, collaborating with large organizations worldwide to hone targeting efforts, and translating preventive practices into community health settings. We have already witnessed many of the strategies at work with the start of four of five large secondary prevention trials in conjunction with the establishment of the Collaboration for Alzheimer Prevention consortium.
2011 was a watershed year for AD in another way. The National Institute of Aging and the Alzheimer’s Association (NIA-AA) collaborated to update clinical and research diagnostic criteria for AD [7–10]. They updated the 1984 criteria to include three stages: preclinical AD, mild cognitive impairment (MCI) due to AD, and AD dementia. The workgroups emphasized the importance of biomarkers when categorizing patients into these stages, and subsequent prevention trials have been able to rely on these criteria, especially those for the preclinical stage. Studies at the Mayo Clinic further operationalized the preclinical stages and demonstrated their effectiveness [11]. Since 2011, new workgroups have also been convened to study potential challenges of AD prevention trials and to propose possible solutions. Some of these challenges will be discussed in this review.
To frame avenues for prevention, many longitudinal observational studies have already established risk factors for AD. As in any preventable disease, risk factors can be divided into modifiable and unmodifiable risk factors. This review will concentrate on modifiable risk factors for AD, many of which are cardiovascular or lifestyle related, and intervention trials targeting these risk factors will be discussed. Five interventional approaches will be described in this review on AD prevention. These include: 1) lifestyle, 2) multidomain, 3) marketed medications for other indications, 4) supplements, and 5) disease-modifying interventions, such as anti-amyloid treatment.
Challenges of Primary and Secondary AD Prevention Trials
Progress in AD research has helped to inform the design and standardization of current prevention trials. Opportunities abound to incorporate new biomarkers into tracking the incidence of AD symptoms and AD pathology. However, in the past five years, researchers have identified new challenges when approaching prevention trials, and several are still pertinent today when looking forward to new trials. It will be important to keep these challenges in mind when reviewing past prevention trials, as many lessons learned from previous trials will help us understand the rationale for many trials currently underway. As the most common cause of dementia, AD is also the most understood, but much of the pathophysiology is still shrouded in mystery. One of the biggest challenges in the field already has been to standardize the language we use when describing AD.
In the span of 27 years, the science of AD accelerated, mainly due to advances in molecular genetics and neuroimaging techniques. Computerized tomography (CT) scans were just coming into use, and the backbone of diagnosing probable, possible, and definite AD stemmed mainly from clinical assessments such as neuropsychological testing. Just to give a sense of the progress of AD research over time, the 1984 guidelines [12] stated, “Information should soon be available about the usefulness of magnetic resonance imaging (MRI) in the diagnosis of Alzheimer’s disease.” Also, “To date, definitive diagnostic information about Alzheimer’s disease from blood or cerebrospinal fluid (CSF) has not been sought consistently, but CSF should be studied to demonstrate neurotransmitters, metabolites, and synthesizing and degradative enzymes.” Incorporating clinically useful biomarkers, the 2011 guidelines expanded on the 1984 framework and greatly enriched our knowledge of the AD syndrome.
Prevention of AD must take into account the entire AD spectrum. This forms the basis for most, if not all, of the challenges when developing preventive interventions. From a regulatory standpoint, secondary prevention trials are considered treatment trials in preclinical AD. The central narrative behind the 2011 guidelines involves what scientists have called, “the amyloid hypothesis,” or more precisely “the A-beta hypothesis,” according to Dennis Selkoe, MD, one of the main proponents of the theory [13]. Selkoe describes in this hypothesis the critical role that abnormal amyloid (A-beta) plays in the brain, wherein the pathophysiology of AD is strongly associated with abnormal accumulation of A-beta in the setting of decreased clearance. The guidelines highlight the evidence for this theory and describe in detail recommendations for monitoring specific biomarkers, including but not limited to positron emission tomography (PET) amyloid status and CSF A-beta level. Although there is also evidence for other pathophysiological mechanisms for AD, such as build-up of abnormal tau, researchers are still validating potential biomarkers and evaluating their usefulness in prevention trials.
Investment in AD prevention trials can be an enormous undertaking so addressing potential challenges early on will optimize trial design and save a lot of time and effort. We have identified seven challenges that have occurred in most AD prevention trials to date. These include: 1) stage of intervention, 2) duration of intervention, 3) subject selection, 4) outcome measure selection, 5) standardization of AD biomarkers, 6) ethics of disclosing biomarker status, and 7) coordination of AD registries.
Stage of intervention
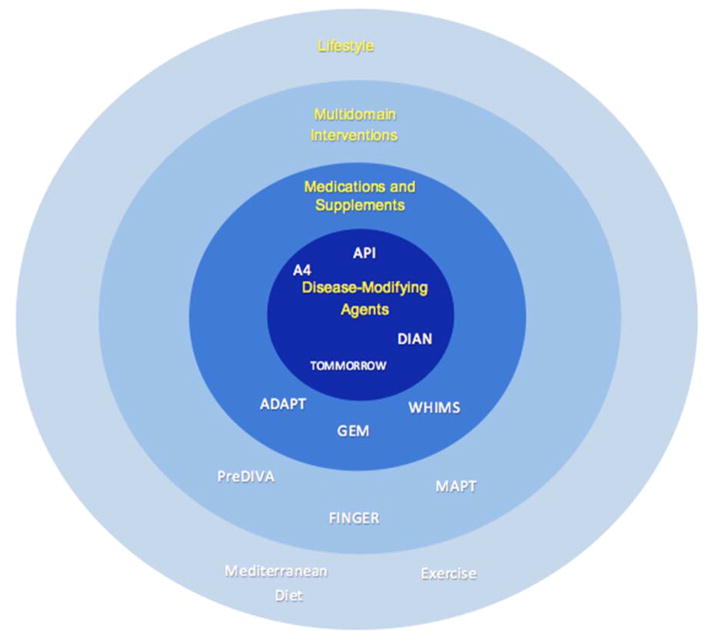
The importance of distinguishing primary and secondary prevention trials cannot be overstated. However, many AD prevention trials in the past decade were not effectively equipped to make that distinction, since the technology for in vivo biomarkers of AD pathology have only recently been developed. The 2011 guideline on preclinical AD recommended dividing AD into clinical symptoms of AD (AD-C) and underlying AD pathology (AD-P). The NIA-AA workgroup postulated that there is a long asymptomatic phase of AD-P before the onset of symptoms that can be tracked by monitoring various biomarkers like amyloid. When conceiving AD prevention trials, it would then make sense to categorize those trials that target individuals with present AD biomarkers as secondary prevention trials and those that intervene before the presence of AD biomarkers as primary prevention trials. Without identification of biomarker status in the selected population, researchers would be unable to label their trials definitively as primary or secondary prevention trials. As the intervention is directed away from AD-P, the trial becomes less targeted and closer to primary prevention (Figure 1).
Figure 1. Levels of Prevention.
This model depicts the targeted approaches to AD prevention, from primary to secondary. The most direct approach to AD prevention is a disease-modifying agent; hence, it is at the center of the diagram. As the circle widens, the preventive approach becomes more indirect, ultimately landing on single lifestyle interventions as described in the text.
A4 = Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease; ADAPT = AD Anti-inflammatory Prevention Trial; API = Alzheimer’s Prevention Initiative; DIAN = Dominantly Inherited Alzheimer Network; FINGER = Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability; GEM = Ginkgo Evaluation of Memory; MAPT = Multidomain Alzheimer Preventive Trial; PreDIVA = Prevention of Dementia by Intensive Vascular Care study; WHIMS = Women’s Health Initiative Memory Study
Duration of intervention
Evidence suggests that the preclinical AD phase can last a decade or even longer before symptoms begin. Timing of certain interventions, therefore, will contribute significantly to primary outcomes of trials. Only with prolonged durations, such as three to five years, will trials be potentially adequately powered to detect a difference in effect from an intervention. Such is the precedence in heart disease and cancer prevention trials thus far [14, 15]. The Mayo Clinic group operationalized the 2011 preclinical AD guidelines and followed subjects in their cohort for at least a year, demonstrating that subjects will progress to MCI or dementia at increasing proportions depending on the severity of preclinical disease stage (0 to 3) [16]. Therefore, researchers should now be precise when describing the stage of AD in which the intervention is being introduced so as to hypothesize the natural progression of their cohort, as well as better measure their clinical or biomarker endpoints.
Subject selection
The negative results of numerous phase III clinical trials using amyloid-modifying drugs at the stage of mild-moderate AD dementia suggested that amyloid-modifying interventions might have been applied too late in the disease course [17]. Testing drugs in those with already moderate AD dementia was unsuccessful, but there remains a glimmer of hope with testing interventions in those with mild AD dementia or MCI due to AD, given the secondary analyses results of cognitive improvement in the mild AD dementia group alone in the solanezumab, amyloid passive immunotherapy, phase III clinical trials [18]. Consistent with our understanding of the preclinical phase of AD, preventive interventions need to be given earlier. Several secondary prevention trials have already begun recruiting cognitively normal individuals for AD prevention trials, such as the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) trial [19]. Screening subjects who are cognitively normal, yet have AD-P, poses another challenge when running one of these trials, since many of the screening eligibility procedures include neuropsychological testing and neuroimaging or CSF. Having a qualified team to recruit and screen a diverse cohort of older subjects with state-of-the-art imaging techniques can be daunting at times. Moreover, recruiting asymptomatic individuals to participate in a clinical trial that poses a certain amount of risk to them could prove difficult since the value of preventative medicine is often underappreciated by lay people and even healthcare professionals.
