Abstract
Despite continued research among men with more sexual partners, little information exists on their seroadaptive behavior. Therefore, we examined seroadaptive anal sex strategies among 719 Vancouver gay and bisexual men (GBM) recruited using respondent driven sampling (RDS). Our objectives were to (1) describe the distribution in frequency of male sexual partnering among Vancouver GBM, and (2) identify important covariates associated with the number of male sexual partners. To this aims, we provide descriptive, univariate, and multivariate adjusted statistics, stratified by HIV status, for the association between having ≥7 male anal sex partners in the past six months (Population Q3, versus <7). Sensitivity Analysis were also performed to assess the robustness of this cut-off point. Results suggest that GBM with more sexual partners are more likely to employ seroadaptive strategies than men with fewer partners. These strategies may be used in hopes of offsetting risk, assessing needs for subsequent HIV testing, and balancing personal health with sexual intimacy. Further research is needed to determine the efficacy of these strategies, assess how GBM perceive their efficacy, and understand the social and health impacts of their widespread uptake.
Keywords: Strength-based, Seroadaptive, HIV, Gay and Bisexual Men, Highly Sexually Active Men
INTRODUCTION
Recent global reviews of HIV literature have demonstrated continued disparities in the sexual health and wellbeing of gay and bisexual men (GBM) (1,2). While many structural and social factors have been explored to explain the disproportionate burden of HIV/STI rates this population faces (3), researchers have often pointed to differences in the sexual partnering patterns of GBM compared with other men and women (4,5). Specifically, studies have shown that, on average, GBM are less likely to discriminate against sexual partners based on age, have greater numbers of lifetime and annual sexual partners, continue to form new partnerships later in life, and report higher prevalence of partner concurrency (6). While these characteristics may contribute to the broader social and cultural identity of GBM, the density and interconnectedness observed in these sexual networks may also play a role in HIV transmission (7,8). That is, men with greater numbers of male anal sex partners may be at greater risk for HIV transmission than men with fewer sexual partners (9–11).
Despite the continued attention that men with more sex partners receive from the academic and public health communities, there is little research on the seroadaptive behavioral strategies of this group. Therefore, GBM and their sexual practices continue to face stigma from what has colloquially been described as “slut-shaming” (12). Such attitudes towards these men and their relationship patterns inhibit constructive communication about sexual risk (13). In contrast, sex-positive community engagement approaches could promote risk reduction strategies which help men with greater numbers of male anal sex partners avoid seroconversion or transmission, while being supportive of the diversity of values and needs found within the GBM communities. Such approaches have focused on reducing the risks associated with anal sex, as it is the most common route of transmission for HIV among GBM (14). While some of these risk reduction strategies are empirically supported (e.g., condom use), other perceived harm reduction strategies may not be fully effective in preventing the transmission of HIV (e.g., withdrawal, negotiated safety agreements) (15). Seroadaptive behaviors, defined as “any modification of sexual behavior based on the person’s (perceived) serostatus, the (perceived) status of the partner and/or HIV transmission risk by type of sex act,” (16), can be broadly divided into three categories: First, those seeking seroconcordant partnerships (i.e., serosorting, where both partners have the same HIV serostatus); Second, those aiming to reduce the probability of HIV transmission (e.g., anal sex abstention, strategic positioning); and Third, those attempting to limit exposure to the virus (e.g., condom use, treatment/viral load sorting) (16). These seroadaptive practices are widely practiced among GBM (17,18) and have been cited as evidence of GBM adaptive resilience in preventing HIV seroconversion (19).
In Vancouver, expanded access to highly active antiretroviral therapy (HAART), and associated individual- and population-level viral load reduction (referred to as “Treatment as Prevention (TasP)”) (20,21), may serve an important role in changing population patterns of these behaviors and practices (18). This closely relates to the theory of risk homeostasis which suggests decreased perceptions of risk corresponding to increased risk-taking behavior (22). This has raised concerns that the benefits associated with biological HIV prevention technologies may be offset by reduced condom use (23). If such is the case, the reinforcement of seroadaptive behaviors along with condom use will serve an important role in helping men with greater numbers of male anal sex partners to manage their sexual health.
