Abstract
Objectives
To examine the influence of acute migraine medication adherence on migraine disability and acute medication satisfaction.
Methods
Adults with migraine completed three months of daily electronic diaries assessing headache symptoms, acute medication taken, acute medication satisfaction, and daily migraine disability. Repeated measures mixed effects models examined the effect of initial medication type [migraine-specific medication (MSM) vs. over-the-counter analgesic (OTC) vs. Opiate/Barbiturate], pain severity at dosing, and their interaction on daily migraine disability and acute medication satisfaction.
Results
Participants (N = 337; 92.5% female, 91.1% Caucasian, Non-Hispanic, 84.0% with episodic migraine) recorded 29,722 diary days. Participants took acute medication on 96.5% of 8,090 migraine days. MSM was taken first most frequently (58.0%), followed by OTC (29.9%) and Opiate/Barbiturate (12.1%). Acute medication was most frequently taken when pain was mild(41.2%), followed by moderate (37.7% ) and severe (11.4%). Initially dosing with MSM while pain was mild was associated with the lowest daily disability [Medication X Pain at Dosing F(4, 6336.12) = 58.73, p < .001] and highest acute medication satisfaction [Medication X Pain at Dosing F(4, 3867.36) = 24.00, p < .001].
Conclusions
Using an MSM (triptan or ergot) first was associated with lowest migraine disability and highest acute medication satisfaction.
Keywords: Migraine, Adherence, Disability, Acute Drug Therapy, Patient Satisfaction
Introduction
Migraine is a prevalent chronic disease characterized by episodes of head pain and associated symptoms (including nausea and sensitivity to light and sound)(1, 2). Migraine is the most disabling neurologic condition worldwide (3), affecting social, occupational, and emotional functioning. Migraine prevalence peaks during mid-life, which contributes to high rates of lost work productivity due to absenteeism (not coming to work) and presenteeism (reduced productivity while at work)(4, 5).
Migraine is most often managed using acute medications, which are taken at the time of the migraine attack (6) to stop or alleviate symptoms. Acute medication is foundational to pharmacologic migraine management: 98% of people with migraine report using at least one type of acute medication to manage their attacks (6). Recommendations for using acute migraine medication adherence (7–9) include using a migraine-specific medication (MSM) first for migraines, taking the acute medication early in the migraine episode (while the pain is still mild), and limiting acute medication overuse.
People with migraine struggle adhering to these optimal acute migraine medication recommendations (10). Patient burden for in-the-moment acute migraine medication use decision-making is high (11). People with migraine describe numerous barriers to adhering to acute migraine medication recommendations (12): differentiating between migraine and other forms of headache is difficult early during a migraine episode, acute medication may not be immediately available at the start of a migraine, and both role conflicts (e.g., employee, mother) and social influences (e.g., stigma associated with medication use) may make adherence challenging.
The majority of these recommendations have emerged from a literature designed to demonstrate the efficacy of acute migraine medications in brief, tightly controlled studies designed to optimize pain-freedom 2 hours after taking the acute medication. A naturalistic diary study could examine both within- and between-person variations in typical acute migraine medication use. Moreover, evaluating episode driven migraine-related disability and patient satisfaction with medication as outcome variables shifts the focus from solely pain reduction to improving the person with migraine’s quality of life, and valuing the personal experience of acutely managing migraine. To our knowledge, no naturalistic diary studies have examined the association between variations in typical acute migraine medication use and daily levels of migraine-related disability.
This study aims to examine how adhering to two key acute migraine medication recommendations [1) Medication Choice: treating migraines with a migraine-specific medication, and avoiding opiates and barbiturates, and 2) Timing of Dosing: taking the first dose of acute medication while the pain is still mild] influences daily fluctuations in migraine-related disability and acute medication satisfaction in a naturalistic daily diary.
Methods
Participants
Adults with migraine were recruited from three sources within Mercy Health, large health system in the United States: a tertiary care headache center, a family medicine practice, and the health system’s research database of people with migraine interested in participating in migraine-related research. Recruitment strategies included physician referral, flyers in waiting and exam rooms, and a letter sent to qualified individuals in the research database. Inclusion criteria were: 1) ≥ 18 years of age, 2) physician diagnosed (using International Classification of Headache Disorders-2 (ICHD-2) episodic or chronic migraine (13), 3) ≥ 4 headache days per month. Exclusion criteria were: 1) no migraine diagnosis, 2) < 4 headache days per month, 3) inability or unwillingness to complete daily electronic recordings. Recruitment was stratified by number of headache days per month, with a target enrollment of 40% 4–8 headache days per month, 40% 9–15 headache days, and 20% 15 or more headache days per month.
Procedures
Study nursing staff obtained basic medical history and vital signs from interested participants. The principal investigator or qualified study clinicians screened interested participants and performed informed consent with those meeting inclusion criteria and then instructed participants on how to use the electronic diary. Participants completed the electronic diary on a web-based portal with response-driven branching logic questionnaire to minimize unnecessary queries.
Participants completed questionnaires evaluating demographics and migraine-related disability at baseline. Participants then, for three months, completed daily electronic diary entries regarding their headache symptoms, medication taken, satisfaction with said medication, and migraine-related disability for that day. The time required to complete the relevant diary questions ranged from a matter of seconds on days where the individual did not have a headache to < 5 minutes on days where multiple medications were taken for their headache. Participants were compensated 1$ per completed diary. The study protocol was approved by the Mercy Health Institutional Review Board.