Outcome measure selection
Since cognitively normal individuals are participating in AD prevention trials, selecting and/or developing the appropriate outcome measures poses a significant challenge. The FDA recently issued new guidance for clinical trials in early AD [20]. In this guidance, the FDA suggests a single cognitive measure as a primary outcome for trials in preclinical AD, which is a shift from the traditional dual cognitive and functional outcome measures required for AD trials at the stage of dementia. In order to be able to capture cognitive decline in individuals who start off with normal cognition, more sensitive assessments than the ones used for traditional trials in AD dementia or even MCI are necessary. For this purpose, the Alzheimer’s Disease Cooperative Study (ADCS) group has recently validated a composite of cognitive assessments, the ADCS Preclinical Alzheimer’s Cognitive Composite (PACC) [21], in hopes of capturing the earliest objective cognitive impairment. Extending beyond objective assessment of cognition, hybrid assessments of subjective cognitive and subjective instrumental activities of daily living (IADL), such as the ADCS Cognitive Function Instrument (CFI), have also been validated [22]. Ultimately the FDA would still like to see a clinically relevant outcome, that is evidence of a benefit in IADL, which is stipulated as a subsequent requirement in the early use of a drug for preclinical AD after meeting the single primary cognitive outcome measure in the clinical trial. This could be potentially addressed by a subjective IADL scale, which the CFI partly represents although it combines subjective IADL questions with subjective cognitive questions. Alternatively, experts have recently suggested that a challenging performance-based IADL assessment may better capture early changes in IADL and would have better psychometric properties and ecological validity than a subjective IADL scale [23].
Standardization of AD biomarkers
According to Jack and colleagues, biomarkers are “parameters (physiological, biochemical, anatomic) that can be measured in vivo and that reflect specific features of disease-related pathophysiological processes [8].” In this framework, which is limited to well-studied AD biomarkers, they can be classified into two main categories: 1) biomarkers of A-beta accumulation and 2) biomarkers of neuronal degeneration or injury. Biomarkers of A-beta accumulation now include CSF A-beta and PET amyloid. Biomarkers of neuronal degeneration or injury include structural imaging with MRI, hypometabolism with 18F-fluorodeoxyglucose (FDG) PET, and CSF total tau and phospho-tau levels. Validated through several longitudinal, observational studies, such as the Alzheimer Disease Neuroimaging Initiative, these biomarkers have been incorporated into the new 2011 diagnostic guidelines and can predict, with different levels of accuracy, onset of AD about a decade before clinical symptoms. Prevention trials targeting AD have varied in their determination of the presence of adequate AD biomarkers in the sample population before intervention. In this review, readers should be mindful of which studies incorporate AD biomarkers into their inclusion criteria, and which merely focus on clinical criteria.
Ethics of biomarker status disclosure
AD prevention trials have started to screen individuals based on certain biomarkers, such as PET amyloid level. Studies operationalizing AD preclinical criteria and following individuals longitudinally have validated the usefulness of these biomarkers in identifying those at risk for AD, as well as quantifying that risk. There is precedence in the medical field for screening individuals with biomarkers, which is done routinely for cancer and heart disease. Potential ethical dilemmas can be encountered when blinding research teams and subjects to AD biomarkers if knowledge is obtained of their biomarker status [24, 25]. For example, if someone has low non-specific amyloid burden on PET, does that mean that he or she will never develop symptoms of AD? Or, if someone has high amyloid burden on PET, would he or she be completely immune from discrimination when applying for long-term care insurance? These are just some of the questions that can be encountered when conducting an AD prevention trial. In addition, precedence exists in healthcare law for genetic and preexisting conditions, but none currently exists for biomarker status. How will society view biomarkers for neurological or any medical conditions? The ethics of biomarker status clearly warrant further research, and at least one AD prevention trial, has decided to incorporate this question into the study, which is the A4 trial [19].
Coordination of AD registries
The AD research community only recently reached a consensus for new AD guidelines outlining stages and biomarkers. Many scientists, who previously had been working in isolation, are now finding themselves collaborating with groups around the nation and the world. Importance of sharing information and coordinating with other registries has been critical in unifying the AD prevention effort. As current trials move forward, researchers will now be able to compare data more easily with the synchronization of clinical and biomarker outcomes [14, 15]. Collaboration of AD experts in academic, governmental, and pharmaceutical bodies may bring about more useful knowledge through prevention trials than if working separately. However, rising above scientific competition can be a challenge of its own, which is one reason why NAPA specifically mentions this in its strategies. The FDA has also been instrumental in outlining guidelines to the research community on potential outcome measures for early AD trials and ways to accelerate the regulatory pathway [20]. Two important practical reasons for these type of AD registries are: 1) sharing data from observational studies to better understand disease progression and inform trial design as has been done with the Alzheimer’s Disease Neuroimaging Initiative (ADNI), Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL), and Harvard Aging Brain Study (HABS) datasets; and 2) for recruitment registries for upcoming trials as has been done with the National Alzheimer’s Coordinating Center (NACC), Dominantly Inherited Alzheimer Network (DIAN), and Alzheimer’s Prevention Initiative (API) apolipoprotein E ε4 (APOE) registries. The latter is particularly relevant for genetic enrichment strategies in trials when trying to enroll participants with susceptibility genes such as APOE4 and TOMM40 or even rarer autosomal dominant AD mutations.
In our review, trials preventing all dementia are included with those just targeting AD, given the fact that in the earliest stages of AD, it often can be challenging to make the distinction between various dementia syndromes accurately. Our targeted population when identifying prevention trials has been for individuals without cognitive impairment, meaning those without clinical AD as described by the 2011 diagnostic guidelines. Our definition of “clinical AD” will encompass the symptoms of “MCI due to AD” and “AD dementia” together.
Lifestyle Interventions
Primary prevention trials have often targeted known risk factors by incorporating specific lifestyle interventions, which have included management of cardiovascular disease risk factors, changes in diet and exercise, cognitive stimulation or training, and social engagement (Table 1). Although many of these trials did not show benefit in preventing AD or dementia, a few select trials did show some benefit in preventing cognitive impairment, and these positive, as well as negative trials, will be reviewed here. A compilation of these interventions would eventually be incorporated into multidomain interventional trials, with each relying more on certain lifestyle interventions than others.
Table 1.
Lifestyle Interventions
| Intervention | Trial | Study Sample | Duration | N | Outcome Measures | Results |
|---|---|---|---|---|---|---|
| Hypertension | Syst-Eur [26] | Aged 60 and older, not demented | 2 years | 2,418 | MMSE | Positive; dementia was reduced by 50% in active treatment |
| Hyperlipidemia | HPS [28] PROSPER [29] |
Aged 40–80 with coronary artery disease or diabetes, not demented Aged 70–82 with vascular disease or risk factors, not demented |
5 years 3.2 years |
20,536 5,804 |
Telephone Interview for Cognitive Status Stroop and MMSE |
Negative; statins did not prevent dementia Negative |
| Diabetes | ACCORD-MIND [30, 31] | Mean age 62, cognitively normal | 3.3 years | 2,977 | Digit Symbol Substitution Test score, total brain volume on MRI | Negative; there was no difference between intensive diabetes therapy vs. standard therapy |
| Mediterranean Diet (observational only) | Three-City Study [32] | Aged 65 and older, not demented | 5 years | 1,410 | MMSE, Isaacs Set Test, Benton Visual Retention Test, Free and Cued Selective Reminding Test | Positive; the Mediterranean diet was associated with slower cognitive decline on MMSE only |
| Exercise | FABS [33] | Aged 50 and older, with subjective memory complaints, no dementia | 1.5 years | 170 | ADAS-Cog | Positive; ADAS-Cog improved after physical activity |
| Cognitive Training | ACTIVE [38–40] | Aged 65–94, cognitively normal | 10 years | 2,832 | Everyday problem-solving, everyday speed, and activities of daily living | Positive; cognitive training, specifically reasoning and speed training, even at 10 years had less decline in ADL |
| Social (observational only) | Kungsholmen [42] | Aged 75 and older, not demented | 3 years | 1,203 | Incidence of dementia | Positive; a large social network was associated with less dementia |
ACCORD-MIND = Action to Control Cardiovascular Risk in Diabetes trial with Memory in Diabetes sub-study; ACTIVE = Advanced Cognitive Training for Independent and Vital Elderly; ADAS-Cog = Alzheimer’s Disease Assessment Scale-Cognitive Subscale; ADL = activities of daily living; FABS = Fitness for the Aging Brain Study; HPS = Heart Protection Study; MMSE = Mini-Mental State Examination; MRI = magnetic resonance imaging; PROSPER = Prospective Study of Pravastatin in the Elderly at Risk
Hypertension was one of the first risk factors to be targeted in preventing AD and dementia. The Systolic Hypertension in Europe (Syst-Eur) trial [26], started in 1988, ended in 1997 and published in 1998, came at the end of a wave of stroke prevention trials, which predominantly concentrated on hypertension as a risk factor. The trial, also called “the vascular dementia project,” had the primary outcomes of dementia and stroke. Because the data had resulted in a significant benefit for stroke, the trial was stopped early. Researchers started with a cohort of patients who did not have dementia, were 60 years of age or older, and who had a blood pressure of 160–219 mm Hg systolic and below 95 mm Hg diastolic. Interventions were nitrendipine (10–40 mg/day), enalapril (5–20 mg/day), hydrochlorothiazide (12.5–25 mg/day), or a combination of these to reduce systolic blood pressure by at least 20 mm Hg to a goal of below 150 mm Hg. Subjects were evaluated cognitively with the Mini-Mental State Exam (MMSE) and dementia criteria as outlined by the Diagnostic and Statistical Manual of Mental Disorders, third edition, revised (DSM-III-R). The active treatment group had 1238 subjects, whereas the placebo group had 1180 subjects, and the median follow-up was two years. The intervention was found to reduce the incidence of dementia by 50%, from 7.7 cases per 1000 patient-years to 3.8 cases, and the analysis included AD dementia as a subcategory. The investigators calculated that out of 1000 persons treated for hypertension for five years, 19 cases of dementia could be prevented.