When combined with condom use, serosorting and strategic positioning were estimated in one prospective cohort study to reduce HIV transmission among GBM by as much as 98% (24). However, more conservative estimates suggest that these strategies result in a 38% to 83% reduction of risk, depending on the strategy being employed (15). Of course, seroadaptive effectiveness requires low prevalence of undiagnosed STI/HIV infection coupled with high levels of partner disclosure and accurate HIV testing (17,25). Further, the effectiveness of seroadaptive strategies relies on an accurate understanding of the protective benefits – or lack thereof – associated with each strategy. For example, while withdrawal may be thought by some men to prevent transmission of HIV, this method is actually associated with a fivefold increase in seroconversions compared with no condomless anal sex (26) and is not considered an effective risk reduction strategy for those engaging in condomless anal sex because HIV is transmissible through pre-ejaculate (27,28).
Consistent with these observations and our understanding of GBM adaptation and resilience, we hypothesized that men with the highest quartile number of male anal sex partners were more likely to utilize seroadaptative behaviors to ameliorate the risks associated with having more sexual partners. We aimed to develop findings that would help shape sex-positive HIV-prevention messaging that will simultaneously reduce the stigma these men face and better encourage the use of more effective risk reduction strategies. To accomplish these goals, we examined reported seroadaptive strategies and individual characteristics relating to HIV-negative and HIV-positive men with the highest quartile number of male anal sex partners in Vancouver, British Columbia, Canada.
METHODS
Study Setting
The Momentum Health Study is a prospective biobehavioral cohort study investigating possible Treatment Optimism (29) and risk compensation associated with British Columbia’s expanded Treatment as Prevention program (21). All data used in this analysis are drawn from the baseline study visit, which occurred between February 2012 and February 2014. Momentum uses respondent-driven sampling (30) to recruit HIV-positive and HIV-negative GBM in Metro Vancouver, British Columbia, Canada. Initially-recruited “seeds” distributed a maximum of 6 paper and/or electronic vouchers to other Vancouver GBM (31). Voucher recipients were screened for study eligibility criteria, which included being 16 years of age and older, identifying as male, having had sex with another man in the past six months, living in Metro Vancouver, and competency to understand a questionnaire written in English. Eligible participants completed a computer-assisted self-interview (CASI) questionnaire and biological tests including point-of-care HIV testing, venipuncture blood tests for hepatitis C and syphilis serology, and optional urine and swab tests for gonorrhoea and chlamydia. Study participants received a $50 CAD honorarium and earned an additional $10 CAD for each eligible recruit who completed the study protocol. All procedures received human ethics clearances from Simon Fraser University, the University of British Columbia, and the University of Victoria. Additional information about the Momentum study questionnaire and protocol can be found elsewhere (32,33).
Dependent Variables
The study’s dependent variable was a dichotomous categorical variable distinguishing men with high numbers of anal sex partners from other men in the sample. While other sources in the literature have described this group as “Highly Sexual Active Men” (9), we have attempted to describe sexual partnering and concurrency with greater sensitivity to the stigma that this group faces, as well as to more accurately describe their partnering pattern compared with frequency of sexual activity. To identify men with higher numbers of sexual partners, we asked this question: “During the past 6 months, with how many males have you had anal sex with (as top or bottom)?” From the resulting distribution, with the total number of anal sex partners in the past six months capped at 100, we used the global third quartile value to divide our sample first into two groups: 1) men with ≥7 anal sex partners (≥Q3, n=195), and 2) men with <7 recent anal sex partners (<Q3, n=523). These results are more conservative than those used by other researchers (9,11), which focused on men having 9 or more anal sex partners in the previous three months. To assess the use of our ≥Q3 versus <Q3 cut-off point, a sensitivity analysis was conducted at the univariate level with cut-off points at the global median (≥3 versus <3) as well as HIV status specific third quartile cut-offs (HIV-positive: ≥15 versus <15, HIV-negative: ≥6 versus <6).
