Abstract
Branching morphogenesis is a fundamental process in the development of diverse epithelial organs such as the lung, kidney, liver, pancreas, prostate, salivary, lacrimal and mammary glands. A unifying theme during organogenesis is the importance of epithelial cell interactions with the extracellular matrix (ECM) and growth factors (GFs). The diverse developmental mechanisms giving rise to these epithelial organs involve many organ-specific GFs, but a unifying paradigm during organogenesis is the regulation of GF activity by heparan sulfates (HS) on the cell surface and in the ECM. This primarily involves the interactions of GFs with the sulfated side-chains of HS proteoglycans. HS is one of the most diverse biopolymers and modulates GF binding and signaling at the cell surface and in the ECM of all tissues. Here, we review what is known about how HS regulates branching morphogenesis of epithelial organs with emphasis on the developing salivary gland, which is a classic model to investigate epithelial-ECM interactions. We also address the structure, biosynthesis, turnover and function of HS during organogenesis. Understanding the regulatory mechanisms that control HS dynamics may aid in the development of therapeutic interventions for diseases and novel strategies for tissue engineering and regenerative medicine.
Keywords: Branching morphogenesis, extracellular matrix, heparan sulfate 3-O-sulfotransferase, FGF10, heparan sulfate, FGFR2b, and growth factors
Introduction
Branching Morphogenesis and Extracellular Matrix (ECM)
Many organs, including the lung, kidney, liver, pancreas, prostate, salivary, lacrimal and mammary glands are formed during embryonic development by the process of branching morphogenesis. Iterative rounds of epithelial branching, driven by the expansion and maintenance of pools of stem and progenitor cells, establish the branched architecture (Fig 1A). This is required to increase the internal surface area for the particular organ function, whether it is gas exchange, filtration, waste excretion or fluid secretion. The main cellular mechanisms involved in branching morphogenesis include cell proliferation, differentiation, migration, and apoptosis. In addition, there are reciprocal interactions among the epithelial stem and progenitor pools and their niche that includes the surrounding ECM and a variety of cell types including mesenchymal, neuronal, immune, lymphatic and endothelial cells (1). The ECM is critical during branching morphogenesis for not only providing structural integrity, but for controlling communication among cell populations by the HS binding secreted growth factors, morphogens, cytokines and other mediators of development (2). For the purposes of this review we will refer to all of these factors simply as growth factors (GFs). The ECM is produced by the mesenchymal cells in the tissue surrounding the developing organ and by the epithelial cells themselves (3). ECM is composed of several distinct families of molecules such as laminins, collagens, and fibronectin, and their role in branching morphogenesis has been previously been reviewed elsewhere (2, 4).
Fig. 1.
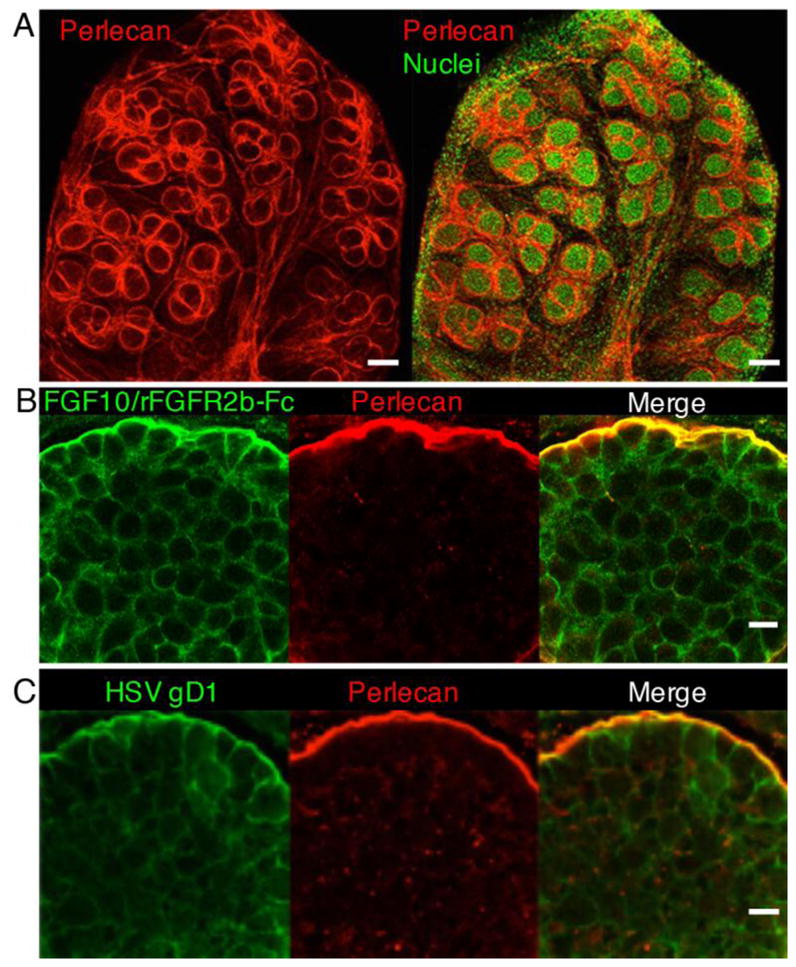
The BM separates epithelial cells from mesenchyme during SMG branching morphogenesis and contains HS that binds growth factors and viral receptors. (A) Whole-mount staining of E14 SMG showing perlecan staining the BM (red) surrounding the branching epithelium, and nuclei (green). Images are 10 μm confocal projections. Scale bar: 100 μm. (B) Higher magnification of the SMG endbud shows that FGF10/rFGFR2b-Fc (LACE assay, green) binds the endogenous HS at the epithelial cell surface and colocalizes with perlecan (red) in BM. Images are single confocal sections. Scale bar: 10 μm. (C) The endbud HS also binds the viral receptor HSV-1 gD285 protein (green) at the epithelial cell surface and colocalizes with perlecan (red) in BM. Images are single 2 μm confocal sections. Scale bar: 10 μm.
The functions of Heparan sulfate (HS) in ECM
The basement membrane (BM), a specialized ECM structure that epithelial cells adhere to, is composed primarily of collagen IV, laminins isoforms, nidogen, and HS proteoglycans, including perlecan (5). An important function of the negatively charged HS in the ECM and BM is to bind and concentrate GFs and thus act as a reservoir for GFs (6). HS is a co-receptor to facilitate signaling complex formation between GFs and their receptors or oligomerization of these receptor complexes. The endogenous HS of any tissue can be tested for its ability to bind growth factor and receptor complexes using the ligand and carbohydrate engagement (LACE) assay (7). As shown in figure 1B, both cell-cell and cell-BM HS binds the FGF10/recombinant FGFR2b-Fc complex. HS is also bound by viral receptors for entry into cells, the herpes simplex virus uses the gD1 protein receptor to bind a 3-O-sulfated epitope of HS on the cell surface and in the BM (8) (Fig. 1C). HS also functions by binding, and storing GFs and then releasing them in a controlled manner (9). HS can generate GF and morphogen gradients through affinity based localization of HS binding components (10). Binding of a GF to HS can enhance its binding and signaling through its receptor, regulate GF activity due to HS cleavage and release of HS fragments, inhibit its function by sequestering it from its receptor, or protect the GF from proteolytic cleavage (2, 11). The HS in the ECM can selectively bind to GFs and, as a consequence, help determine the binding specificity between ligands and receptors and the signaling direction of epithelial-mesenchyme crosstalk during development. For example, during limb development, specific HS structures selectively bind FGF10 derived from the mesenchyme but not FGF8 derived from the ectodermal cells (12). Thus, HS can dictate growth factor selectivity in a developing organ. Overall, the resulting function of a GF during branching morphogenesis is dependent on the context in which it interacts with HS to control its biological activity.