Hyperlipidemia has continued to receive attention as a potential cardiovascular risk factor linked to the onset of AD dementia. Several prevention trials have evaluated the effect of intervention with statins, and the Cochrane Dementia and Cognitive Improvement Group recently performed a systematic review of these trials [27], determining that there was good evidence for the inability of statins given in late life to prevent dementia. The two trials that met inclusion criteria were the Heart Protection Study (HPS) 2002 trial and the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) 2002 trial. The HPS trial [28], treated 20,536 British adults aged 40–80 who had coronary artery disease or diabetes, for five years with simvastatin 40 mg daily or placebo. Primary outcomes were all-cause mortality and various vascular outcomes. The subjects were also evaluated with the Telephone Interview for Cognitive Status (TICS) at the final follow-up visit, and the mean scores were compared between the groups. Although simvastatin significantly improved mortality and vascular morbidity, there was no difference in the cognitive scores, and the incidence of dementia in both groups was comparable. Thirty-one subjects in each group developed dementia, which was about 0.3% of the total group.
The PROSPER trial [29], was a randomized, controlled trial that treated non-demented (MMSE≥24) 5,804 subjects, aged 70–82 years and who had history of vascular disease or risk factors, with either pravastatin 40 mg daily or placebo for three years. Endpoints were similar to the HPS trial, and the results pointed to a protective effect of pravastatin on cardiovascular outcomes. However, cognitive function declined at the same rate in the two groups. Scores on the MMSE, various neuropsychological tests including the Stroop test, and questionnaires of IADL were similar between the two groups by the end of the trial. Similar to HPS, PROSPER had solely relied on cognitive testing as markers for cognitive change and did not pursue underlying AD pathology in the brain with either imaging or CSF testing. The PROSPER study in particular also included older subjects with already known cerebrovascular disease, thus likely skewing potential cognitive decline toward vascular etiologies rather than pure AD. Unfortunately, neither of these studies was able to demonstrate benefit of statins for AD prevention or cognitive function.
Diabetes, in addition to hypertension and hyperlipidemia, has been the third main cardiovascular focus of AD prevention trials. The most prominent trial has been the Action to Control Cardiovascular Risk in Diabetes trial with Memory in Diabetes (ACCORD-MIND) sub-study [30]. At 52 sites in North America, the study randomized 2,977 cognitively normal individuals, with mean age 62.3 years, to intensive glycemic control of hemoglobin A1c less than 6% or standard of care with hemoglobin A1c between 7% and 7.9%. In the duration of 40 months, the researchers measured total brain volume (TBV) by MRI and cognitive status through the Digit Symbol Substitution Test (DSST), the Rey Auditory Verbal Learning Test (RAVLT), and the Stroop test at various time points in the study. They found that although the intensive treatment group had greater TBV, there were no statistically significant differences between the cognitive scores.
In a follow-up ACCORD-MIND study, they used the standard diabetes group with hemoglobin A1c less than 7.5% and randomized them to one of two sub-studies: 1) intensive hypertension management versus standard of care, or 2) intensive lipid management versus placebo [31]. The intensive hypertension goal was to a systolic blood pressure of less than 120 mm Hg, whereas the standard blood pressure goal was less than 140 mm Hg. The intensive lipid goal was low-density lipoprotein less than 100 mg/dL. The cognitive and MRI outcomes were the same as the previous study. Unfortunately, the results were the same for cognitive scores, demonstrating the lack of cognitive protection for intensive blood pressure or lipid management in diabetic patients. Although TBV declined in the intensive hypertension group, the long-term cognitive effects are still unknown.
Diet and exercise, often linked to cardiovascular outcomes, have been two behavioral interventions studied in AD prevention trials. The most promising diet intervention at this time has been the Mediterranean diet, which is a diet rich in fruits and vegetables, combined with olive oil and fish, as well as low amounts of dairy, meat, and wine [32]. A notable prospective study was by Féart and colleagues in JAMA, 2009, also known as the Three-City (3C) study, where 1,410 non-demented adults aged 65 years or older in Bordeaux, France were followed for five years on their adherence to a Mediterranean diet. Cognitive testing included four neuropsychological tests, which were the MMSE, Isaacs Set Test (IST), Benton Visual Retention Test (BVRT), and Free and Cued Selective Reminding Test (FCSRT). Researchers found that those who adhered to the Mediterranean diet had a slower rate of decline on the MMSE, but not on the other cognitive tests.
In a recent randomized-controlled trial of physical activity known as the Fitness for the Aging Brain Study (FABS) [33], subjects with subjective memory complaints but no dementia, who underwent a 6-month exercise program had modest cognitive improvement at 18 months. The cohort included 170 subjects aged 50 years and older, and the primary cognitive outcome was the Alzheimer Disease Assessment Scale-Cognitive Subscale (ADAS-Cog). Secondary analyses included word list delayed recall and Clinical Dementia Rating sum of boxes (CDR-SB), which also improved. The intervention group improved 0.26 points on the ADAS-Cog, whereas the usual care group declined 1.04 points. When combined with the Mediterranean diet, physical activity was shown to be associated with reduced incidence of AD in a cohort study of 1,880 elders in New York when followed for over five years [34]. For those with a high score for both Mediterranean diet and physical activity, the hazard ratio for AD was 0.65, which continues to underscore the importance of a healthy lifestyle.
Finally, cognitive and social interventions have received much attention by the AD prevention community. As part of the 21-year Bronx Aging Study, leisure activities that included reading, playing board games, playing musical instruments, and dancing were associated with a reduced risk of dementia [35]. Cognitive reserve and its highly correlated education level have often been found to protect individuals from future onset of dementia, but studies evaluating this effect have sometimes been variable in quality and mixed in their results [36]. Limitations of many studies have included having participants with already high cognitive reserve, heterogeneous modes of cognitive training, absence of follow up or poor follow up, and lack of understanding of how one trained cognitive domain affects other cognitive domains [37].
The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial has been the most robust cognitive intervention trial to date. Started in 1998, this randomized, controlled trial assigned 2,832 persons aged 65 to 94 into one of four groups: 1) memory training, 2) reasoning training, 3) speed of processing, or 4) no training as control group. These three cognitive domains were selected based on earlier research that showed these domains to be most related to IADL. Each intervention consisted of 10 sessions with a certified trainer. Booster training sessions were offered periodically for the duration of ten years, and results of the ACTIVE trial were reported at 2-year [38], 5-year [39], and 10-year follow up [40]. Cognitive outcomes included the Hopkins Verbal Learning Test, Rey Auditory-Verbal Learning Testing, and the Rivermead Behavioral Paragraph Recall Test, as well as other validated reasoning and speed of processing tests. From the second to the tenth year, subjects with training sessions had continued improvement in their respective cognitive domains, except for memory training, which no longer had a protective effect by year 10. All subjects who continued in the study and were in the intervention groups noted less difficulty in IADL when they were asked at the tenth year of follow up. The results of the ACTIVE trial give the strongest evidence for a benefit of cognitive intervention in the prevention of cognitive impairment.
The stress hypothesis, similar to the vascular hypothesis and cognitive reserve hypothesis, contends that having a healthy social network may help to prevent cognitive decline and AD. Conversely, having more stress activates the glucocorticoid axis and adversely affects hippocampal volume. In a systematic review in Lancet Neurology, Fratiglioni and colleagues conclude that the evidence suggests a protective effect of social engagement [41]. Results from mortality studies have continued to demonstrate a decrease in mortality with people with high-quality social relations, whereas those with low-quality social relations have a two to four-times higher mortality rate from all causes. The authors reviewed 13 large observational cohort studies on the effects of social relations on cognition or dementia in older adults, with most studies suggesting a benefit. Being single, having been widowed, or living alone has been associated with increased dementia. An example of a cohort study suggesting a benefit of social networks is the Kungsholmen study [42].