Measures
Baseline Questionnaires
Demographics
Participants reported their age, gender, race/ethnicity, employment status, education, and household income. Certain responses were condensed due to the sampling distribution; race/ethnicity (Caucasian, Non-Hispanic vs. Other), employment status (currently employed vs. not currently employed), education (college degree vs. no college degree), and household income (≥$50,000/year vs. <$50,000/year).
Disability
Participants completed the original (90 day) version of the Migraine Disability Assessment Scale (MIDAS), a five-item measure of headache-related role interference in the following three domains: Work/School, Household Work, and Family/Social/Leisure Activities. Higher scores indicate higher levels of disability. The MIDAS has demonstrated good internal consistency (Cronbach’s alpha = .83), test-retest reliability (Pearson’s r = .80)(14), and has been consistently associated with higher headache diary scores (15, 16).
Electronic Diary
Participants who took preventive medication indicated if they took their preventive medication each day. All participants indicated if they experienced a headache on each diary day. On headache days, participants provided the following:
Headache diagnosis
Participants reported headache duration, severity at its worst (mild, moderate severe), pain quality, pain location, and presence of migraine associated symptoms; nausea, vomiting, photophobia, and phonophobia. ICHD-2 criteria were used to categorize each headache (13). Headache day diagnosis was categorized as “migraine,” “probable migraine,” “presumed migraine treated with MSM,” “tension-type headache,” and “other headache.” For the purpose of analyses, migraine, probable migraine, and presumed migraine were all considered “migraine days.” Only migraine days were examined for the remainder of the analyses. Participants were classified as “chronic migraine” if they met migraine criteria and reported 15 or more headache days during the first month of monitoring. Participants were classified as having medication overuse if they reported both behavioral medication overuse (MSM days/month ≥ 10; Non-Steroidal Anti-Inflammatory (NSAID) days/month ≥ 15; Opiate or Barbiturate days/month ≥ 10) and 15 or more headache days/month.
Medication type
Participants recorded each type of acute medication taken on each headache day. For the purpose of these analyses, only the first medication recorded was examined. Medications were categorized as “None” if no medication was recorded, “MSM” for triptans and ergot derivatives, “Over the Counter (OTC)” for non-prescription medications, and “Opiate/Barbiturate” for opioid and barbiturate medications.
Pain at dosing
For each medication, participants recorded head pain severity at first medication dosing, with three response options: “Mild,” “Moderate,” and “Severe.”
Medication use
We characterized medication use as: “MSM,” “OTC,” and “Opiate/Barbiturate.” When a MSM was the first medication used, medication was coded as “MSM.” Participants that met criteria for migraine, probable migraine, or used MSM for presumed migraine but used an OTC medication first were categorized as “OTC.” Similarly, participants who met criteria for migraine, probable migraine, or used MSM for presumed migraine but used an opiate or barbiturate first were categorized as “Opiate/Barbiturate.”
Satisfaction with medication
Participants recorded their satisfaction with the first medication taken on each headache day on an 11-point Likert-type scale, ranging from 0 “None” to 10 “Total Satisfaction.”
Daily disability
On each headache day, participants completed the Migraine Disability Index (MIDI) (17). The MIDI is a four-item scale assessing the degree to which a headache episode interferes with role functioning in four domains: Family/Home, Recreation, Social, and Occupational. Participants respond on an 11-point Likert-type scale assessing degree of headache-related interference ranging from 0 “Not at all” to 10 “Totally.” Participants only responded to role functioning that would have occurred on that day (e.g., one cannot have occupational impairment on non-work days). The MIDI total score is an average of scored items; higher scores indicated higher levels of disability. The MIDI has demonstrated good internal consistency (Cronbach’s alpha = .89) and test-retest reliability (Intraclass Correlation Coefficient = 0.88; 95% CI = .85 - .91) across severity and symptom matched migraines.
Analyses
Descriptive analyses characterized participants on demographics and diary entries on number of headache days, headache diagnoses, medication types used, satisfaction with acute medication, and migraine-related disability. Repeated measures mixed effects models were used to examine whether MIDI scores and satisfaction with acute headache medication varied by each demographic variable, to determine whether these variables should be controlled in the final models. Repeated measures mixed effects models were used to examine the effect of head pain severity at first medication dose, type and medication used, and their interaction on the MIDI and patient satisfaction with the first acute headache medication. Only diary days on which participants reported taking at least one medication for the current headache were included in the analysis. MIDI and patient satisfaction served as outcome variables in two separate analyses. Fixed effects included pain at dosing, medication use, their interaction, and diary day to account for potential changes in the outcome variables over time due to monitoring or treatment. Random effects included the intercept and medication use, accounting for person-level variance in the intercept and medication use (which, due to the coding of the variable, included variance in headache diagnosis). Identity matrices were associated with the lowest Aikike’s Information Criteria, and were therefore used to model the random effects. Diary day served as the repeated effect. A first-order autoregressive matrix was associated with the lowest Aikike’s Information Criterion, and was therefore used to model the repeated effect. Post-hoc subgroup analyses evaluated repeated measures mixed effects models separately in people with chronic vs. episodic migraine, and in people who took preventive medication vs. people who did not take preventive medication. Alpha was set at .05, two-tailed, for all analyses. Multiple comparisons were accounted for by a Bonferroni correction for post-hoc tests for mixed models analyses. SPSS version 21 was used for all analyses.