HS in the ECM as a component of stem cell niche
Epithelial stem cell fate and stem cell expansion during branching morphogenesis can be regulated by signals provided by the local microenvironment (niche), which includes mesenchyme cells, nerves, blood vessels, lymphatics and the GFs bound to the HS-containing ECM. It is possible that tissue-specific HS chains serve to integrate autocrine and paracrine signals from the resident niche cells with signals from the HS-ECM , together with signals from the stem cells themselves.
The niche contains all the signals required to program stem cells, for example a single mammary stem cell can reproduce an organ when transplanted into a cleared fat pad, the endogenous niche for gland development (13). In addition, ex vivo three-dimensional (3D) organoid culture in Matrigel has increased our understanding of the role of HS in the ECM for the development of many branching organs. When a single KIT+ prostate cell grafted with stromal cells was transplanted into the renal capsule in vivo could regenerate a prostate (14). Also, a single highly expressing EpCAM+ or c-Kit salivary gland stem cell can form organoids in a HS containing ECM and be induced by growth factors to undergo a branching program (15, 16). Although organ-specific GFs regulate branching in specific organs, a common paradigm between organ systems is the critical requirement and function of HS-binding GFs. In this review, we will mainly focus on the salivary gland as an example of a branching organ where glycosaminoglycans and HS function were shown to be critical for epithelial branching morphogenesis (17–20).
Salivary gland branching morphogenesis
There are three major pairs of salivary glands, the sublingual, the submandibular, and the parotids, which are morphologically and functionally distinct but their embryonic development by branching morphogenesis share common mechanisms. We will focus on studies of the mouse submandibular gland (SMG), which has been used to investigate the role of HS during branching morphogenesis. The SMG initiates as a thickening of the oral epithelium on a stalk that invaginates into a condensed mesenchyme at E12. Clefts in the epithelium result in 3–5 epithelial buds at E13.5, and branching morphogenesis occurs to form a highly branched gland. Functional differentiation begins in vivo after E15 and continues to birth (21). Postnatal development involves maturation of both acinar cells and ductal structures. Fibroblast growth factors (FGFs), a family of 23 ligands, and their transmembrane protein tyrosine kinase receptors (FGFRs) (22) are critical for SMG development. HS proteoglycans are also essential for FGF/FGFR binding and activation (23). Deletion of either Fgf10 or Fgfr2b in mice results in multiple abnormalities, but importantly agenesis of branching organs such as the lung, salivary, and lacrimal glands (24–26). The overlapping phenotypes of Fgf10- and Fgfr2b-null mutants confirm Fgfr2b as the major receptor for Fgf10. FGFR2b signaling requires HS to increase the affinity of FGF10 for FGFR2b, stabilizing the ternary signaling complex. In this review we will focus on FGF10-FGFR2b signaling as an example of a well-characterized GF signaling pathway that requires HS for function.
Heparan sulfate proteoglycans
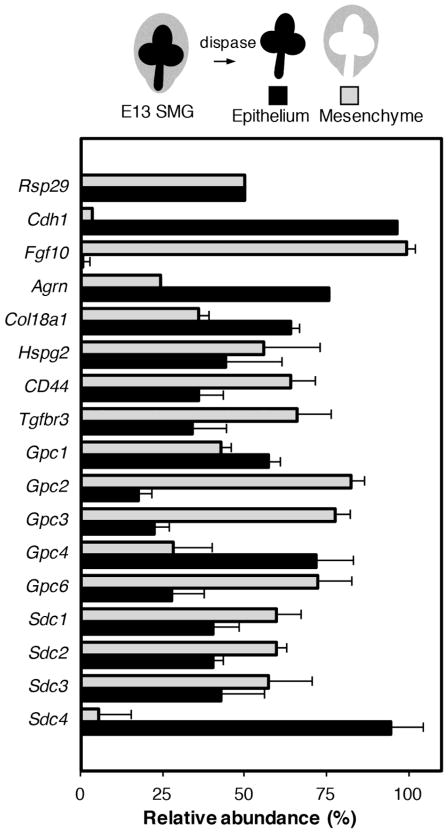
HS is a linear polysaccharide consisting of alternating glucosamine and uronic acid residues (27) that is attached to a protein core of a proteoglycan. The negative charge of HS is due to sulfates that are added to the chain. This is in contrast to heparin, which is a highly negatively charged linear polysaccharide stored in the secretory granules of mast cells that plays a role in anticoagulation and may be a defense mechanism against invading bacteria (27). HS is attached to heparan sulfate proteoglycans (HSPGs), which constitute a large family of glycoproteins with a core protein and highly variable in terms of number and size of heparan sulfate moieties attached. There are three classes of HSPGs: those that are bound to the cell membrane by the GPI-anchor (the glypicans), those contain a transmembrane domain (the syndecans and betaglycan) and those that are secreted into the BM (perlecan, agrin and collagen XVIII). The HS chains of HSPGS are responsible for many of the functional characteristics of HSPGs as described above. HSPGs also function as both a pro- and anti-angiogenic factors. This function is mediated by their HS chains, which can bind to, and concentrate angiogenic growth factors in proximity to cell-surface receptors, where they are presented in a biologically active form. On the other hand, these BM HSPGs could also restrict the diffusion of growth factors over extended distances. In this review, we will particularly address the role of the secreted HSPGs during branching morphogenesis. HS is ubiquitous on cell membranes, in the BM, and in the ECM therefore all cell-cell and cell-matrix interaction, may be influenced by HS activity. However, the transcripts for HSPGs are differentially expressed during branching morphogenesis in an E13 SMG (Fig. 2). Importantly any biological activity involving an HS-binding GF may be dependent on HS in the local microenvironment. It is the complexity of HS structures and biosynthesis that has made it difficult to study, as a result many researchers either ignore HS interactions or treat HS as a “black box” which can be addressed by simply adding heparin into an assay.
Fig. 2.
HSPGs are differentially expressed during SMG branching morphogenesis. The epithelium and mesenchyme of E13 SMGs were separated by dispase, and the relative expression of HSPGs was compared in epithelium and mesenchyme by qPCR analysis. Gene expression was normalized to Rps29. Cdh1 and Fgf10 serve as controls for the separation of the epithelium and mesenchyme, respectively. Agrn, Col18a1, Gpc4 and Sdc4 are mainly expressed in the epithelium, whereas Gpc2, Gpc3, and Gpc6 are mainly expressed in the mesenchyme.
Collagen type XVIII is a hybrid collagen-proteoglycan that contains a frizzled and an endostatin domain (28). Targeted disruption of Col18a1 affects the integrity of basement membranes of various tissues including the kidney leading to an abnormally loosened network structure (29, 30). Col18a1 null mice show increase in the width of various BMs such as those in the kidney proximal tubules (31). Collagen type XVIII is expressed throughout the epithelial bud at the initiation of lung and kidney organogenesis, but becomes localized to the epithelial tips in the lungs during early stages of epithelial branching, whereas its expression in the kidney is confined to the epithelial stalk region and is lost from the newly formed ureter tips (32). The addition of collagen XVIII endostatin to ureteric cultures inhibits branching of the explants, whereas neutralizing antibody to endostatin increases ureteric bud outgrowth and branching. These data indicates that local expression of endostatin at the tips of the ureteric bud may play a role in its regulation (33). Interestingly, glypican 3 (Gpc-3) knockout mice display enhanced ureteric bud branching early in development (34) and it is suggested that Gpc-3 may bind unique factors such as endostatin, BMP2 or FGF7 that limit GF-mediated ureteric bud growth and branching. Gpc-3 is highly expressed in the ureteric bud during embryogenesis (35) then down-regulated in the adult kidney (36, 37). Lung branching morphogenesis is decreased when a function blocking collagen XVIII antibody is added to lung explant culture with a decrease in Wnt2 expression (32). However, the function of collagen XVIII during salivary gland morphogenesis has not been reported.