Multidomain Interventions
In 2011, the European Dementia Prevention Initiative (EDPI) [43] met in Stockholm to highlight three new large dementia prevention trials: 1) the Prevention of Dementia by Intensive Vascular Care study (PreDIVA), 2) the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), and 3) the Multidomain Alzheimer Preventive Trial (MAPT) (Table 2). These multi-centered trials emphasize the importance of international collaboration and standardization of study design. They are also unique in their multidomain interventions targeting vascular and lifestyle risk factors, as described in the previous section. Although many studies have evaluated individual lifestyle interventions, these are the first randomized, controlled trials looking into a combination of treatments. Two of the three trials are still ongoing and none have published their results yet. We will therefore primarily elaborate on study design and nuances of measure outcomes.
Table 2.
Multidomain Interventions – The European Dementia Prevention Initiatives
| Trial | Intervention | Study Sample | Duration | N | Outcome Measures | Results |
|---|---|---|---|---|---|---|
| PreDIVA [44] | Nurse-led intensive management of cardiovascul ar risk factors | Aged 70–78, MMSE>23, absence of dementia | 6 years | 3,700 | Incidence of dementia | Trial ongoing; results pending |
| FINGER [45] | Nutrition, exercise, cognitive training, social activity, management of cardiovascul ar risk factors | Aged 60–75, average or low-average cognitive scores with high CAIDE risk score | 2 years | 1,200 | Modified Neuropsychological Battery, Stroop Test, Trailmaking Test A and B | Preliminary results positive |
| MAPT [47] | Nutritional, physical, cognitive, as well as omega-3 | Aged 70 and older, frail elderly, MMSE>24 | 3 years | 1,200 | Change in cognitive function by Grober and Buschke Test | Trial ongoing; results pending |
FINGER = Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability; MAPT = Multidomain Alzheimer Preventive Trial; MMSE = Mini-Mental State Examination; PreDIVA = Prevention of Dementia by Intensive Vascular Care
PreDIVA [44] is a 6-year trial that started in 2006 with a sample size of 3,534 subjects, aged 70–78, who were not demented with an MMSE >23. They follow up for a cognitive assessment every two years for six years, and the intervention is nurse-led vascular care every four months for two years for management of risk factors, diet, and exercise. The primary outcome is incidence of dementia and disability. Secondary outcomes include cognitive decline as measured by MMSE or Visual Association Test (VAT), depression, and cardiovascular events. Baseline data have included a mean age of 74.4, with 45% male, 36.2% with cardiovascular history, 20.3% with diabetes, 75.9% with systolic blood pressure over 140 mm Hg, 44.4% with untreated hypertension, 27.7% with body mass index (BMI) greater than 30, 14% who smoke, and mean MMSE 28.0.
FINGER [45] started in 2009 and includes 1,200 subjects aged 60–75 for a 2-year intervention of group and individual management of vascular disease, diet, exercise, and cognitive training. Inclusion criteria include a dementia risk score greater than 6 on the Cardiovascular Risk Factors, Aging and Incidence of Dementia (CAIDE) and a cognitive performance that is average or slightly lower than average. Primary outcome during the 2-year follow up period is cognitive decline as measured by a neuropsychological test battery that includes the Stroop test and Trailmaking Test A and B. Secondary outcomes include incidence of dementia, cardiovascular events, depression, disability, quality of life, and health resources utilization. Some exploratory outcomes have included MRI structural imaging for 100 subjects and amyloid PET, FDG-PET, and CSF measures for 60 subjects. Baseline data are notable for mean age of 68.6, 53.4% male, mean MMSE 26.7, average systolic blood pressure 141.2 mm Hg, and average BMI 28.8. The trial was recently completed and preliminary results were presented at the Alzheimer’s Association International Conference in July 2014 indicating that cognition in the intervention group was significantly better than in the control group [46].
MAPT [47] is a 3-year trial that started in 2008 and enrolled 1,680 subjects aged greater than 70, who meet frailty criteria for subjective memory complaint, limitation of one IADL, or slow walking speed. MMSE was greater than 24. Subjects were randomized into one of four groups: 1) omega-3 supplementation alone, 2) multidomain intervention alone, 3) omega-3 supplementation plus multidomain intervention, or 4) placebo. The multidomain intervention includes management of vascular risk factors, diet, exercise, and cognitive training. The primary outcome is change in cognition on the Grober and Buschke test, an enhanced cue recall memory test. Secondary outcomes include MMSE, CDR, functional assessment, depression, and health resources utilization. Baseline data have not been published yet.
Medication Interventions
Medications with non-AD or non-dementia indications have sometimes been used in AD prevention trials, and they have mainly included non-steroidal anti-inflammatory drugs (NSAIDs) and hormone replacement therapy (HRT) (Table 3). The goals of these studies have been to target underlying AD pathophysiology as suggested in observational studies. Unfortunately, these studies have not demonstrated a benefit for AD prevention, as well as sometimes leading to potential harm for those in the intervention arm. Follow up for these studies usually have been many years after the intervention, so researchers are better able to accurately measure the longitudinal effect of exposure to these study drugs.
Table 3.
Medication Interventions
| Intervention | Trial | Study Sample | Duration | N | Outcome Measures | Results |
|---|---|---|---|---|---|---|
| Anti-Inflammatory | ADAPT [49] | 70 and older, cognitively normal | 7 years | 2,528 | Incidence of MCI and AD dementia | Negative; there were concerns of adverse cardiovascular effects, and there was no evidence that NSAIDs prevented AD or slowed cognitive decline |
| Estrogen and Progesterone | WHIMS [55] | 65 and older women, without probable dementia | 4 years | 4,532 | Incidence of dementia, 3MSE | Negative; estrogen alone and both hormones together increased the risk for probable dementia |
3MSE = Modified Mini-Mental State Examination; ADAPT = AD Anti-inflammatory Prevention Trial; NSAIDs = non-steroidal anti-inflammatory drugs; WHIMS = Women’s Health Initiative Memory Study
Interest in NSAID therapy for AD began in the early 1990s with a small randomized, double-blinded controlled trial by Rogers and colleagues [48], in which indomethacin was given to 44 subjects with mild to moderate AD dementia for six months and yielded improved scores on cognitive exams versus placebo. Leading up to the trial, previous data pertaining to immunologic markers and retrospective studies with patients with rheumatoid arthritis had suggested a link between inflammation and AD. Subsequent trials with NSAIDs unfortunately have been unable to reproduce these positive results.
The most recent and largest trial to date using NSAIDs for AD prevention was the AD Anti-inflammatory Prevention Trial (ADAPT) [49], which started in March 2001. It was the first trial to assess whether NSAIDs can be useful for primary prevention of AD. Six American dementia research clinics randomized 2,528 cognitively normal subjects 70 years of age and older into three groups: 1) celecoxib 200 mg bid, 2) naproxen 220 mg bid, or 3) placebo. Subjects needed to have at least one first-degree relative with AD dementia. Baseline and annual cognitive evaluations utilized a Cognitive Assessment Battery that included the Modified Mini-Mental State Examination (3MSE), the Hopkins Verbal Learning Test-Revised, the informant-based Dementia Severity Rating Scale, Digit Span Test, Generative Verbal Fluency, narratives from the Rivermead Behavioral Memory Test, and the Brief Visuospatial Test-Revised. For those suspected to have dementia, a more thorough evaluation with expert physicians, nurses, and psychometrists occurred. In such cases, subjects underwent more assessments including the Neuropsychiatric Inventory, the Consortium to Establish A Registry for Alzheimer’s Disease (CERAD) cognitive test battery, Trailmaking Test, Logical Memory, Benton Visual Retention Test, Controlled Oral Word Association Test, Symbol Digit Modalities Test, Shipley Vocabulary, and Self-rating of Memory Functions. Laboratory tests and neuroimaging were also ordered to complete the evaluation. The researchers diagnosed dementia based on DSM-IV or NINDS-ADRDA criteria and MCI using standard criteria.
The trial stopped prematurely because on December 17, 2004, the National Cancer Institute-sponsored Adenoma Prevention Celecoxib (APC) trial declared that celecoxib significantly increased cardiovascular risk. On the same day, the ADAPT Steering Committee suspended treatment for celecoxib and naproxen, and this decision was later made permanent. By that time, median follow up was about 734 days, and treatment with NSAIDs was about 560 days. Time after randomization was almost four years [49], and the published results showed no benefit of celecoxib or naproxen for AD prevention. The numbers of individuals diagnosed with AD dementia, MCI, or prodromal AD (those who did not meet specific criteria for MCI) were similar across all three arms. Since then, the ADAPT group has continued to report on follow up cognitive results of their cohort. Six months after study cessation, NSAIDs did not demonstrate a benefit [50]. In a two-year follow up, 117 participants underwent CSF testing for tau and Aβ1-42. From the CSF results, the researchers revised their ADAPT hypothesis by noting that NSAIDs have an adverse effect on patients with AD in later stages, whereas for those without symptoms, conventional NSAIDs like naproxen can lead to a reduction in AD 2–3 years after treatment [51]. A follow up analysis of the ADAPT cohort identified three classes: 1) “no-decline,” 2) “slow-decline,” and 3) “fast-decline.” They found contrasting effects of naproxen and celecoxib in their influence of cognitive decline in the subjects [52]. A 7-year follow up report of the original ADAPT cohort and ADAPT Follow-up Study (ADAPT-FS) cohort of 1,537 subjects concluded that celecoxib and naproxen cannot prevent AD in those with a family history of AD [53].