Results
Participant Descriptive Statistics
Three hundred seventy participants consented to participate in the study; 337 recorded at least one migraine day in the daily headache diary (including days when the participant met criteria for migraine, probable migraine, or used MSM) and were included in these analyses. Descriptive statistics for person-level variables are presented in Table 1. Participants were predominantly female (92.5%) and Caucasian, Non-Hispanic (91.1%) with a mean age of 41.72 (SD = 11.27). No demographic variable was significantly associated with the MIDI (ps > .40) or Satisfaction with Acute Medication (ps > .08), therefore the following mixed effect models were not controlled for any demographic variable.
Table 1.
Participant Characteristics
| Mean (SD)/ N (Percentage) |
|
|---|---|
| Demographics | |
| Gender | |
| Women | 312 (92.6%) |
| Men | 25 (7.4%) |
| Age | 41.72 (11.27) |
| Education | |
| High School | 31 (9.2%) |
| Some College | 88 (26.1%) |
| Undergraduate Degree | 128 (38.0%) |
| Masters Degree | 74 (22.0%) |
| Professional Degree | 16 (4.7%) |
| Income | |
| <25,000 | 44 (13.1%) |
| 25,000–49,000 | 80 (23.8%) |
| 50,000–74,000 | 69 (20.5%) |
| 75,000–99,000 | 59 (17.6%) |
| >100,000 | 84 (25.0%) |
| Ethnicity | |
| Caucasian, Non-Hispanic | 307 (91.1%) |
| Other | 30 (8.9%) |
| Employment | |
| Working | 259 (76.9%) |
| Not Working | 78 (23.1%) |
| Migraine Characteristics | |
| Frequency | |
| Chronic Migraine | 54 (16.0%) |
| Episodic Migraine | 283 (84.0%) |
| MIDAS | 19.39 (19.18) |
| Preventive Medication Use | |
| No Preventive Medication | 98 (29.1%) |
| Preventive Medication | 239 (70.9%) |
| Acute Medication Overuse | |
| No Overuse | 279 (82.8%) |
| Any Overuse | 58 (17.2%) |
| Years with Headache | 22.42 (11.77) |
Fifty-four participants (16.0%) met criteria for chronic migraine (Table 1). On average, participants reported a moderate level of disability (MIDAS M = 19.39, SD = 19.18) and reported experiencing headache on average for 22.42 years (SD = 11.77). Fifty-eight participants (17.2%) reported any medication overuse; 45 reported only MSM overuse, 6 reported only Opiate/Barbiturate overuse, and 2 reported only NSAID overuse; 2 reported overuse of both Opiate/Barbiturate and MSM, and 2 reported overuse of NSAID and MSM, and 1 participant reported overuse of all three classes of medication.
Diary Descriptive Statistics
On average, each participant completed 88.20 (SD = 41.94) diary days, for a total of 29,722 diary days. Each participant recorded an average of 29.96 (SD = 21.75) headache days, for a total of 10,097 headache days, with 57.5% of headache days meeting full migraine criteria, 3.1% meeting probable migraine criteria, 19.5% being presumed migraine days due to MSM use, 18.6% meeting tension-type headache criteria, and 1.3% meeting criteria for other headache diagnosis (Table 2). Thus, according to the criteria set forth by this study that days meeting full criteria for migraine, probable migraine, and presumed migraine would be considered “migraine days,” participants recorded a total of 8,090 migraine days, representing 80.0% of all headache days recorded.
Table 2.
Headache Diagnosis by Headache Day
| Diagnosis | Frequency of Headache Days |
Percent of Headache Days |
|---|---|---|
| Migraine | 5801 | 57.5% |
| Probable Migraine | 315 | 3.1% |
| Presumed Migraine (MSM Treated) | 1974 | 19.5% |
| Tension-Type Headache | 1879 | 18.6% |
| Other Headache | 128 | 1.3% |
Participants recorded taking an acute headache medication on a total of 7,803 migraine days, representing 96.5% of the migraine days recorded; no acute medication use was recorded on only 3.5% (n = 287) of migraine days. A triptan or ergot derivative was taken first on 58.0% of migraine days on which an acute medication was recorded; on 29.9% of these days an OTC medication was taken first; and on 12.1% of these days an opiate or barbiturate was taken first (Table 3). Although an opiate or barbiturate was taken first for a minority of migraine days, the majority of participants (n = 218, 64.7%) used an opiate or barbiturate at least once during the study. Participants who used an opiate or barbiturate at least once were more likely to have chronic migraine (21.1%) and higher migraine-related disability (M MIDAS = 21.63, SD = 22.04) than those who never used an opiate or barbiturate (Chronic Migraine = 6.7%; M MIDAS = 15.30, SD – 11.31).
Table 3.
Medication Variables by Migraine Days with Acute Medication
| Frequency of Migraine Days with Acute Medication |
Percent of Migraine Days with Acute Medication |
|
|---|---|---|
| Type of Medication First Used | ||
| MSM | 4526 | 58.0% |
| OTC | 2335 | 29.9% |
| Opiate/Barbiturate | 942 | 12.1% |
| Pain Severity at First Dose† | ||
| Mild | 3213 | 5.6% |
| Moderate | 2945 | 41.8% |
| Severe | 888 | 12.6% |
Taken from the total of 7046 migraine days on which participants recorded pain severity at first dose of medication
On 7,046 (90.3%) of migraine days that participants reported taking an acute headache medication, pain severity at first dose of medication taken was recorded. Of those days, pain was mild 41.2% of the days, moderate 37.7% of the days, and severe 11.4% of the days (Table 3).