Perlecan is the most prevalent BM HSPG and is complex multidomain protein with a number of discrete binding partners. It is synthesized and secreted as a single molecule composed of five distinct domains, I–V. Domain I contains binding sites for HS chains, which is important for cell adhesion and anchoring the BM via interaction with laminin, collagen IV, and fibronectin; and for GFs such as FGF2, PDGF, VEGF, angiopoietin-3 and FGF10/FGFR2b complex (28, 38). The HS chains of perlecan also bind FGF10 and this HS can be cleaved by heparanase. In addition, the protein core can be cleaved by various proteases, again releasing various pro-angiogenic factors. For example, heparanase-mediated cleavage of perlecan HS in the BM releases FGF10, which increases salivary gland branching morphogenesis (38). One of the key roles of perlecan in the BM is to bind to laminin and collagen IV to bring together the two supramolecular networks ((39), thus acting as a scaffolding protein. Perlecan can act to regulate the bioavailability of FGFs in the extracellular environment (40). Inactivation of perlecan in mice leads to embryonic lethality at E11.5 and mutant embryos are characterized by a broad range of abnormalities in the heart, brain, kidney and skeleton (41). Targeted deletion of exon 3 in Hspg2 results in deletion of the three GAG attachment sites in the N-terminal domain I of perlecan (42). These mice display severe structural defects of the lens capsule degeneration, and increased smooth muscle cell proliferation (reviewed in (43, 44)).
Agrin is a multimodular HSPG with up to three HS and has an endorepellin-like domain at its C-terminus, which interacts with several receptors including integrins and dystroglycan (45, 46). Interestingly, genetic studies on mice suggest that dystroglycan function is required for the organization and assembly of ECM components into a BM structure (47, 48). Dystroglycan is expressed during early stages of developing lung and salivary gland, and inhibiting laminin-1 dystroglycan interactions using a function-blocking antibody retards salivary gland and lung epithelial branching ex vivo (49).
Together, HSPGs contribute to the structural assembly and maintenance of BMs and importantly the HS chains carry out important roles in the signaling pathways that lead to cell survival, motility and tissue morphogenesis through their ability to modulate the biological activity of GFs and surface receptors.
Heparan sulfate biosynthesis
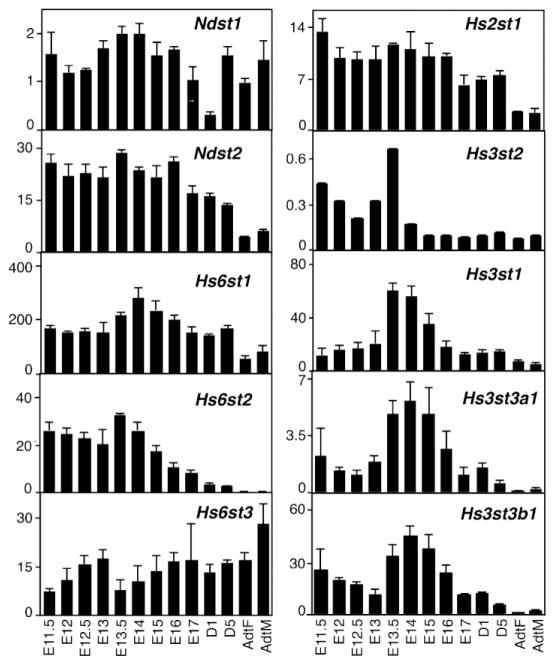
The specificities of the interactions between HS and its protein ligands are due to the fine structure of HS, which is characterized by the specific sulfation patterns and hexoronic acid isoform residues (27). The divergent fine structures of the HS are generated through the coordinated action of sulfotransferases in the Golgi apparatus. Biosynthesis of HS begins with the formation of the tetrasaccharide glycan linker on a cognate serine residue of the proteoglycan core protein by xylosyltransferases, galactosyltransferases, and glucuronosyltransferase-1 (50). After the formation of the linkage region, the HS backbone is synthesized by a set of glycosyltransferases that belong to the EXT gene family (exostosin proteins EXT1, EXT2, EXTL1, EXTL2, and EXTL3). The backbone is then modified by a semi-ordered series of reactions, which generate distinct HS motifs. During polymerization of the HS chain, HS N-deacetylase/N-sulfotransferase (NDST) deacetylates and N-sulfates subsets of N-acetylglucosamine residues of the growing polymer. Four NDST isoforms have been identified, with NDST-1 and the less abundant NDST2 being widely expressed in a variety of tissues, whereas NDST3 and NDST4 are expressed mainly in the brain (51). The subsequent modification step is C-5 epimerization of GlcA to IdoA by the enzyme D-glucuronyl C5-epimerase (52), the only known isoform. Next the HS backbone is O-sulfated at various positions (C-2, C-3 and C-6) by different sulfotransferases. 2-O-sulfation is mediated by a sole 2-O-sulfotransferase enzyme, 6-O-sulfation is mediated by three isoenzymes whereas 3-O-sulfation, which is the least abundant sulfate modification, is catalyzed by seven isoforms in mouse and human. The expression of individual sulfotransferases isoforms throughout organogenesis is dynamic and tissue specific, which raises unanswered questions about the specific regulation and function of HS sulfated epitopes in specific developmental processes such as branching morphogenesis. As an example, the expression of sulfotransferases isoforms during SMG development is included (Fig. 3).
Fig. 3.
HS sulfotransferase isoform expression is developmentally regulated during SMG organogenesis. Graphs show the relative expression in arbitrary units (AU x 100) of sulfotransferase isoforms expressed in the SMG at 12 different stages from E11.5 to adult. Interestingly, Hs3st1, Hs3st3a1 and Hs3st3b1 expression peaks during stages when branching morphogenesis peaks. Gene expression was obtained using Agilent microarrays. Gene expression profiles are available online at http://sgmap.nidcr.nih.gov. AdtF, adult female; AdtM, adult male; E, embryonic day; D, postnatal day.
HS is one of the most complex biopolymers due to the diverse sulfation patterns established by the sulfotransferase isoforms, which results in extreme structural heterogeneity (reviewed in (53)). The epimerase and sulfotransferase reactions along the GAG chain are not uniform, resulting in a domain-type arrangements with some areas of the GAG chain being highly modified and some regions with low-density of modifications. The mechanisms that control where areas of high and low sulfate density are located is not known, and unraveling the mechanisms that lead to such a diverse structure is a challenge for the field.
Defects in HSPG assembly can result in complete loss of FGF, Hedgehog, and Wingless/Wnt signaling and result in severe abnormalities or lethality during embryonic development. We will next summarize the abnormalities observed in mice that are deficient in different HS biosynthesis enzymes, giving particularly emphasis on defects associated with morphogenesis. More recently the function of specific sulfation patterns has been addressed using molecular genetic approaches in multiple organisms. Here we focus on the mouse and genetic deletions in genes associated with HSPGs and HS that result in a branching phenotype (Table 1).
Table 1.
Genetic deletions of HSPGs and HS sulfotransferases in mice that display a branching phenotype
| Gene/mutation | Branching phenotype |
|---|---|
| Col18a1−/− | Defective basement membrane formation-leading to hydrocephalus and anterior ocular defects (30) Thickened basement membrane with reduced filtration capacity (31) |
| Sdc1−/− | Reduced secondary and tertiary branching in mammary gland (102) |
| Gpc3−/− | Enhanced ureteric bud branching in kidney (34) |
| Ext1fl/fl(MMTVcre+) | Defective primary ductal branching in mammary gland (56) |
| Ndst1−/− | Lens development (59), lacrimal gland induction (60), immature lungs (57) |
| Ndst1fl/fl(MMTVcre+) | Defective lobuloalveolar expansion in mammary gland (64) |
| Ndst2−/− | Mild increase in mammary branching (89) |
| Ndst1f/f MMTV cre+; Ndst2−/− | Hyperbranching of mammary gland (89) |
| Glce−/− | Kidney agenesis, immature lungs (67) |
| Hs2st1−/− | Renal agenesis (68) |
| Hs2st1fl/fl(MMTV cre+) | Reduced secondary ductal side branches in mammary gland (56) |
| Hs6st1−/− | Impaired lung development (72) |
| HpseTg | Mammary hyperbranching (103) |
| Hpse−/− | Increased branching morphogenesis (91) |
Mice deficient in Ext1 lack an organized mesoderm and extra embryonic tissues, and die at the gastrulation stage (54). Similarly, loss of Ext2 results in early embryonic lethality due to a gastrulation defect and abnormalities in the formation of extra-embryonic structures (55). This highlights the importance of the Ext genes in HS synthesis and the necessity of HS for mammalian life. Mammary epithelial-specific inactivation of Ext1 results in markedly reduced mammary ductal growth and branching morphogenesis indicating a requirement for HS (56).