HRT is another medication intervention that has been tested in AD prevention trials. Based on the fact that women have a higher risk of AD than men, estrogen deficiency has been theorized to contribute to this difference. In 2008, the Cochrane database performed a systematic review of HRT for cognitive function in postmenopausal women [54]. The authors included 24 studies, but only 16 had analyzable data, which included 10,114 women. They concluded that there was good evidence to show that HRT with estrogen or combined estrogen and progesterone does not prevent cognitive decline in postmenopausal women.
The Women’s Health Initiative Memory Study (WHIMS), was one of the more prominent randomized-controlled trials that evaluated the effects of HRT for the prevention of AD [55]. However, similar to the ADAPT trial, their intervention of estrogen plus progesterone was found to increase risk for heart disease, stroke, pulmonary embolism, and breast cancer in the larger Women’s Health Initiative cohort. Hence, the planned 8.5-year trial was truncated to 5.6 years, and the intervention of estrogen plus progesterone was discontinued on July 8, 2002, but an estrogen-alone intervention was continued. The trial randomized 4,532 post-menopausal women aged 65 and older who were free of probable dementia to 0.625 mg of conjugated estrogen plus 2.5 mg of medroxyprogesterone acetate or placebo. The primary outcome was incidence of probable dementia, and the secondary outcome was incidence of MCI. Cognitive assessments were performed with the 3MSE and a modified CERAD neuropsychological battery. By the study’s end, 61 women in the HRT group developed probable dementia versus 40 women in the placebo group, which was statistically significant at p<0.01, yielding a hazard ratio of 2.05. There was no difference for MCI in both groups. With these results, the researchers concluded that the combination of estrogen and progesterone increases the risk of dementia for post-menopausal women. In the follow up study with the estrogen alone arm, results showed that estrogen increased the risk of dementia and MCI as well [56], with a statistically significant hazard ratio of 1.38. This result of cognitive decline with HRT was confirmed again four years after study cessation [57].
Supplemental Interventions
Some of the most popular treatments in the lay community for AD prevention have been the use of supplements, such as Ginkgo biloba and omega-3 fatty acids (Table 4). In the past decade, more attention has been paid to these supplements as potential interventions, and several trials have been completed or are still ongoing to evaluate their effects. Common concerns for complementary and alternative medicine have been standardization of preparation and dosing of the drug, as well as clarity of mechanism of action.
Table 4.
Supplemental Interventions
| Intervention | Trial | Study Sample | Duration | N | Outcome Measures | Results |
|---|---|---|---|---|---|---|
|
| ||||||
| Ginkgo | GEM [58] | 75 and older, cognitively normal or MCI | 6 years | 3,069 | 3MSE, ADAS-Cog | Negative; Ginkgo did not prevent cognitive impairment |
| GuidAge [59] | Subjective memory complaints | 5 years | 2,854 | Incidence of probable AD dementia | Negative; Ginkgo did not reduce incidence of AD dementia | |
|
| ||||||
| Omega-3 | MIDAS [61] | MMSE>26, logical memory>1 standard deviation below younger adults | 24 weeks | 485 | CANTAB Paired Associate Learning | Positive; DHA was shown to improve learning and memory in age-related cognitive decline |
3MSE = Modified Mini-Mental State Examination; ADAS-Cog = Alzheimer’s Disease Assessment Scale-Cognitive Subscale; CANTAB = Cambridge Neuropsychological Test Automated Battery; DHA = docasahexaenoic acid; GEM = Ginkgo Evaluation of Memory; MIDAS = Memory Improvement with Docasahexaenoic Acid Study; MCI = mild cognitive impairment; MMSE = Mini-Mental State Examination
The Ginkgo Evaluation of Memory (GEM) study, was a randomized, double blind, controlled trial at five U.S. academic centers that started in 2000 and ended in 2008, with median follow up of 6.1 years [58]. 3,069 cognitively normal subjects aged 75 or older and 482 subjects with MCI were enrolled in the study with cognitive assessments every six months to evaluate for the onset of dementia. The study was sponsored by the National Center for Complementary and Alternative Medicine and the National Institute on Aging. All subjects randomized to either the intervention arm or placebo arm were given identical blister packs manufactured by a designated company and administered by the National Center for Complementary and Alternative Medicine, ensuring quality of the chemical composition. The intervention was Ginkgo biloba extract, also known as EGb 761, 120 mg twice a day. Primary outcome was incidence of dementia, but Ginkgo biloba was found not to prevent cognitive decline in those with normal cognition or MCI. Another trial called GuidAge had the same results in a group of 2,854 subjects with subjective memory complaints [59] who were given the same Ginkgo biloba extract for five years.
Omega-3 fatty acids have a complex association with AD, as noted in a recent systematic review [60] of 11 observational studies and four clinical trials. Only seven of the observational studies had positive findings, but none of the four clinical trials had demonstrated any benefit for prevention or treatment of dementia. One trial evaluated those with vascular dementia, one with MCI or AD, another with AD, and one with MMSE scores >21 who were not demented. The observational studies that were positive included the French Personnes Agees QUID (PAQUID) study, the Chicago Health and Aging Project, the Cardiovascular Health and Cognition Study, the Framingham Heart Study, the French Three-City cohort study, and the Bordeaux subset of the Three-City cohort study. Clinical trials that have incorporated omega-3 fatty acids include the Older People and n-3 Long-chain polyunsaturated fatty acids (OPAL) study, Memory Improvement with Docasahexaenoic Acid Study (MIDAS), MAPT, and Enzymotec study with Omega-3. Time will tell whether omega-3 fatty acids will prove useful in large clinical trials for AD prevention. An example of a clinical trial that has shown benefit of omega-3 fatty acids is the MIDAS study [61].
Secondary Prevention Trials
Since the publication of the new NIA-AA diagnostic guidelines and the implementation of NAPA in 2011, enthusiasm for new AD research has soared, and a new wave of AD prevention trials is already underway (Table 5). Different from primary prevention trials that seek to decrease risk factors for AD, secondary prevention trials hope to treat underlying pathophysiology so as to prevent cognitive symptoms from ever developing [62]. The five large secondary AD prevention trials include: 1) the API Autosomal-Dominant AD (ADAD), 2) API APOE4 Trial, 3) the DIAN-Trials Unit (DIAN-TU), 4) the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) trial, and 5) the TOMMORROW Trial. API, DIAN, and A4 have already formed an umbrella group called the Collaboration for Alzheimer’s Prevention (CAP), in order to maintain regular dialogue about study design and outcomes validation.
Table 5.
Disease-Modifying Interventions
| Trial | Location | Intervention | Study Sample | Duration | N | Outcome | Start |
|---|---|---|---|---|---|---|---|
|
| |||||||
| API- ADAD [65] | Colombia | Crenezumab | Aged 30–60, presenilin-1 | 5 years | 600 | API-Composite [83] | 2013 |
| API-APOE4 [69] | North America and Europe | Anti-amyloid vaccine CAD106 or BACE inhibitor | APOE4 homozygotes | 5 years | 1,300 | API-Composite [84] | 2015 |
|
| |||||||
| DIAN-TU [72] | U.S. and Canada | Gantenerumab Solanezumab | Cognitively normal, MCI, or mild AD dementia; ADAD mutations | 2 years | 210 | Cogstate, CSF Abeta and PET amyloid deposition | 2013 |
|
| |||||||
| A4 [19] | 60 centers in U.S., Canada, and Australia | Solanezumab | Aged 65–85, cognitively normal with elevated amyloid burden on amyloid PET | 3.25 years | 1,000 | ADCS-PACC, C3 | 2014 |
|
| |||||||
| TOMMORROW [80] | 50 centers around the world | Pioglitazone | Cognitively normal; genetic risk of TOMM40 and APOE4 | 5 years | 6,000 | Incidence of MCI due to AD | 2014 |
A4 = Anti-Amyloid Treatment for Asymptomatic AD; ADCS-PACC = Alzheimer’s Disease Cooperative Study-Preclinical Alzheimer’s Cognitive Composite; API = Alzheimer’s Prevention Initiative; BACE = Beta-site amyloid precursor protein cleaving enzyme; C3 = Computerized Cognitive Composite; DIAN = Dominantly Inherited Alzheimer’s Network
Various national and international workgroups have come together to decide on best practices for AD clinical trials, especially with regard to safety of investigational drugs, realistic cognitive and biomarker outcomes, and adequate duration of trials. Critical decision points in preparation for AD secondary prevention trials have included: 1) further investment in testing the amyloid hypothesis with anti-amyloid treatment [63], 2) guidelines for safe monitoring of subjects for amyloid-imaging related abnormalities with amyloid-modifying drugs [64], 3) timing of interventions for maximal benefit of prevention, and 4) innovation by the FDA to consider cognitive outcomes for approval of investigational drugs for early AD with post-approval monitoring [20]. At this time, four of the five trials above have agreed to continue testing the amyloid hypothesis and are actively working together to make sure that the cognitive outcomes are comparable and meaningful. With regard to the TOMMORROW trial, we are seeing a creative new direction in AD prevention that may generate further hypotheses about AD pathophysiology.