Mixed Effect Models for Repeated Measures
Daily headache-related disability
Pain at dosing, F(2, 6460.78) = 1118.88, p < .001, medication, F(2, 519.63) = 93.78, p < .001, and their interaction, F(4, 6336.12) = 58.73, p < .001, were significantly associated with the MIDI. The diary day fixed effect was not significant, F (1, 4000.88) = 0.66, p = .416, indicating that monitoring over time did not significantly influence the MIDI.
Overall, initially dosing with acute headache medication when the pain was mild was associated with lower daily disability than when the pain was moderate or severe; initially dosing when the pain was moderate was also associated with lower daily disability than when pain was severe (Table 4). Overall, initially dosing with an MSM was associated with lower daily disability than an OTC or Opiate/Barbiturate; initially dosing with an OTC was associated with lower disability than an Opiate/Barbiturate (Table 4).
Table 4.
Mixed Effects Models for Repeated Measures
| Fixed Effects | MIDI | Satisfaction with Acute Medication |
|||||
|---|---|---|---|---|---|---|---|
| Estimate | SE | 95% CI* | Estimate | SE | 95% CI* | ||
| Intercept | 7.21 | −.11 | 7.00, 7.41 | 3.77 | 0.20 | 3.38, 4.17 | |
| Diary Day | −0.001 | 0.001 | −0.002, 0.001 | 0.003 | 0.001 | −0.0001, 0.006 | |
| Pain at Dosing | |||||||
| Mild vs. Severe | −3.42 | 0.07 | −3.60, −3.25 | 2.07 | 0.15 | 1.72, 2.42 | |
| Moderate vs. Severe | −1.84 | 0.07 | −2.01, −1.67 | 1.33 | 0.14 | 1.00, 1.66 | |
| Mild vs. Moderate | −1.59 | 0.06 | −1.72, −1.45 | 0.74 | 0.12 | 0.46, 1.02 | |
| Medication Type | |||||||
| OTC vs. MSM | 0.73 | 0.08 | 0.54, 0.93 | −1.59 | 0.19 | −2.05, −1.14 | |
| Opiate/Barbiturate vs. MSM | 1.24 | 0.10 | 1.01, 1.48 | −1.60 | 0.24 | −2.18, −1.02 | |
| OTC vs. Opiate/Barbiturate | −0.51 | 0.10 | −0.76, −0.26 | 0.01 | 0.26 | −0.61, 0.63 | |
| Pain at Dosing X Medication† | |||||||
| Mild Pain at Dosing | OTC vs. MSM | 1.57 | 0.09 | 1.40, 1.75 | −2.61 | 0.20 | −3.10, −2.12 |
| Opiate/Barbiturate vs. MSM | 2.20 | 0.13 | 1.89, 2.52 | −3.03 | 0.31 | −3.77, −2.29 | |
| OTC vs. Opiate/Barbiturate | −0.63 | 0.14 | −0.96, −0.30 | 0.42 | 0.32 | −0.36, 1.20 | |
| Moderate Pain at Dosing | OTC vs. MSM | 0.52 | 0.09 | 0.30, 0.74 | −1.40 | 0.21 | −1.91, −0.90 |
| Opiate/Barbiturate vs. MSM | 1.43 | 0.13 | 1.13, 1.73 | −1.42 | 0.29 | −2.12, −0.73 | |
| OTC vs. Opiate/Barbiturate | −0.91 | 0.13 | −1.23, −0.60 | 0.02 | 0.31 | −0.72, 0.75 | |
| Severe Pain at Dosing | OTC vs. MSM | 0.11 | 0.15 | −0.45, 0.47 | −0.77 | 0.32 | −1.53, −0.01 |
| Opiate/Barbiturate vs. MSM | 0.10 | 0.16 | −0.28, 0.48 | −0.36 | 0.32 | −1.13, 0.41 | |
| OTC vs. Opiate/Barbiturate | 0.01 | 0.17 | −0.40, 0.42 | −0.41 | 0.37 | −1.30, 0.48 | |
| OTC | Mild vs. Severe | −3.15 | 0.12 | −3.44, −2.86 | 1.73 | 0.26 | 1.11, 2.35 |
| Moderate vs. Severe | −2.01 | 0.12 | −2.30, −1.72 | 1.26 | 0.26 | 0.64, 1.88 | |
| Mild vs. Moderate | −1.14 | 0.08 | −1.33, −0.95 | 0.47 | 0.16 | 0.09, 0.85 | |
| Opiate/Barbiturate | Mild vs. Severe | −2.51 | 0.15 | −2.87, −2.14 | 0.90 | 0.31 | 0.17, 1.64 |
| Moderate vs. Severe | −1.09 | 0.14 | −1.43, −0.74 | 0.83 | 0.28 | 0.18, 1.49 | |
| Mild vs. Moderate | −1.42 | 0.14 | −1.75, −1.09 | 0.07 | 0.30 | −0.62, 0.76 | |
| MSM | Mild vs. Severe | −4.61 | 0.10 | −4.85, −4.37 | 3.57 | 0.17 | 3.15, 4.00 |
| Moderate vs. Severe | −2.42 | 0.10 | −2.66, −2.18 | 1.90 | 0.17 | 1.48, 2.31 | |
| Mild vs. Moderagte | −2.20 | 0.06 | −2.34, −2.05 | 1.68 | 0.11 | 1.42, 1.94 | |
Pair-wise comparisons displayed by levels of Pain at Dosing and Medication Use. Comparisons across levels of Pain at Dosing and Medication Use are not displayed.