Disruption of Ndst1 results in severe phenotypes in lungs, brain, cranial facial, lens, vascular, skeletal, and lacrimal gland development, suggesting that NDST1 is an essential in mouse embryonic development and for branching morphogenesis. NDST1 deficient mice experience severe respiratory difficulties and die shortly after birth due to immaturation of the type II pneumocytes, which results in insufficient production of surfactants (57, 58). NDST1 deficient mice have undersulfated HS in which N-sulfation and 2-O-sulfation are reduced, whereas 6-O-sulfation is normal (58). NDST1 has been shown to be required for FGF signaling during lens induction (59) and lacrimal gland induction (60) in mice. The lack of NDST2 in mice does not affect normal life span and fertility, but have severe mast cell defects, caused by a complete lack of heparin but do not display any obvious HS defects (61). A lack of both NDST1 and NDST2 results in early embryonic lethality (62). Mice lacking NDST3 are viable and fertile and do not show any obvious phenotype (63). Furthermore, selective inactivation of Ndst1 in mice mammary epithelia does not affect branching morphogenesis but causes a defect in lobuloalveolar expansion during pregnancy and lactation (64). These data demonstrate a clear connection between sulfation and branching morphogenesis. What often remains to be determined is biochemical analysis of the HS structures that are made in the absence of one enzyme. This has been hampered in the past by the requirement of large amounts of HS to be analyzed, recently more sensitive techniques such as LC-MS/MS have been used to sequence complex mixtures of HS tetrasacharides (65).
Targeted disruption of D-glucoronyl C5-epimerase gene (Glce) results in neonatal lethality accompanied by multiple defects in mice (66). All Glce knockout pups have kidney agenesis, and have immature and poorly inflated lungs that have thickened and cell-rich alveolar septa (67). Disruption of Glce results in lack of IdoA, which alters the sulfation pattern resulting in a large reduction in 2-O-sulfation (66).
Mice with a deletion of HS 2-O-sulfotransferase (Hs2st1) exhibit bilateral renal agenesis suggesting that this type of sulfation is critical in the modulation of the activity of one or more key growth factors involved in kidney development (68). These mice have normal UB outgrowth, but mesenchymal condensation and subsequent UB branching is absent primarily due to a failure of MM induction (69). In addition, these mice also display defects in the eye and skeleton (68). Although, Hs2st1 mutant embryos have normal lungs and the pups remain viable for a few hours after birth. However, analysis of the HS structures shows that loss of 2-O-sulfation is compensated by sulfation at other positions, specifically at the 6-O-position, which preserves the overall HS charge (70).
In the lacrimal gland, HS in the distal tip of the epithelium is differentially modified with uniquely sulfated HS to restrict and potentiate FGF signaling within the tip to promote directional outgrowth of the lacrimal gland bud (60). Deletion of Hs2st1 and Hs6st1 increases the diffusion of Fgf10 in the ECM, decreasing FGF signaling in the lacrimal gland bud and prevents lacrimal gland development (71). Thus, the HS sulfation controls lacrimal gland branching morphogenesis. In addition, based on the sequence of HS biosynthetic reactions, it would be predicted that a loss in Hs2st1 would result in a reduction in the amount of one type of 3-O-sulfated epitope in the HS. The formation of an Hs3st3-like epitope on a glucosamine requires an adjacent 2-O-sulfated iduronic acid. It remains to be determined whether there is any compensation in 3-O-sulfation, with loss of Hs2st1.
Mice deficient in Hs6st1 exhibit neonatal lethality, decreased growth and aberrant lung morphology (72). In addition, lungs from the adult Hs6st1 mutant mice show enlarged airspaces associated with fragmentation and irregular deposition of elastin in alveoli (73). The expression of Hs6st1 is strong at the tips of branching tubules in the developing lung and Fgf10 preferentially binds 6-O-sulfated residues rather than 2-O-sulfated residues in HS (74, 75). Overall these studies all indicate that 6-O-sulfation is essential for homeostasis of lung alveoli. In addition, the requirement of 6-O-sulfated HS is also critical for Fgf10-Fgfr2b signaling during lacrimal gland branching morphogenesis and development (71).
3-O-sulfotransferases are the largest family of sulfotransferase enzymes, and it is not clear why we need seven isoforms. Out of the seven HS 3-O-sulfotransferase isoforms present in the mouse, only knockout mice for Hs3st1 and Hs3st2 have been generated. The Hs3st1−/− mice experience intrauterine growth retardation and spontaneous eye degeneration, which is dependent on the mouse background (76). Mice deficient in Hs3st2 are viable and do not display any abnormalities (77).
The vast array of phenotypes as a result of loss of different HS biosynthetic enzymes provides important insight into the diverse roles of HS and the necessity of tight regulation of HS sulfation patterning (53).
Role of HS in branching morphogenesis
Many experimental models where HS function is broadly perturbed using chemical and/or enzymatic methods suggest that HS and HS sulfation are critical for cellular functions and the process of branching morphogenesis. The use of Xylosides to inhibit HS chain extension, chlorate to inhibit HS sulfation, bacterial heparin-lyases to depolymerize HS chains and HS mimetics to compete function of specific structures, have been widely used to investigate HS function. Particularly when these types of perturbations are combined with ex vivo culture of fetal branching organs such as the mouse SMG, lungs, kidneys, lacrimal glands, mammary glands, the critical role for HS during branching morphogenesis is apparent. During lung morphogenesis, in vitro and ex vivo studies performed on isolated embryonic lung buds show that treatment with either heparin lyase or sodium chlorate markedly inhibits branching (78). This suggests that the overall HS plays a role in lung branching. Indeed, the alveolar BM beneath type I cells is shown to be highly sulfated when compared to that beneath type II cells (79, 80). Furthermore, it has been shown that HS low in O-sulfation, as shown using 10E4 antibody that recognizes relatively low-O-sulfated, N-acetylated HS epitopes, is dynamically expressed in the lung mesenchyme at the sites of prospective budding near FGF10-expressing areas. In turn, highly sulfated HS is present in the BM of branching epithelial tubules as shown by a strong alcian blue and 3G10 antibody staining (78), where it has been suggested to function as an effective FGF10 sink to effectively activate FGFR2b signaling. The dynamic pattern of HS expression suggests that low O-sulfated HS may facilitate diffusion and direct FGF10, and other distal signals from their source to target cells. Indeed it has been shown that highly sulfated HS in BM reduces the rate of FGF2 movement through matrix by several fold (100–1000) compared to when HS binding does not occur (81). Genetic deletion of Hs6st1 in mice shows lung abnormalities, as described in the previous section, highlights the importance of this modification in lung morphogenesis.