API-ADAD and API-APOE4 Treatment Trial
API-ADAD is the first secondary prevention trial with the goal of treating cognitively normal individuals who have a genetic-based risk of developing AD. Led by Banner Alzheimer’s Institute, University of Antioquia and Genentech, API has been following a group of Colombian subjects aged 30 to 60 with the autosomal dominant presenilin-1 (PSEN1 E280A) mutation in a Phase 2 randomized, controlled clinical trial testing the effect of crenezumab on AD prevention [65]. The cohort has been followed at the University of Antioquia in Colombia [66]. Subjects will either receive crenezumab or placebo subcutaneously every two weeks for 260 weeks (five years). The researchers plan to enroll 300 subjects. The trial was started in September 2013 with an estimated completion date of September 2020 [67]. Primary endpoint is change on the API composite cognitive test score.
In July 2014, the Banner’s Alzheimer’s Institute announced at the Alzheimer’s Association International Conference that the API-APOE4 trial will start in 2015. The trial will follow 1,300 cognitively normal subjects who are homozygous for the APOE4 allele. Study drug will consist of an active amyloid vaccine, CAD106 [68], developed by Novartis, as well as a beta-site amyloid precursor protein cleaving enzyme (BACE) inhibitor yet to be announced [69]. Only two to three percent of the general population is homozygous for the APOE4 gene, and Reiman and colleagues have been conducting research on the cognitive effect of APOE4 on carriers for the past two decades [70]. Another unique aspect of the trial will be evaluating the effect of APOE4 disclosure on the subject population, as this may lead to further potential emotional, cognitive, and ethical consequences [71].
DIAN-TU
DIAN is a 14-site international registry based out of Washington University at St. Louis, Missouri under the direction of John Morris, MD [72]. The network has been actively tracking subjects and families with ADAD mutations, and thus far, it has recruited 336 subjects as of July 2013, with a target of 400 subjects. Subjects with these autosomal-dominant mutations have a near 100% certainty of getting AD at a mean age of about 45 so many of the age-related challenges with sporadic AD are removed. Rigorously testing subjects with a wide array of biomarkers, DIAN has been unique in its ability to extrapolate biomarker changes for decades prior to the age of onset of AD [73]. DIAN-TU under the direction of Randall Bateman, MD will enroll 210 cognitively normal, MCI, or mild AD dementia subjects with ADAD mutations and randomize them to receive one of two passive amyloid immunizations, Eli Lilly’s solanezumab or Roche’s gantenerumab, or placebo and follow them for two years [74]. A sequential randomization technique will allow for a pooled placebo group while testing the two drugs. Various cognitive assessments, neuroimaging, and CSF biomarkers will be obtained, including functional MRI, volumetric MRI, FDG-PET, amyloid PET, CSF A-beta, CSF tau, and CSF phospho-tau. The safety and mechanism of solanezumab and gantenerumab have already been validated in several reports prior to the onset of this trial [75]. Like API-ADAD, DIAN-TU will include cognitively normal and impaired subjects with mutations in PSEN-1, but in addition will also include those with presenilin-2 (PSEN-2) and amyloid precursor protein (APP) mutations.
A4 Trial
A4 is the first randomized, controlled trial focused on cognitively normal elderly who already have high levels of amyloid in the brain [10, 19, 62, 76]. Testing whether solanezumab can prevent sporadic AD, the trial will follow subjects who are aged 65 to 85 who are cognitively normal and who are determined to have elevated amyloid burden on an amyloid PET scan [77]. Directed by Reisa Sperling, MD, MMSc through the ADCS with the collaboration of the National Institute of Aging and Eli Lilly, it will take place in 60 centers in the United States, Canada, and Australia. To ensure equity among the elderly and to have a representative sample population, 20% of screened subjects must come from a minority population. The A4 trial is powered to see a 30% change in cognitive decline. The plan is to screen 5,000 individuals in order to randomize 1,000 to received either solanezumab or placebo. Subjects will receive infusions monthly for three years and three months. As with dementia trials, subjects will require a study partner, who in this trial will also ensure social support during the amyloid disclosure process that takes place during screening. Amyloid disclosure is one of the areas that will also be studied in A4, and information about the perceptions of biomarker positivity will be invaluable for future trials [78]. The primary outcome measure will be a composite of cognitive tests, while secondary outcome measures will consist of other sensitive cognitive and IADL assessments, neuroimaging, and CSF biomarkers. The trial has developed a unique set of highly sensitive cognitive instruments to detect the earliest change in cognition, known as the ADCS PACC [21], and the C3, an iPad-based computerized cognitive composite [19]. The A4 trial was started in February 2014, and the A5 Trial is already being planned.
TOMMORROW Trial
TOMMORROW, sponsored by Takeda in collaboration with Zinfandel Pharmaceuticals, targets a different AD mechanism. The TOMM40 gene, (translocase of outer mitochondrial membrane 40 homolog gene) is linked to APOE4 and has been shown to predict the age of late-onset AD [79]. A longitudinal study has demonstrated the importance of each gene on future cognitive functioning [70]. Incorporating the genetic risks from the TOMM40 and APOE4 genes along with the risk associated with increasing age, this trial will follow 6,000 cognitively normal subjects and randomize them to either pioglitazone or placebo [80]. Pioglitazone, a thiazolidinedione and peroxisome proliferator-activated receptor γ (PPAR-γ) traditionally used for diabetes mellitus, has recently been shown to have effects on patients with AD [81, 82]. The TOMMORROW trial will take place in 50 centers around the world and run for five years. Primary outcome will be the incidence of MCI due to AD based on clinical diagnosis, since as of now, neuroimaging and CSF biomarkers have yet to be incorporated into the trial.
Future of AD Trials
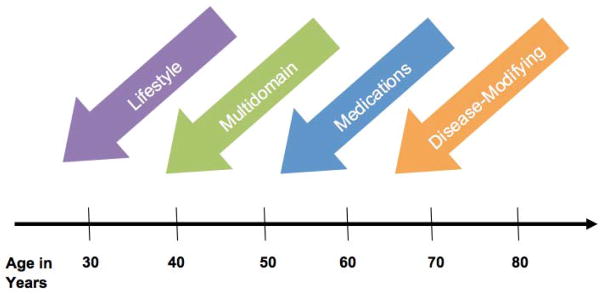
As research in preclinical AD progresses, knowledge of underlying AD pathophysiology will help guide future prevention trials. Perhaps, there will be a conceptual switch from prevention to outright treatment, similar to the field of cancer therapeutics where ductal carcinoma in situ or colon polyps are treated with early intervention. As the field stands right now, prevention trials are divided between more direct approaches versus indirect approaches (Figure 1) since AD prevention has resided on a spectrum. We are learning that amyloid may not be the only critical step toward the path of AD or the only mechanism of action to target. Tau-modifying agents are being developed, as well as many other targets, which eventually may open the door for combination therapy as has been the case in cancer, cardiovascular, and infectious disease treatment approaches. Tau PET imaging is now emerging on the research scene and could help further refine the preclinical stages of AD, as well as serve as an important outcome measure in AD prevention trials. Instead of a single trajectory based on amyloid, preclinical AD therapy could conceivably require a triple cocktail of treatments that target amyloid, tau, and neurodegeneration, similar to the situation in breast cancer where therapy is guided by the pattern of estrogen receptor (ER), progesterone receptor (PR), and Her2/neu receptors. Primary prevention trials may also incorporate AD biomarkers, so that precise timing of interventions can be elucidated (Figure 2).
Figure 2. Times for Prevention.
This diagram illustrates potential future times of intervention for longitudinal prevention clinical trials that could yield the maximal benefit. The timeline may differ for autosomal-dominant AD versus late-onset/sporadic AD.
The trials highlighted in this review represent the beginnings of the field of AD prevention. Depending on how the science of preclinical AD progresses, clinical researchers will need to decide how to translate this information for the general public. Examples of translational AD research have included the development of appropriate use criteria for amyloid PET imaging and the investment of the Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) study, which was recently added to the A4 trial [19]. The LEARN study will follow those subjects who screened failed for the A4 trial due to having low non-specific amyloid burden on amyloid PET imaging and will give researchers the opportunity to see if those individuals will eventually develop amyloid and tau build-up or other pathologies in conjunction with their clinical status. Questions of timing for screening of individuals and dosing of interventions are just two of the myriad of questions that will emerge as these clinical trials continue to evolve. Combination therapies will also have to be tested and in what order. With the new development of blood-based biomarkers for AD, genetic profiling, multiple therapies with different targets, preventive AD therapeutics will become more individualized and hopefully more effective [85].