For pairwise comparisons, 95% Confidence Intervals adjusted using Bonferroni correction
The interaction between pain at initial dosing and medication is depicted in Figure 1. When the pain was mild or moderate, initially dosing with an MSM was associated with lower daily disability than either an OTC or Opiate/Barbiturate and initially dosing with an OTC was also associated with lower daily disability than initially dosing with an Opiate/Barbiturate (Figure 1, Table 4). However, no significant differences in daily disability were detected between initially dosing with an MSM, OTC or Opiate/Barbiturate when the pain was severe (Figure 1, Table 4). For all types of medication, initially dosing when the pain was mild was associated with lower daily disability than when the pain was moderate or severe, and initially dosing when the pain was moderate was associated with lower disability than when the pain was severe (Figure 1, Table 4).
Figure 1.
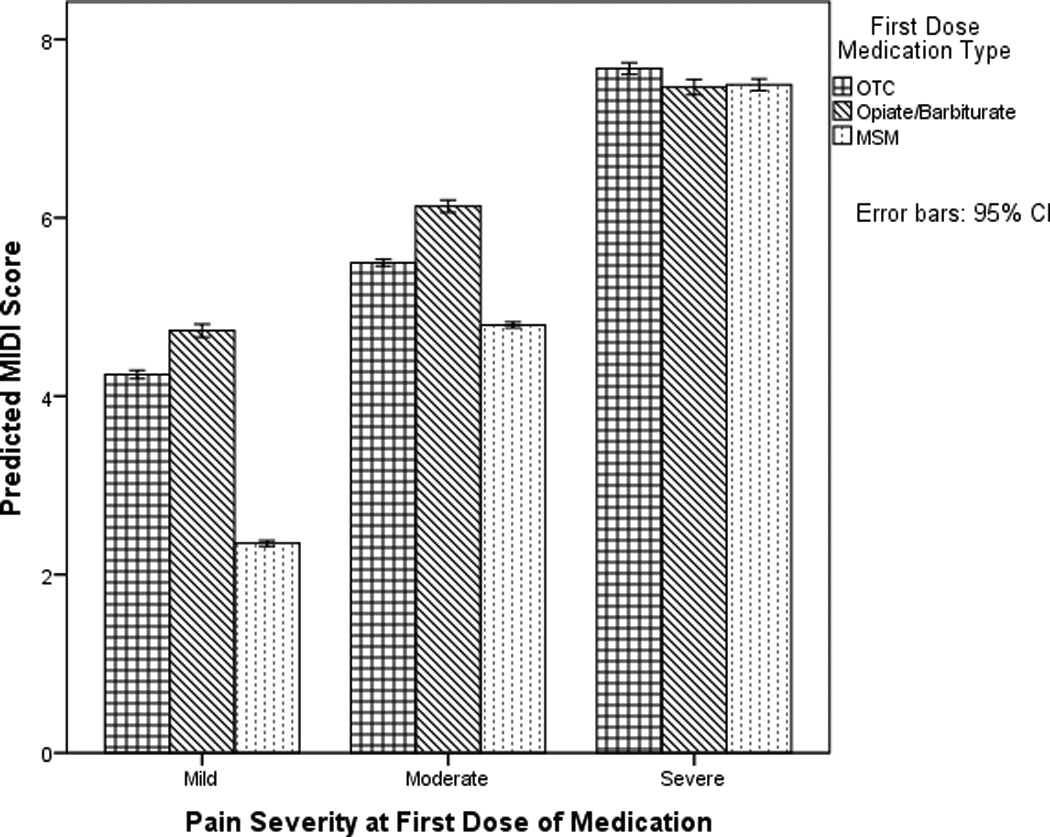
This figure depicts the interaction between pain at initial dosing and medication type on daily Migraine Disability Index (MIDI) scores. Model-predicted MIDI scores are visualized.
Post-hoc subgroup analyses revealed results were broadly comparable among people with episodic and chronic migraine. Among with chronic migraine, MIDI scores slightly increased over time, t(670.49) = .006, p < .001, whereas for people with episodic migraine, MIDI scores slightly decreased over time, t(3177.79) = −.002, p = .013. For chronic migraine, when the pain was moderate, initially dosing with an MSM was no longer significantly associated with lower disability than an OTC (estimate = −0.25, 95% CI = −0.70, 0.19).
Post-hoc subgroup analyses revealed results were broadly comparable among people with and without preventive medication. Among people with no preventive medication, the relative benefit of dosing with an OTC vs. Opiate/Barbiturate when the pain was mild was no longer significant (estimate = −0.18, 95% CI = −.037, 0.72).
Satisfaction with acute medication
Pain at dosing, F(2, 3903.51) = 99.58, p < .001, medication, F(2, 326.55) = 44.53, p < .001, and their interaction, F(4, 3867.36) = 24.00, p < .001, were significantly associated with satisfaction with acute medication. The diary day fixed effect was not significant, F (1, 1842.59) = 3.56, p = .059, but did approach significance; the estimate indicates that there was a small, nonsignificant increase in satisfaction with acute medication over the course of the study (estimate = 0.003, 95% CI = −0.0001, 0.006); inclusion of this effect in the model described above adjusted for this small, non-significant time-related increase.
Overall, initially dosing when the pain was mild was associated with higher satisfaction with the medication than initially dosing when the pain was moderate or severe; initially dosing when the pain was moderate was also associated with higher satisfaction than initially dosing when pain was severe (Table 4). Overall, initially dosing with an MSM was associated with higher satisfaction than initially dosing with an OTC or Opiate/Barbiturate; no difference in satisfaction was reported between initially dosing with an OTC or Opiate/Barbiturate (Table 4).