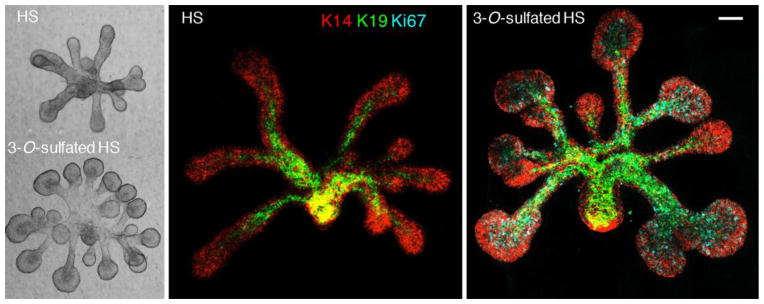
Differences in HS has also been shown during SMG branching whereby GAGs accumulate at sites of rapid proliferation on the branching SMG epithelium (20), but are rapidly degraded on the distal lobules to allow epithelial growth and expansion (19). In addition, HS but not chondroitin sulfate, has been shown to be required for SMG branching (reviewed previously in (21)). Early in vitro studies found that treatment of cultured salivary glands with β-xylosidase, a competitive inhibitor of GAG synthesis, inhibited branching of cultured embryonic SMG (18). Differences in sulfation patterns of the HS have been shown to influence both ductal elongation and endbud expansion during SMG development (82). In addition, variation in binding affinities that different FGFs have for HS regulates epithelial morphogenesis by affecting the diffusion of FGFs through the ECM, creating morphogenic gradients (10). Specific sulfate modifications create specific epitopes that influence signaling in progenitor cells. For example, K14+KIT+ progenitors found in epithelial endbuds are enriched in Hs3st3 enzyme isoforms. These enzymes are rapidly upregulated in response to FGF10/FGFR2b signaling, resulting in an autocrine increase in MAPK signaling and expression of Hs3st3 genes, KIT and transcription factors downstream of FGFR2b signaling. The rapid response to 3-O-sulfated HS increased the number of K14+KIT+ progenitors, proliferation and endbud morphogenesis (Fig. 4) (83). Thus, specific 3-O-sulfated epitope on HS regulate a number of processes during SMG branching morphogenesis.
Fig. 4.
3-O-sulfated-HS increases FGF10-dependent epithelial morphogenesis by increasing proliferation. E13 SMG epithelia were cultured in a 3D laminin matrix and treated with FGF10 and either HS with low endogenous 3-O-sulfation or 3-O-sulfated-HS for 28 hr. Epithelia were were stained for K14 (red), K19 (green), and Ki67 (cyan). Images are confocal projections. Scale bars: 100 μm.
A number of studies performed in vitro using isolated embryonic kidney cultures have shown the importance of HS in kidney development (reviewed in (84)). Removal of HS by treatment with heparin lyase or addition of sodium chlorate, a competitive inhibitor of sulfate, to kidney cultures perturbed UB branching (85, 86). In addition, treatment of these cultures with heparin compounds with variable sulfation patterns indicated a requirement of 6-O-sulfation in growth and branching of UB (87). The use of heparin mimetics, or heparin fragments, of defined size and with specific sulfation patterns have been used in many culture systems to compete the endogenous HS for GF binding.
During mammary gland morphogenesis, the GAGs bind to growth factors and accumulate specifically in the BM surrounding the ducts, which may serve as a reservoir for growth factors such as TGFβ to inhibit the formation of new branches (88). Using mice in various HS biosynthetic enzymes have been conditionally deleted in the mammary epithelial cells, it has been proposed that specific sulfates on HS can influence GF interactions during mammary gland development. Selective deletion of Ext1 in mammary epithelium results in failure of primary ductal branching morphogenesis whereas selective Hs2st1 deletion in mammary epithelium markedly decreases secondary and ductal side-branches and reduces bifurcated terminal end buds without inhibiting ductal formation (56). Mammary epithelial-specific inactivation of Ndst1 does not affect earlier stages of mammary development but results in defective lobuloalveolar development leading to insufficient milk production (64), whereas Ndst2 deletion results in a mild increase in ductal branched structures. Interestingly, deletion of both Ndst1 and Ndst2 results in significantly more primary and secondary branching (89). Together, these data demonstrate that variably sulfated HS and their specific interactions with GFs regulate individual stages of epithelial branching morphogenesis.
Post-synthesis modification of HS: Heparanase and Sulfatase
HS chains can be modified or removed by enzymes that specifically target them, altering the HS function on either the cell surface or in the ECM. These enzymes include heparanases and sulfatases, which can function within and outside the cell.
Extracellular HS turnover provides additional level of complexity to the role of HS. The biological activity of HS in vivo is regulated by heparanase, which cleaves HS only at a few sites, resulting in HS fragments of 5–10 kDa to regulate ECM degradation and remodeling (90). Understanding the role of heparanase during branching morphogenesis is complicated by the fact that there is co-regulation of heparanase and matrix metalloproteinases (91). Simply knocking out heparanase did not result in a reduction in branching morphogenesis. Heparanase is robustly localized within the growing end buds of the branching mammary gland. Surprisingly, mammary glands of heparanase knockout mice, which are viable and fertile, show increased branching morphogenesis (91). However, an increase in expression of MMP-2 and MMP-14 was associated with the loss of heparanase, suggesting that compensation may occur by the release of HS via proteolytic cleavage of the HSPGs by proteases or sheddases (91). Interestingly, mammary glands from transgenic virgin mice overexpressing heparanase show precocious alveolar development and maturation, with primary and secondary ducts, similar to pregnant mice (90). This suggests that HS cleavage by excess heparanase increases GF activity to increase branching. Recently, mammary branching morphogenesis has been shown to require reciprocal signaling by heparanase and MMP-14, whereby the levels of MMP-14 are directly proportional to that of heparanase levels (92). Although it is not clear how heparanase and MMP-14 regulate each other’s levels, it can be speculated that release of GF from ECM by heparanase may allow initiation of cell signaling that results in MMP-14 expression (92).
In ex vivo assays of SMG branching morphogenesis, endogenous heparanase colocalizes with perlecan in the BM and within the cleft of the SMG. Inhibition or addition of active form of heparanase decreases or increases branching of ex vivo SMG cultures, respectively (38). In addition, heparanase specifically releases FGF10 from perlecan and activates mitogen-activated protein kinase (MAPK) signaling, clefting and endbud formation. These data show that ECM remodeling through HS degradation can release growth factors from ECM reservoir, which can affect epithelial proliferation and regulate organ morphogenesis. However, the heparanase 1, null mouse, does not have a developmental phenotype in the SMG (91), however the compensation by other proteases has not been investigated.
The role of heparanase in kidney morphogenesis has not been explored, however its over-expression has been implicated in renal fibrosis (reviewed in (93). This may be due to the regulation of TGFβ function by heparanase. It was also speculated that cleavage of the BM HSPGs by heparanase could unmask ECM molecules and liberate HS-bound growth promoting factors to stimulate cell proliferation and differentiation (90).
Sulfatases
The two glucosamine-6-sulfatases sulfatases, Sulf1 and Sulf2, are extracellular 6-O-endosulfatases that remove 6-O-sulfates from HS either in the ECM and cell surface or within the Golgi apparatus (94, 95). These enzymes alter a number of GF signaling events either positively or negatively by removing 6-O-sulfates (96). For example, Sulf activates BMP signaling by releasing the HS-associated BMP agonist Noggin and allowing BMP to interact with its cognate receptor (97). On the other hand, Sulf activity disrupts the ability of FGF2 to induce signaling by removing 6-O-sulfate moiety that is necessary to form the functional FGF2-HS-FGFR ternary complex (98). However, the Sulf1 knockout mice show no obvious phenotype (99) yet mice with a homozygous deletion of the sulf2 gene display strain-specific defects in viability, growth and lung development (100). The lack of major developmental phenotypes in these mice suggested functional redundancy. As predicted, the Sulf1 and Sulf2 double knockout mice have a developmental defect; 50% of mice die around birth, and among the survivors only 20% become adults that display a short stature, renal and skeletal defects (95). Closer look at the glomerular membranes in these mice show glomerular hypercellularity, matrix accumulation, mesangiolysis and glomerular BM irregularity as a result of impaired growth factor signaling (Takashima et al., 2016). This latter study indicates that fine-tuning of 6-O-sulfation by Sulfs may control multiple functions of HS chains during morphogenesis. Less is known about sulfatases that can remove other sulfate moieties, a glucosamine-3-O-sulfatase called arylsulfatase G, was identified and shown to be required for complete lysosomal degradation the HS (101). It is not known if there are extracellular enzymes that can remove N-, 2- and 3-O sulfates. In summary, modification of HS sulfation, may control tissue regeneration by fine-tuning precise and specific molecular interactions during branching morphogenesis.