Acknowledgments
The authors would like to thank Reisa Sperling, MD, MMSc for inspiration and Marwan Sabbagh, MD for review of the manuscript. The authors are supported by the following grants: K23 AG033634, R01 AG027435, K24 AG035007, the Massachusetts Alzheimer’s Disease Research Center (P50 AG005134), and the Harvard Aging Brain Study (P01 AGO36694, R01 AG037497).
Footnotes
Disclosures
Dr. Hsu is a co-investigator and Dr. Marshall is a site principal investigator for the A4 Trial. Drs. Hsu and Marshall have received research salary support from Eisai Inc. and Eli Lilly and Company. Dr. Marshall has also received research salary support from Janssen Alzheimer Immunotherapy and Wyeth/Pfizer Pharmaceuticals.
References
- 1.Association As. 2014 Alzheimer’s Disease Facts and Figures. Alzheimer’s and Dementia. 2014;10:e47–e92. doi: 10.1016/j.jalz.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. The New England journal of medicine. 2013;368(14):1326–34. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Association As. Changing the Trajectory of Alzheimer’s Disease. A National Imperative. 2010 [Google Scholar]
- 4.Gorelick PB, Sacco RL, Smith DB, Alberts M, Mustone-Alexander L, Rader D, et al. Prevention of a first stroke: a review of guidelines and a multidisciplinary consensus statement from the National Stroke Association. Jama. 1999;281(12):1112–20. doi: 10.1001/jama.281.12.1112. [DOI] [PubMed] [Google Scholar]
- 5.Mrazek PJHRJ. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. Institute of Medicine, National Academies Press; 1994. [PubMed] [Google Scholar]
- 6.Services USDoHaH. National Plan to Address Alzheimer’s Disease. 2014 Update2014. [Google Scholar]
- 7.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(3):270–9. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jack CR, Jr, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(3):257–62. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(3):263–9. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(3):280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jack CR, Jr, Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Annals of neurology. 2012;71(6):765–75. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 13.Selkoe DJ. Toward a comprehensive theory for Alzheimer’s disease. Hypothesis: Alzheimer’s disease is caused by the cerebral accumulation and cytotoxicity of amyloid beta-protein. Annals of the New York Academy of Sciences. 2000;924:17–25. doi: 10.1111/j.1749-6632.2000.tb05554.x. [DOI] [PubMed] [Google Scholar]
- 14.Carrillo MC, Brashear HR, Logovinsky V, Ryan JM, Feldman HH, Siemers ER, et al. Can we prevent Alzheimer’s disease? Secondary “prevention” trials in Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9(2):123–31 e1. doi: 10.1016/j.jalz.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 15.Carrillo MC, Rowe CC, Szoeke C, Masters CL, Ames D, O’Meara T, et al. Research and standardization in Alzheimer’s trials: reaching international consensus. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9(2):160–8. doi: 10.1016/j.jalz.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Knopman DS, Jack CR, Jr, Wiste HJ, Weigand SD, Vemuri P, Lowe V, et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology. 2012;78(20):1576–82. doi: 10.1212/WNL.0b013e3182563bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karran E, Hardy J. Antiamyloid therapy for Alzheimer’s disease--are we on the right road? The New England journal of medicine. 2014;370(4):377–8. doi: 10.1056/NEJMe1313943. [DOI] [PubMed] [Google Scholar]
- 18.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. The New England journal of medicine. 2014;370(4):311–21. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 19.Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. The A4 study: stopping AD before symptoms begin? Science translational medicine. 2014;6(228):228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kozauer N, Katz R. Regulatory innovation and drug development for early-stage Alzheimer’s disease. The New England journal of medicine. 2013;368(13):1169–71. doi: 10.1056/NEJMp1302513. [DOI] [PubMed] [Google Scholar]
- 21.Donohue MC, Sperling RA, Salmon DP, Rentz DM, Raman R, Thomas RG, et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA neurology. 2014;71(8):961–70. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amariglio RE, DM, Marshall GA, Rentz DM, Salmon DP, Ferris SH, Karantzoulis S, Aisen P, Sperling RA. Alzheimer’s Disease Cooperative Study. Tracking early decline in cognitive function in older individuals at risk for Alzheimer’s disease dementia: the ADCS-Cognitive Function Instrument. JAMA Neurology. doi: 10.1001/jamaneurol.2014.3375. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marson D. Investigating functional impairment in preclinical Alzheimer’s disease. J Prev Alzheimers Dis. 2015;2:4–6. doi: 10.14283/jpad.2015.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grill JD, Karlawish J, Elashoff D, Vickrey BG. Risk disclosure and preclinical Alzheimer’s disease clinical trial enrollment. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9(3):356–9 e1. doi: 10.1016/j.jalz.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlawish J. Addressing the ethical, policy, and social challenges of preclinical Alzheimer disease. Neurology. 2011;77(15):1487–93. doi: 10.1212/WNL.0b013e318232ac1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forette F, Seux ML, Staessen JA, Thijs L, Birkenhager WH, Babarskiene MR, et al. Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet. 1998;352(9137):1347–51. doi: 10.1016/s0140-6736(98)03086-4. [DOI] [PubMed] [Google Scholar]
- 27.McGuinness B, Craig D, Bullock R, Passmore P. Statins for the prevention of dementia. The Cochrane database of systematic reviews. 2009;(2):CD003160. doi: 10.1002/14651858.CD003160.pub2. [DOI] [PubMed] [Google Scholar]
- 28.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 29.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 30.Launer LJ, Miller ME, Williamson JD, Lazar RM, Gerstein HC, Murray AM, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label substudy. The Lancet Neurology. 2011;10(11):969–77. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson JD, Launer LJ, Bryan RN, Coker LH, Lazar RM, Gerstein HC, et al. Cognitive function and brain structure in persons with type 2 diabetes mellitus after intensive lowering of blood pressure and lipid levels: a randomized clinical trial. JAMA internal medicine. 2014;174(3):324–33. doi: 10.1001/jamainternmed.2013.13656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feart C, Samieri C, Rondeau V, Amieva H, Portet F, Dartigues JF, et al. Adherence to a Mediterranean diet, cognitive decline, and risk of dementia. Jama. 2009;302(6):638–48. doi: 10.1001/jama.2009.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. Jama. 2008;300(9):1027–37. doi: 10.1001/jama.300.9.1027. [DOI] [PubMed] [Google Scholar]
- 34.Scarmeas N, Luchsinger JA, Schupf N, Brickman AM, Cosentino S, Tang MX, et al. Physical activity, diet, and risk of Alzheimer disease. Jama. 2009;302(6):627–37. doi: 10.1001/jama.2009.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verghese J, Lipton RB, Katz MJ, Hall CB, Derby CA, Kuslansky G, et al. Leisure activities and the risk of dementia in the elderly. The New England journal of medicine. 2003;348(25):2508–16. doi: 10.1056/NEJMoa022252. [DOI] [PubMed] [Google Scholar]
- 36.Valenzuela M, Sachdev P. Can cognitive exercise prevent the onset of dementia? Systematic review of randomized clinical trials with longitudinal follow-up. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2009;17(3):179–87. doi: 10.1097/JGP.0b013e3181953b57. [DOI] [PubMed] [Google Scholar]
- 37.Papp KV, Walsh SJ, Snyder PJ. Immediate and delayed effects of cognitive interventions in healthy elderly: a review of current literature and future directions. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2009;5(1):50–60. doi: 10.1016/j.jalz.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. Jama. 2002;288(18):2271–81. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. Jama. 2006;296(23):2805–14. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebok GW, Ball K, Guey LT, Jones RN, Kim HY, King JW, et al. Ten-year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. Journal of the American Geriatrics Society. 2014;62(1):16–24. doi: 10.1111/jgs.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. The Lancet Neurology. 2004;3(6):343–53. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 42.Fratiglioni L, Wang HX, Ericsson K, Maytan M, Winblad B. Influence of social network on occurrence of dementia: a community-based longitudinal study. Lancet. 2000;355(9212):1315–9. doi: 10.1016/S0140-6736(00)02113-9. [DOI] [PubMed] [Google Scholar]
- 43.Richard E, Andrieu S, Solomon A, Mangialasche F, Ahtiluoto S, Moll van Charante EP, et al. Methodological challenges in designing dementia prevention trials - the European Dementia Prevention Initiative (EDPI) Journal of the neurological sciences. 2012;322(1–2):64–70. doi: 10.1016/j.jns.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 44.Richard E, Van den Heuvel E, Moll van Charante EP, Achthoven L, Vermeulen M, Bindels PJ, et al. Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer disease and associated disorders. 2009;23(3):198–204. doi: 10.1097/WAD.0b013e31819783a4. [DOI] [PubMed] [Google Scholar]
- 45.Kivipelto M, Solomon A, Ahtiluoto S, Ngandu T, Lehtisalo J, Antikainen R, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9(6):657–65. doi: 10.1016/j.jalz.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Solomon ALE, Soininen H, Tuomilehto J, Lindstrom J, Lehtisalo J. A multidomain, two-year, randomized controlled trial to prevent cognitive impairment: The finger study. Alzheimer’s and Dementia. 2014;10(4):137–8. [Google Scholar]