The interaction between pain at dosing and medication is depicted in Figure 2. Initially dosing with an MSM when the pain was mild or moderate was associated with higher satisfaction than an OTC or Opiate/Barbiturate when the pain was mild or moderate; no difference in satisfaction was detected between an OTC or Opiate/Barbiturate when the pain was mild or moderate (Figure 2, Table 4). Initially dosing with an MSM when the pain was severe was associated with higher satisfaction than initially dosing with an OTC; no difference in satisfaction was detected between initially dosing with an MSM or Opiate/Barbiturate when the pain was severe. For MSM and OTC, initially dosing when the pain was mild was associated with higher satisfaction than initially dosing when the pain was moderate or severe; similarly, initially dosing with an MSM or OTC when the pain was moderate was associated with higher satisfaction than initially dosing with an MSM or OTC when the pain was severe (Figure 2, Table 4). Initially dosing with an Opiate/Barbiturate when the pain was severe was associated with lower satisfaction than initially dosing with an Opiate/Barbiturate when the pain was mild or moderate; no difference in satisfaction was detected between an Opiate/Barbiturate when the pain was mild or moderate (Figure 2, Table 4).
Figure 2.
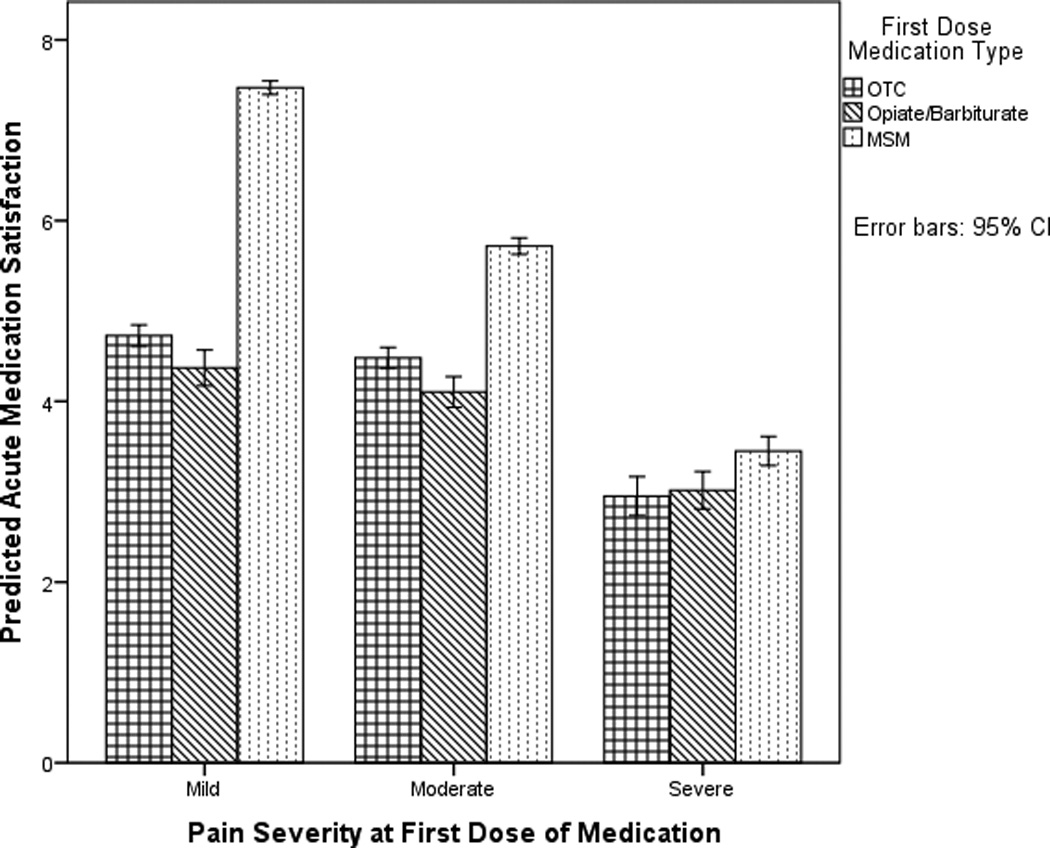
This figure depicts the interaction between pain at initial dosing and medication type on satisfaction with the initial acute medication. Model-predicted acute medication satisfaction is visualized.
Post-hoc subgroup analyses found comparable results among people with chronic and episodic migraine, and among people with no preventive medication and with preventive medication.
Discussion
Overall, this naturalistic daily electronic diary study found that adhering to current acute migraine medication recommendations was associated with lower daily disability and higher patient satisfaction with acute migraine medication. Initially dosing with an MSM while the pain was mild was associated with lower daily disability and higher patient satisfaction compared to other medication-taking strategies, implying that this approach may be the optimal acute medication-taking strategy. Overall, using an MSM (triptan or ergot) first was associated with notably lower migraine-related disability on that day, and higher satisfaction with the medication, than using either OTC or Opiate/Barbiturate class medications. Further, for all medication types, initially dosing when the pain was mild was associated with the lowest reported disability and the highest reported patient satisfaction.
These data suggest that timing of acute medication use is critical to reducing daily migraine disability and increasing satisfaction with acute medication, across the variety of acute medication types observed in this study. The type of medication used initially to treat any given migraine attack may be driven by medication-related factors (MSM may be contraindicated, MSM may have previously failed, and formulary restrictions for MSM) and patient factors (not having an MSM on hand, not recognizing the episode as a migraine, preferring other medication types, wanting to save the MSM for a “bad” headache, and frequent migraines leading to concerns about medication overuse). In this study, initially dosing with any medication type while the pain was mild was associated with lower daily migraine disability, and higher patient satisfaction with treatment, than initially dosing while the pain is severe.