Conclusion
Together, HSPGs of the matrix actively contribute to cell-to-cell signaling via multiple mechanisms. HS is instrumental in the storage and concentration of heparan-binding GFs in the vicinity of cells that bear the relevant signaling receptor. The degree of specificity of GF signaling is influenced by the vast polymorphism of HS sequences. Therefore understanding heparan sulfate’s role during branching morphogenesis has therapeutic implications for cell- and GF-based regeneration and repair of branching organs.
Highlights.
Heparan sulfates (HS) are major modulators of growth factor binding and signaling at cell surfaces and in the extracellular matrix
HS have extreme structural heterogeneity due to diverse sulfation patterns established by sulfotransferase enzymes
Sulfate structures on HS are critical determinants of growth factor specificity and function during branching morphogenesis.
Understanding the function of HS has therapeutic implications for cell- and GF-based regeneration and repair of branching organs.
Acknowledgments
The authors would like to thank Drs. Jennifer Symonds and Belinda Hauser for comments and critical reading of this manuscript. This work was supported by the Intramural Research Program of the National Institute of Dental and Craniofacial Research at the National Institutes of Health.
Abbreviations
- E
Embryonic day
- ECM
extracellular matrix
- BM
basement membrane
- GAGs
glycosaminoglycans
- HS
heparan sulfate
- FGF
fibroblast growth factor
- GF/s
growth factor/s
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Patel VN, Hoffman MP. Salivary gland development: a template for regeneration. Semin Cell Dev Biol. 2014;25–26:52–60. doi: 10.1016/j.semcdb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol. 2014;15:786–801. doi: 10.1038/nrm3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daley WP, Yamada KM. ECM-modulated cellular dynamics as a driving force for tissue morphogenesis. Curr Opin Genet Dev. 2013;23:408–414. doi: 10.1016/j.gde.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rozario T, DeSimone DW. The extracellular matrix in development and morphogenesis: a dynamic view. Dev Biol. 2010;341:126–140. doi: 10.1016/j.ydbio.2009.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vlodavsky I, Bar-Shavit R, Ishai-Michaeli R, Bashkin P, Fuks Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends Biochem Sci. 1991;16:268–271. doi: 10.1016/0968-0004(91)90102-2. [DOI] [PubMed] [Google Scholar]
- 7.Allen BL, Rapraeger AC. Spatial and temporal expression of heparan sulfate in mouse development regulates FGF and FGF receptor assembly. J Cell Biol. 2003;163:637–648. doi: 10.1083/jcb.200307053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shukla D, et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]
- 9.Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Current Opinion in Biotechnology. 2003;14:526–532. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Makarenkova HP, et al. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci Signal. 2009;2:ra55. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitelock JM, Iozzo RV. Heparan sulfate: a complex polymer charged with biological activity. Chem Rev. 2005;105:2745–2764. doi: 10.1021/cr010213m. [DOI] [PubMed] [Google Scholar]
- 12.Norton WH, Ledin J, Grandel H, Neumann CJ. HSPG synthesis by zebrafish Ext2 and Extl3 is required for Fgf10 signalling during limb development. Development. 2005;132:4963–4973. doi: 10.1242/dev.02084. [DOI] [PubMed] [Google Scholar]
- 13.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 14.Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- 15.Maimets M, et al. Long-Term In Vitro Expansion of Salivary Gland Stem Cells Driven by Wnt Signals. Stem Cell Reports. 2016;6:150–162. doi: 10.1016/j.stemcr.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pringle S, et al. Human Salivary Gland Stem Cells Functionally Restore Radiation Damaged Salivary Glands. Stem Cells. 2016;34:640–652. doi: 10.1002/stem.2278. [DOI] [PubMed] [Google Scholar]
- 17.Thompson HA, Spooner BS. Inhibition of branching morphogenesis and alteration of glycosaminoglycan biosynthesis in salivary glands treated with beta-D-xyloside. Dev Biol. 1982;89:417–424. doi: 10.1016/0012-1606(82)90330-x. [DOI] [PubMed] [Google Scholar]
- 18.Thompson HA, Spooner BS. Proteoglycan and glycosaminoglycan synthesis in embryonic mouse salivary glands: effects of beta-D-xyloside, an inhibitor of branching morphogenesis. J Cell Biol. 1983;96:1443–1450. doi: 10.1083/jcb.96.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernfield M, Banerjee SD. The turnover of basal lamina glycosaminoglycan correlates with epithelial morphogenesis. Dev Biol. 1982;90:291–305. doi: 10.1016/0012-1606(82)90378-5. [DOI] [PubMed] [Google Scholar]
- 20.Bernfield MR, Banerjee SD. Acid mucopolysaccharide (glycosaminoglycan) at the epithelial-mesenchymal interface of mouse embryo salivary glands. J Cell Biol. 1972;52:664–673. doi: 10.1083/jcb.52.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patel VN, Rebustini IT, Hoffman MP. Salivary gland branching morphogenesis. Differentiation. 2006;74:349–364. doi: 10.1111/j.1432-0436.2006.00088.x. [DOI] [PubMed] [Google Scholar]
- 22.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2:REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin X, Buff EM, Perrimon N, Michelson AM. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development. 1999;126:3715–3723. doi: 10.1242/dev.126.17.3715. [DOI] [PubMed] [Google Scholar]
- 24.De Moerlooze L, et al. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development. 2000;127:483–492. doi: 10.1242/dev.127.3.483. [DOI] [PubMed] [Google Scholar]
- 25.Min H, et al. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–3161. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sekine K, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 27.Thacker BE, Xu D, Lawrence R, Esko JD. Heparan sulfate 3-O-sulfation: a rare modification in search of a function. Matrix Biol. 2014;35:60–72. doi: 10.1016/j.matbio.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Biol. 2005;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 29.Fukai N, et al. Lack of collagen XVIII/endostatin results in eye abnormalities. EMBO J. 2002;21:1535–1544. doi: 10.1093/emboj/21.7.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ylikarppa R, et al. Lack of type XVIII collagen results in anterior ocular defects. FASEB J. 2003;17:2257–2259. doi: 10.1096/fj.02-1001fje. [DOI] [PubMed] [Google Scholar]
- 31.Utriainen A, et al. Structurally altered basement membranes and hydrocephalus in a type XVIII collagen deficient mouse line. Hum Mol Genet. 2004;13:2089–2099. doi: 10.1093/hmg/ddh213. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, et al. Induced repatterning of type XVIII collagen expression in ureter bud from kidney to lung type: association with sonic hedgehog and ectopic surfactant protein C. Development. 2001;128:1573–1585. doi: 10.1242/dev.128.9.1573. [DOI] [PubMed] [Google Scholar]
- 33.Karihaloo A, et al. Endostatin regulates branching morphogenesis of renal epithelial cells and ureteric bud. Proc Natl Acad Sci U S A. 2001;98:12509–12514. doi: 10.1073/pnas.221205198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cano-Gauci DF, et al. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson-Golabi-Behmel syndrome. J Cell Biol. 1999;146:255–264. doi: 10.1083/jcb.146.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grisaru S, Cano-Gauci D, Tee J, Filmus J, Rosenblum ND. Glypican-3 modulates BMP- and FGF-mediated effects during renal branching morphogenesis. Dev Biol. 2001;231:31–46. doi: 10.1006/dbio.2000.0127. [DOI] [PubMed] [Google Scholar]
- 36.Pellegrini M, et al. Gpc3 expression correlates with the phenotype of the Simpson-Golabi-Behmel syndrome. Dev Dyn. 1998;213:431–439. doi: 10.1002/(SICI)1097-0177(199812)213:4<431::AID-AJA8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Saikali Z, Sinnett D. Expression of glypican 3 (GPC3) in embryonal tumors. Int J Cancer. 2000;89:418–422. [PubMed] [Google Scholar]