- 47.Gillette-Guyonnet S, Andrieu S, Dantoine T, Dartigues JF, Touchon J, Vellas B, et al. Commentary on “A roadmap for the prevention of dementia II. Leon Thal Symposium 2008.” The Multidomain Alzheimer Preventive Trial (MAPT): a new approach to the prevention of Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2009;5(2):114–21. doi: 10.1016/j.jalz.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 48.Rogers J, Kirby LC, Hempelman SR, Berry DL, McGeer PL, Kaszniak AW, et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology. 1993;43(8):1609–11. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- 49.Group AR, Lyketsos CG, Breitner JC, Green RC, Martin BK, Meinert C, et al. Naproxen and celecoxib do not prevent AD in early results from a randomized controlled trial. Neurology. 2007;68(21):1800–8. doi: 10.1212/01.wnl.0000260269.93245.d2. [DOI] [PubMed] [Google Scholar]
- 50.Group AR, Martin BK, Szekely C, Brandt J, Piantadosi S, Breitner JC, et al. Cognitive function over time in the Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT): results of a randomized, controlled trial of naproxen and celecoxib. Archives of neurology. 2008;65(7):896–905. doi: 10.1001/archneur.2008.65.7.nct70006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, et al. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(4):402–11. doi: 10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leoutsakos JM, Muthen BO, Breitner JC, Lyketsos CG, Team AR. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: findings from the randomized controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial. International journal of geriatric psychiatry. 2012;27(4):364–74. doi: 10.1002/gps.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alzheimer’s Disease Anti-inflammatory Prevention Trial Research G. Results of a follow-up study to the randomized Alzheimer’s Disease Anti-inflammatory Prevention Trial (ADAPT) Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9(6):714–23. doi: 10.1016/j.jalz.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lethaby A, Hogervorst E, Richards M, Yesufu A, Yaffe K. Hormone replacement therapy for cognitive function in postmenopausal women. The Cochrane database of systematic reviews. 2008;(1):CD003122. doi: 10.1002/14651858.CD003122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289(20):2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 56.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004;291(24):2947–58. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 57.Espeland MA, Brunner RL, Hogan PE, Rapp SR, Coker LH, Legault C, et al. Long-term effects of conjugated equine estrogen therapies on domain-specific cognitive function: results from the Women’s Health Initiative study of cognitive aging extension. Journal of the American Geriatrics Society. 2010;58(7):1263–71. doi: 10.1111/j.1532-5415.2010.02953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeKosky ST, Williamson JD, Fitzpatrick AL, Kronmal RA, Ives DG, Saxton JA, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. Jama. 2008;300(19):2253–62. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vellas B, Coley N, Ousset PJ, Berrut G, Dartigues JF, Dubois B, et al. Long-term use of standardised Ginkgo biloba extract for the prevention of Alzheimer’s disease (GuidAge): a randomised placebo-controlled trial. The Lancet Neurology. 2012;11(10):851–9. doi: 10.1016/S1474-4422(12)70206-5. [DOI] [PubMed] [Google Scholar]
- 60.Fotuhi M, Mohassel P, Yaffe K. Fish consumption, long-chain omega-3 fatty acids and risk of cognitive decline or Alzheimer disease: a complex association. Nature clinical practice Neurology. 2009;5(3):140–52. doi: 10.1038/ncpneuro1044. [DOI] [PubMed] [Google Scholar]
- 61.Yurko-Mauro K, McCarthy D, Rom D, Nelson EB, Ryan AS, Blackwell A, et al. Beneficial effects of docosahexaenoic acid on cognition in age-related cognitive decline. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2010;6(6):456–64. doi: 10.1016/j.jalz.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 62.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Science translational medicine. 2011;3(111):111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2012;8(4):261–71. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 64.Sperling RA, Jack CR, Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(4):367–85. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, et al. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. Journal of Alzheimer’s disease : JAD. 2011;26 (Suppl 3):321–9. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gomez CM, Jaramillo-Elorza MC, Moreno S, Aguirre-Acevedo DC, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer’s disease: a retrospective cohort study. The Lancet Neurology. 2011;10(3):213–20. doi: 10.1016/S1474-4422(10)70323-9. [DOI] [PubMed] [Google Scholar]
- 67.Health USNIo. A Study of Crenezumab Versus Placebo in Preclinical PSEN1 E280A Mutation Carriers to Evaluate Efficacy and Safety in the Treatment of Autosomal-Dominant Alzheimer Disease, Including a Placebo-Treated Noncarrier Cohort. 2014 Jul 24; doi: 10.1016/j.trci.2018.02.002. Available from: http://www.clinicaltrials.gov/show/NCT01998841. [DOI] [PMC free article] [PubMed]
- 68.Winblad B, Andreasen N, Minthon L, Floesser A, Imbert G, Dumortier T, et al. Safety, tolerability, and antibody response of active Abeta immunotherapy with CAD106 in patients with Alzheimer’s disease: randomised, double-blind, placebo-controlled, first-in-human study. The Lancet Neurology. 2012;11(7):597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 69.Friedrich MJ. Researchers test strategies to prevent Alzheimer disease. Jama. 2014;311(16):1596–8. doi: 10.1001/jama.2014.3891. [DOI] [PubMed] [Google Scholar]
- 70.Caselli RJ, Dueck AC, Huentelman MJ, Lutz MW, Saunders AM, Reiman EM, et al. Longitudinal modeling of cognitive aging and the TOMM40 effect. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2012;8(6):490–5. doi: 10.1016/j.jalz.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Green RC, Roberts JS, Cupples LA, Relkin NR, Whitehouse PJ, Brown T, et al. Disclosure of APOE genotype for risk of Alzheimer’s disease. The New England journal of medicine. 2009;361(3):245–54. doi: 10.1056/NEJMoa0809578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moulder KL, Snider BJ, Mills SL, Buckles VD, Santacruz AM, Bateman RJ, et al. Dominantly Inherited Alzheimer Network: facilitating research and clinical trials. Alzheimer’s research & therapy. 2013;5(5):48. doi: 10.1186/alzrt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. The New England journal of medicine. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Health USNIo. Dominantly Inherited Alzheimer Network Trial: An Opportunity to Prevent Dementia. A Study of Potential Disease Modifying Treatments in Individuals at Risk for or With a Type of Early Onset Alzheimer’s Disease Caused by a Genetic Mutation (DIAN-TU) 2014 Jul 22; Available from: http://www.clinicaltrials.gov/show/NCT01760005.
- 75.Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Archives of neurology. 2012;69(2):198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 76.Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease-the challenges ahead. Nature reviews Neurology. 2013;9(1):54–8. doi: 10.1038/nrneurol.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Health USNIo. Clinical Trial of Solanezumab for Older Individuals Who May be at Risk for Memory Loss (A4) 2014 Jul 24; Available from: http://clinicaltrials.gov/show/NCT02008357.
- 78.Lingler JH, Klunk WE. Disclosure of amyloid imaging results to research participants: has the time come? Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2013;9(6):741–4 e2. doi: 10.1016/j.jalz.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roses AD, Lutz MW, Amrine-Madsen H, Saunders AM, Crenshaw DG, Sundseth SS, et al. A TOMM40 variable-length polymorphism predicts the age of late-onset Alzheimer’s disease. The pharmacogenomics journal. 2010;10(5):375–84. doi: 10.1038/tpj.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Health USNIo. Biomarker Qualification for Risk of Mild Cognitive Impairment (MCI) Due to Alzheimer’s Disease (AD) and Safety and Efficacy of Pioglitazone in Delaying Its Onset. 2014 Jul 24; Available from: http://clinicaltrials.gov/show/NCT01931566.
- 81.Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Archives of neurology. 2011;68(1):45–50. doi: 10.1001/archneurol.2010.229. [DOI] [PubMed] [Google Scholar]
- 82.Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiology of aging. 2011;32(9):1626–33. doi: 10.1016/j.neurobiolaging.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 83.Ayutyanont N, Langbaum JB, Hendrix SB, Chen K, Fleisher AS, Friesenhahn M, et al. The Alzheimer’s prevention initiative composite cognitive test score: sample size estimates for the evaluation of preclinical Alzheimer’s disease treatments in presenilin 1 E280A mutation carriers. The Journal of clinical psychiatry. 2014;75(6):652–60. doi: 10.4088/JCP.13m08927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Langbaum JB, Hendrix SB, Ayutyanont N, Chen K, Fleisher AS, Shah RC, et al. An empirically derived composite cognitive test score with improved power to track and evaluate treatments for preclinical Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2014;10(6):666–74. doi: 10.1016/j.jalz.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Holtzman DM, Mandelkow E, Selkoe DJ. Alzheimer disease in 2020. Cold Spring Harbor perspectives in medicine. 2012;2(11) doi: 10.1101/cshperspect.a011585. [DOI] [PMC free article] [PubMed] [Google Scholar]