These data also suggest that the relative benefit of choosing one type of acute migraine medication over another may depend on pain severity at the time of initial dosing, at least for outcomes of daily migraine-related disability and satisfaction with acute medication. If initially dosing when the pain is mild or moderate, taking an MSM was associated with lower disability than OTCs, and both MSM and OTCs were associated with lower disability than an opiate/barbiturate. This difference was most pronounced when the acute medication was taken while the pain was still mild. However, the relative benefit of using an MSM or OTC rather than an opiate/barbiturate disappeared if the participants initially dosed when the pain was severe. These data indicate the combination of initially dosing with an MSM and doing so while the pain is still mild, is associated with lowest reported daily migraine-related disability and highest satisfaction with acute medication in this naturalistic examination of people with migraine.
Acute medication-taking strategies varied widely across participants. These results provide continuing evidence that assessing how people with migraine take acute medications is a key aspect of clinical care and clinical research. Further, participants in this study reported many migraine days with poor adherence to current acute migraine medication recommendations. This emphasizes the need for interventions to improve patient adherence to physician recommendations regarding acute medication use. Optimal migraine management places significant burden on the patient, and requires in-the-moment decision-making type and timing of acute medication dosing for each migraine episode. Although patients describe numerous barriers to performing these recommendations (12), this study suggests that adhering to these recommendations is associated with the lowest migraine-related disability and highest patient satisfaction in a naturalistic setting. This data aligns with previous research that suggests adherence to these recommendations is associated with better clinical outcomes (e.g., pain freedom at 2 hours) (18–21) and potentially reduces chronification (22, 23).
This study has a number of strengths. Data were naturalistic, allowing us to evaluate typical day-to-day variations in acute medication-taking behavior, migraine-related disability and satisfaction with acute medication. Participants represented a broad sample of people with migraine and were recruited across both specialty and primary care settings in a large health system in the United States. Data collection was intensive and specific to the stated purpose of this study; on each headache day, participants reported migraine-related disability, acute medication use, and satisfaction with acute medication. This resulted in a large number of longitudinal observations, both within and between participants, designed specifically to answer questions regarding acute migraine medication adherence and relationships with daily variations in migraine disability and patient satisfaction (rather than a secondary analysis of a study primarily designed to answer questions regarding acute medication efficacy). Thus, results reflected the intra-person, as well as inter-person, variation in medication-taking patterns, daily migraine-related disability and satisfaction with acute medication use over the course of three months. Repeated measures mixed effects models allowed us to take into account person-level variance in the intercept and medication use and any changes in the outcome variable across time due to monitoring or treatment.
On the other hand, naturalistic data limits causal interpretation of findings. Higher satisfaction with acute medication observed on days when participants reported initially dosing with an MSM may occur simply because people who respond best to MSMs tend to take them first. Similarly, the higher migraine-related disability observed on days when participants reported taking opiates or barbiturates first may simply be because people who take opiates or barbiturates have more severe (and therefore more disabling) migraine than people who do not take these classes of medications. Although people who used an opiate or barbiturate at least once over the course of the study were in the majority, they were more likely to have chronic migraine and report higher migraine-related disability; thus, these patients may differ qualitatively compared to patients who never used an opiate or barbiturate. These results may not be generalizable to people with migraine where acute medication use requires restraint, such as medication overuse headache, formulary restrictions, or significant medication side effects. In these cases, the use of a more nuanced and stratified acute treatment paradigm according to attack severity would be a key component of a more comprehensive treatment plan (24). Further, participants in this study, on average, had headache for over two decades; these results may not generalize to patients who are newly prescribed acute migraine medications.
Patient adherence is a prerequisite to attaining clinical response to acute migraine medication and to properly gauge therapeutic responsiveness. It is therefore of critical importance to 1) empirically examine factors associated with each aspect of patient adherence with acute migraine medications, 2) develop and test behavior change interventions to improve patient adherence with acute migraine medications, and 3) disseminate these interventions into practices which prescribe acute migraine medications.
Clinical Implications.
All medication types were associated with the lowest reported disability and the highest reported patient satisfaction if taken while head pain was still mild.
Initially dosing with an MSM (triptan or ergot) was associated with lower migraine-related disability on that day, and higher satisfaction with acute medication, than initially using either an OTC or Opiate/Barbiturate.
Clinicians should routinely assess how patients are taking acute medications for migraine, and directly address suboptimal adherence when it arises in clinical practice.
Acknowledgments
This work was supported by the NINDS (NS048288: PI Nicholson; 1K23NS096107-01: PI Seng). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank the Mercy Health Research and Mercy Clinic Headache Center clinicians and staff who assisted with all aspects of this study from subject recruitment to data management.
Dr. Seng receives research support from the National Institute of Health [1K23NS096107-01 (PI: Seng)] and the International Headache Academy (PI: Seng); she serves as a consultant or has received honoraria or travel funding from the American Academy of Neurology, American Psychological Association Commission on Accreditation, and GlaxoSmithKline.
Dr. Robbins has received honoraria for educational activities with the American Headache Society, Springer, and book royalties from Wiley.
Footnotes
Conflict of Interest Statement
Dr. Nicholson has nothing to disclose.