- 38.Patel VN, et al. Heparanase cleavage of perlecan heparan sulfate modulates FGF10 activity during ex vivo submandibular gland branching morphogenesis. Development. 2007;134:4177–4186. doi: 10.1242/dev.011171. [DOI] [PubMed] [Google Scholar]
- 39.Wiradjaja F, DiTommaso T, Smyth I. Basement membranes in development and disease. Birth Defects Res C Embryo Today. 2010;90:8–31. doi: 10.1002/bdrc.20172. [DOI] [PubMed] [Google Scholar]
- 40.Ornitz DM. FGFs, heparan sulfate and FGFRs: complex interactions essential for development. Bioessays. 2000;22:108–112. doi: 10.1002/(SICI)1521-1878(200002)22:2<108::AID-BIES2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 41.Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y. Perlecan is essential for cartilage and cephalic development. Nat Genet. 1999;23:354–358. doi: 10.1038/15537. [DOI] [PubMed] [Google Scholar]
- 42.Rossi M, et al. Heparan sulfate chains of perlecan are indispensable in the lens capsule but not in the kidney. EMBO J. 2003;22:236–245. doi: 10.1093/emboj/cdg019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kirn-Safran C, Farach-Carson MC, Carson DD. Multifunctionality of extracellular and cell surface heparan sulfate proteoglycans. Cell Mol Life Sci. 2009;66:3421–3434. doi: 10.1007/s00018-009-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Z, et al. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 2004;64:4699–4702. doi: 10.1158/0008-5472.CAN-04-0810. [DOI] [PubMed] [Google Scholar]
- 45.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whitelock JM, Melrose J, Iozzo RV. Diverse cell signaling events modulated by perlecan. Biochemistry. 2008;47:11174–11183. doi: 10.1021/bi8013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williamson RA, et al. Dystroglycan is essential for early embryonic development: disruption of Reichert's membrane in Dag1-null mice. Hum Mol Genet. 1997;6:831–841. doi: 10.1093/hmg/6.6.831. [DOI] [PubMed] [Google Scholar]
- 48.Henry MD, Campbell KP. A role for dystroglycan in basement membrane assembly. Cell. 1998;95:859–870. doi: 10.1016/s0092-8674(00)81708-0. [DOI] [PubMed] [Google Scholar]
- 49.Durbeej M, et al. Dystroglycan binding to laminin alpha1LG4 module influences epithelial morphogenesis of salivary gland and lung in vitro. Differentiation. 2001;69:121–134. doi: 10.1046/j.1432-0436.2001.690206.x. [DOI] [PubMed] [Google Scholar]
- 50.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 51.Humphries DE, Sullivan BM, Aleixo MD, Stow JL. Localization of human heparan glucosaminyl N-deacetylase/N-sulphotransferase to the trans-Golgi network. Biochem J. 1997;325(Pt 2):351–357. doi: 10.1042/bj3250351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crawford BE, Olson SK, Esko JD, Pinhal MA. Cloning, Golgi localization, and enzyme activity of the full-length heparin/heparan sulfate-glucuronic acid C5-epimerase. J Biol Chem. 2001;276:21538–21543. doi: 10.1074/jbc.M100880200. [DOI] [PubMed] [Google Scholar]
- 53.Lamanna WC, et al. The heparanome--the enigma of encoding and decoding heparan sulfate sulfation. J Biotechnol. 2007;129:290–307. doi: 10.1016/j.jbiotec.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 54.Lin X, et al. Disruption of gastrulation and heparan sulfate biosynthesis in EXT1-deficient mice. Dev Biol. 2000;224:299–311. doi: 10.1006/dbio.2000.9798. [DOI] [PubMed] [Google Scholar]
- 55.Stickens D, Zak BM, Rougier N, Esko JD, Werb Z. Mice deficient in Ext2 lack heparan sulfate and develop exostoses. Development. 2005;132:5055–5068. doi: 10.1242/dev.02088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garner OB, et al. Stage-dependent regulation of mammary ductal branching by heparan sulfate and HGF-cMet signaling. Dev Biol. 2011;355:394–403. doi: 10.1016/j.ydbio.2011.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fan G, et al. Targeted disruption of NDST-1 gene leads to pulmonary hypoplasia and neonatal respiratory distress in mice. FEBS Lett. 2000;467:7–11. doi: 10.1016/s0014-5793(00)01111-x. [DOI] [PubMed] [Google Scholar]
- 58.Ringvall M, et al. Defective heparan sulfate biosynthesis and neonatal lethality in mice lacking N-deacetylase/N-sulfotransferase-1. J Biol Chem. 2000;275:25926–25930. doi: 10.1074/jbc.C000359200. [DOI] [PubMed] [Google Scholar]
- 59.Pan Y, Woodbury A, Esko JD, Grobe K, Zhang X. Heparan sulfate biosynthetic gene Ndst1 is required for FGF signaling in early lens development. Development. 2006;133:4933–4944. doi: 10.1242/dev.02679. [DOI] [PubMed] [Google Scholar]
- 60.Pan Y, et al. Bud specific N-sulfation of heparan sulfate regulates Shp2-dependent FGF signaling during lacrimal gland induction. Development. 2008;135:301–310. doi: 10.1242/dev.014829. [DOI] [PubMed] [Google Scholar]
- 61.Forsberg E, et al. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- 62.Holmborn K, et al. Heparan sulfate synthesized by mouse embryonic stem cells deficient in NDST1 and NDST2 is 6-O-sulfated but contains no N-sulfate groups. J Biol Chem. 2004;279:42355–42358. doi: 10.1074/jbc.C400373200. [DOI] [PubMed] [Google Scholar]
- 63.Grobe K, et al. Heparan sulfate and development: differential roles of the N-acetylglucosamine N-deacetylase/N-sulfotransferase isozymes. Biochim Biophys Acta. 2002;1573:209–215. doi: 10.1016/s0304-4165(02)00386-0. [DOI] [PubMed] [Google Scholar]
- 64.Crawford BE, et al. Loss of the heparan sulfate sulfotransferase, Ndst1, in mammary epithelial cells selectively blocks lobuloalveolar development in mice. PLoS One. 2010;5:e10691. doi: 10.1371/journal.pone.0010691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang R, et al. De Novo Sequencing of Complex Mixtures of Heparan Sulfate Oligosaccharides. Anal Chem. 2016 doi: 10.1021/acs.analchem.6b00519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li J-p. Glucuronyl C5-Epimerase. 2010;93:59–78. doi: 10.1016/S1877-1173(10)93004-4. [DOI] [PubMed] [Google Scholar]
- 67.Li JP, et al. Targeted disruption of a murine glucuronyl C5-epimerase gene results in heparan sulfate lacking L-iduronic acid and in neonatal lethality. J Biol Chem. 2003;278:28363–28366. doi: 10.1074/jbc.C300219200. [DOI] [PubMed] [Google Scholar]
- 68.Bullock SL, Fletcher JM, Beddington RS, Wilson VA. Renal agenesis in mice homozygous for a gene trap mutation in the gene encoding heparan sulfate 2-sulfotransferase. Genes Dev. 1998;12:1894–1906. doi: 10.1101/gad.12.12.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shah MM, et al. Hs2st mediated kidney mesenchyme induction regulates early ureteric bud branching. Dev Biol. 2010;339:354–365. doi: 10.1016/j.ydbio.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Merry CL, et al. The molecular phenotype of heparan sulfate in the Hs2st-/- mutant mouse. J Biol Chem. 2001;276:35429–35434. doi: 10.1074/jbc.M100379200. [DOI] [PubMed] [Google Scholar]
- 71.Qu X, et al. Lacrimal gland development and Fgf10-Fgfr2b signaling are controlled by 2-O- and 6-O-sulfated heparan sulfate. J Biol Chem. 2011;286:14435–14444. doi: 10.1074/jbc.M111.225003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Habuchi H, et al. Mice deficient in heparan sulfate 6-O-sulfotransferase-1 exhibit defective heparan sulfate biosynthesis, abnormal placentation, and late embryonic lethality. J Biol Chem. 2007;282:15578–15588. doi: 10.1074/jbc.M607434200. [DOI] [PubMed] [Google Scholar]
- 73.Izvolsky KI, Lu J, Martin G, Albrecht KH, Cardoso WV. Systemic inactivation of Hs6st1 in mice is associated with late postnatal mortality without major defects in organogenesis. Genesis. 2008;46:8–18. doi: 10.1002/dvg.20355. [DOI] [PubMed] [Google Scholar]
- 74.Izvolsky KI, et al. Heparan sulfate–FGF10 interactions during lung morphogenesis. Dev Biol. 2003;258:185–200. doi: 10.1016/s0012-1606(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 75.Izvolsky KI, et al. Heparan sulfates expressed in the distal lung are required for Fgf10 binding to the epithelium and for airway branching. Am J Physiol Lung Cell Mol Physiol. 2003;285:L838–846. doi: 10.1152/ajplung.00081.2003. [DOI] [PubMed] [Google Scholar]
- 76.<Hs3st1 null 2003.pdf>.
- 77.Hasegawa H, Wang F. Visualizing mechanosensory endings of TrkC-expressing neurons in HS3ST-2-hPLAP mice. J Comp Neurol. 2008;511:543–556. doi: 10.1002/cne.21862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Izvolsky KI, et al. Heparan sulfate FGF10 interactions during lung morphogenesis. Developmental Biology. 2003;258:185–200. doi: 10.1016/s0012-1606(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 79.van Kuppevelt TH, Cremers FP, Domen JG, Kuyper CM. Staining of proteoglycans in mouse lung alveoli. II. Characterization of the Cuprolinic blue-positive, anionic sites. Histochem J. 1984;16:671–686. doi: 10.1007/BF01003394. [DOI] [PubMed] [Google Scholar]
- 80.Sannes PL. Differences in basement membrane-associated microdomains of type I and type II pneumocytes in the rat and rabbit lung. J Histochem Cytochem. 1984;32:827–833. doi: 10.1177/32.8.6747274. [DOI] [PubMed] [Google Scholar]
- 81.Dowd CJ, Cooney CL, Nugent MA. Heparan sulfate mediates bFGF transport through basement membrane by diffusion with rapid reversible binding. J Biol Chem. 1999;274:5236–5244. doi: 10.1074/jbc.274.8.5236. [DOI] [PubMed] [Google Scholar]
- 82.Patel VN, et al. Specific heparan sulfate structures modulate FGF10-mediated submandibular gland epithelial morphogenesis and differentiation. J Biol Chem. 2008;283:9308–9317. doi: 10.1074/jbc.M709995200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Patel VN, et al. Hs3st3-modified heparan sulfate controls KIT+ progenitor expansion by regulating 3-O-sulfotransferases. Dev Cell. 2014;29:662–673. doi: 10.1016/j.devcel.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nigam SK, Bush KT. Growth factor-heparan sulfate "switches" regulating stages of branching morphogenesis. Pediatr Nephrol. 2014;29:727–735. doi: 10.1007/s00467-013-2725-z. [DOI] [PubMed] [Google Scholar]
- 85.Davies J, Lyon M, Gallagher J, Garrod D. Sulphated proteoglycan is required for collecting duct growth and branching but not nephron formation during kidney development. Development. 1995;121:1507–1517. doi: 10.1242/dev.121.5.1507. [DOI] [PubMed] [Google Scholar]
- 86.Steer DL, et al. Regulation of ureteric bud branching morphogenesis by sulfated proteoglycans in the developing kidney. Dev Biol. 2004;272:310–327. doi: 10.1016/j.ydbio.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 87.Shah MM, et al. Growth factor-dependent branching of the ureteric bud is modulated by selective 6-O sulfation of heparan sulfate. Dev Biol. 2011;356:19–27. doi: 10.1016/j.ydbio.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silberstein GB, Daniel CW. Glycosaminoglycans in the basal lamina and extracellular matrix of the developing mouse mammary duct. Dev Biol. 1982;90:215–222. doi: 10.1016/0012-1606(82)90228-7. [DOI] [PubMed] [Google Scholar]
- 89.Bush KT, et al. N-sulfation of heparan sulfate regulates early branching events in the developing mammary gland. J Biol Chem. 2012;287:42064–42070. doi: 10.1074/jbc.M112.423327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zcharia E, et al. Molecular properties and involvement of heparanase in cancer progression and mammary gland morphogenesis. J Mammary Gland Biol Neoplasia. 2001;6:311–322. doi: 10.1023/a:1011375624902. [DOI] [PubMed] [Google Scholar]
- 91.Zcharia E, et al. Newly generated heparanase knock-out mice unravel co-regulation of heparanase and matrix metalloproteinases. PLoS One. 2009;4:e5181. doi: 10.1371/journal.pone.0005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gomes AM, et al. Mammary Branching Morphogenesis Requires Reciprocal Signaling by Heparanase and MMP-14. J Cell Biochem. 2015;116:1668–1679. doi: 10.1002/jcb.25127. [DOI] [PubMed] [Google Scholar]
- 93.Masola V, Zaza G, Onisto M, Lupo A, Gambaro G. Impact of heparanase on renal fibrosis. J Transl Med. 2015;13:181. doi: 10.1186/s12967-015-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dhoot GK, et al. Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 2001;293:1663–1666. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- 95.Holst CR, et al. Secreted sulfatases Sulf1 and Sulf2 have overlapping yet essential roles in mouse neonatal survival. PLoS One. 2007;2:e575. doi: 10.1371/journal.pone.0000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rosen SD, Lemjabbar-Alaoui H. Sulf-2: an extracellular modulator of cell signaling and a cancer target candidate. Expert Opin Ther Targets. 2010;14:935–949. doi: 10.1517/14728222.2010.504718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S. Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J Biol Chem. 2004;279:5604–5611. doi: 10.1074/jbc.M310691200. [DOI] [PubMed] [Google Scholar]
- 98.Wang S, et al. QSulf1, a heparan sulfate 6-O-endosulfatase, inhibits fibroblast growth factor signaling in mesoderm induction and angiogenesis. Proc Natl Acad Sci U S A. 2004;101:4833–4838. doi: 10.1073/pnas.0401028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lamanna WC, et al. Heparan sulfate 6-O-endosulfatases: discrete in vivo activities and functional co-operativity. Biochem J. 2006;400:63–73. doi: 10.1042/BJ20060848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lum DH, Tan J, Rosen SD, Werb Z. Gene trap disruption of the mouse heparan sulfate 6-O-endosulfatase gene, Sulf2. Mol Cell Biol. 2007;27:678–688. doi: 10.1128/MCB.01279-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kowalewski B, et al. Arylsulfatase G inactivation causes loss of heparan sulfate 3-O-sulfatase activity and mucopolysaccharidosis in mice. Proc Natl Acad Sci U S A. 2012;109:10310–10315. doi: 10.1073/pnas.1202071109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu BY, McDermott SP, Khwaja SS, Alexander CM. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc Natl Acad Sci U S A. 2004;101:4158–4163. doi: 10.1073/pnas.0400699101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zcharia E, et al. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J. 2004;18:252–263. doi: 10.1096/fj.03-0572com. [DOI] [PubMed] [Google Scholar]