References
- 1.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 2.Lipton R, Bigal M, Diamond M, Freitag F, Reed M, Stewart W. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343–349. doi: 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- 3.Leonardi M, Raggi A. Burden of migraine: international perspectives. Neuro Sci. 2013;34:S117–S118. doi: 10.1007/s10072-013-1387-8. [DOI] [PubMed] [Google Scholar]
- 4.Munakata J, Hazard E, Serrano D, Klingman D, Rupnow MF, Tierce J, et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2009;49:498–508. doi: 10.1111/j.1526-4610.2009.01369.x. [DOI] [PubMed] [Google Scholar]
- 5.Landy SH, Runken MC, Bell CF, Higbie RL, Haskins LS. Assessing the impact of migraine onset on work productivity. J Occup Env Med. 2011;53:74–81. doi: 10.1097/JOM.0b013e31812006365. [DOI] [PubMed] [Google Scholar]
- 6.Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache. 2007;47:355–363. doi: 10.1111/j.1526-4610.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 7.Holland S, Silberstein SD, Freitag F, Dodick DW, Argoff C, Ashman E. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346–1353. doi: 10.1212/WNL.0b013e3182535d0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silberstein S. Practice Parameter: evidence-based guidelines for migraine headache (an evidence-based review) Neurology. 2000;55:754–762. doi: 10.1212/wnl.55.6.754. [DOI] [PubMed] [Google Scholar]
- 9.Worthington I, Pringsheim T, Gawel MJ, Gladstone J, Cooper P, Dilli E, et al. Canadian Headache Society Guideline: acute drug therapy for migraine headache. Can J Neurol Sci. 2013;40:S1–S80. [PubMed] [Google Scholar]
- 10.Ramsey RR, Ryan JL, Hershey AD, Powers SW, Aylward BS, Hommel KA. Treatment adherence in patients with headache: a systematic review. Headache. 2014;54:795–816. doi: 10.1111/head.12353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters M, Abu-Saad HH, Vydelingum V, Dowson A, Murphy M. Patients’ decision-making for migraine and chronic daily headache management. A qualitative study. Cephalalgia. 2003;23:833–841. doi: 10.1046/j.1468-2982.2003.00590.x. [DOI] [PubMed] [Google Scholar]
- 12.Seng EK, Holroyd KA. Optimal use of acute headache medication: a qualitative examination of behaviors and barriers to their performance. Headache. 2013;53(9):1438–1450. doi: 10.1111/head.12157. [DOI] [PubMed] [Google Scholar]
- 13.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. 2nd. Suppl 1. Vol. 24. Cephalalgia: 2004. pp. 9–160. [DOI] [PubMed] [Google Scholar]
- 14.Stewart WF, Lipton RB, Kolodner K, Liberman J, Sawyer J. Reliability of the migraine disability assessment score in a population-based sample of headache sufferers. Cephalalgia. 1999;19:107–114. doi: 10.1046/j.1468-2982.1999.019002107.x. [DOI] [PubMed] [Google Scholar]
- 15.Stewart WF, Lipton RB, Whyte J, Dowson A, Kolodner K, Liberman JN, et al. An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology. 1999;53:988–994. doi: 10.1212/wnl.53.5.988. [DOI] [PubMed] [Google Scholar]
- 16.Lipton RB, Hamelsky SW, Kolodner KB, Steiner TJ, Stewart WF. Migraine, quality of life, and depression: a population-based case-control study. Neurology. 2000;55:629–635. doi: 10.1212/wnl.55.5.629. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson RA, Chibnall JCWBJ, Smith TR. Annual Scientific Meeting of the American Headache Society. Washington, DC: 2011. The Migraine Disaility Index (MIDI): A new measure of within-attack migraine-related disability. [Google Scholar]
- 18.Cady RK, Lipton RB, Hall C, Stewart WF, O’Quinn S, Gutterman D. Treatment of mild headache in disabled migraine sufferers: results of the Spectrum Study. Headache. 2000;40:792–797. doi: 10.1046/j.1526-4610.2000.00144.x. [DOI] [PubMed] [Google Scholar]
- 19.Cady RK, Sheftell F, Lipton RB, O’Quinn S, Jones M, Putnam DG, et al. Effect of early intervention with sumatriptan on migraine pain: retrospective analyses of data from three clinical trials. Clin Ther. 2000;22:1035–1048. doi: 10.1016/s0149-2918(00)80083-1. [DOI] [PubMed] [Google Scholar]
- 20.Pascual J, Cabarrocas X. Within-patient early versus delayed treatment of migraine attacks with almotriptan: the sooner the better. Headache. 2002;42:28–31. doi: 10.1046/j.1526-4610.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- 21.Mathew NT, Kailasam J, Meadors L. Early treatment of migraine with rizatriptan: a placebo-controlled study. Headache. 2004;44:669–673. doi: 10.1111/j.1526-4610.2004.04125.x. [DOI] [PubMed] [Google Scholar]
- 22.Bigal ME, Lipton RB. What predicts the change from episodic to chronic migraine? Curr Opin Neurol. 2009;22:269–276. doi: 10.1097/WCO.0b013e32832b2387. [DOI] [PubMed] [Google Scholar]
- 23.Bigal ME, Lipton RB. Overuse of acute migraine medications and migraine chronification. Curr Pain Headache Rep. 2009;13:301–307. doi: 10.1007/s11916-009-0048-3. [DOI] [PubMed] [Google Scholar]
- 24.Becker WJ. Acute migraine treatment in adults. Headache. 2015;55:778–793. doi: 10.1111/head.12550. [DOI] [PubMed] [Google Scholar]