Independent Variables
Seroadaptive strategies were represented in the study questionnaire (32) by a series of yes/no responses with necessary variation in the question wording to be applicable to HIV-negative versus HIV-positive participants. The question block was introduced by saying, “Some guys use strategies to prevent getting/transmitting HIV. Do you do any of the following to prevent getting/transmitting HIV? (check ALL that apply).” Three strategies were asked the same for HIV-negative and HIV-positive men: condom use (“Always using condoms for anal sex”), anal sex avoidance (“Having sex which doesn’t include anal sex“), and serostatus inquiry (“Asking my sex partners about their HIV status before sex”). Four strategies were asked in a manner specific to participants’ serostatus: HIV-negative men were asked if they used strategic positioning (“Being a top for anal sex”), serosorting (“Having anal sex without condoms only with guys I know are HIV-negative”), viral load sorting (“Having anal sex without condoms with HIV-positive guys who have low viral loads or are on HIV treatment”), and withdrawal (“not letting my sex partners cum inside me”). Likewise, HIV-positive men were asked about strategic positioning (“Being the bottom for anal sex”), serosorting (“Having anal sex without condoms only with guys I know are HIV-positive”), viral load sorting (“Having anal sex without condoms if my viral load is low or I’m on HIV treatment”), and withdrawal (“Not cumming inside my sex partners”).
Additional independent variables pertained to sociodemographic factors, substance use, psychosocial traits, and sexual behavior in order to gain insights into the characteristics of men with the highest quartile number of male anal sex partners and to assess possible confounding variables. Socio-demographic variables included measures of age, education, annual income, race/ethnicity, residence, sexual orientation, and anal sex role preference (i.e., top, bottom or versatile). Substance use questions pertaining to alcohol use classified participants as “harmful drinkers” via the AUDIT Scale (34) and whether they used erectile dysfunction drugs (EDD), poppers, crystal methamphetamine, and/or Ecstasy in the past six months. Previously reported associations between EDD and crystal methamphetamine (35,36), led us to test the inclusion of an interaction term (EDD × crystal methamphetamine).
Psychosocial measures included three validated scales. The first was the Escape Motivation Scale (12 questions, study Cronbach’s α = 0.90) (37), assessing if GBM used alcohol and illicit substances to diminish cognitive recognition of sexual risk, (e.g., “When I am high, I find it difficult to stay within my sexual limits”). Each item of the Escape Motivation Scale was scored on a 4-point Likert scale (Strongly disagree, Disagree, Agree, Strongly Agree), meaning the total score possible was 12–48 points with higher scores indicating greater escape motivations. The second was the Sexual Sensation Seeking Scale (revised, 11 questions, study α = 0.73) (38), which measured respondents’ attitudes towards sexual thrill-seeking (e.g., “I like wild, ‘uninhibited’ sexual encounters”). Each item on the Sexual Sensation Seeking Scale was scored on a 4-point Likert Scale (Not at all like me, Not like me, Like me, Very much like me.), meaning the total score possible was 11–44 points, with higher scores indicates greater sensation seeking. The last scale used was the Treatment Optimism Scale (12 questions, study α = 0.85) (39), examining possible changing sexual risk perceptions associated with (e.g., “HIV/AIDS is a less serious threat than it used to be because of new treatments”). Each item on the treatment optimism scale was scored on a 4-point Likert scale (Strongly disagree, Disagree, Agree, Strongly Agree), meaning the total score possible was 12–48 points with higher scores indicating greater treatment optimism.
Analysis
All analyses were conducted using SAS version 9.3 (40) and stratified by HIV serostatus. The data for this analysis were adjusted by the respondent driven sampling program (RDSAT) version 7.1.46 to generate point estimates and 95% confidence intervals (41). Independent variables with probability values < 0.20 were selected from initial univariable models for inclusion in subsequent multivariable models. Final multivariable models were determined using backward selection elimination procedure based on the optimisation (minimization) of two criteria at each step: Akaike Information Criterion (AIC) (42) and Type-III p-values (43). These two criteria balance model selection between finding an explanatory model with lower p-values (indicating greater significance) and lower AIC values (indicating goodness-of-fit). This model building procedure is described in greater detail elsewhere (44). A central premise of RDS is that respondents’ social network size can be used to estimate sampling probabilities and generate population estimates (45).
RESULTS
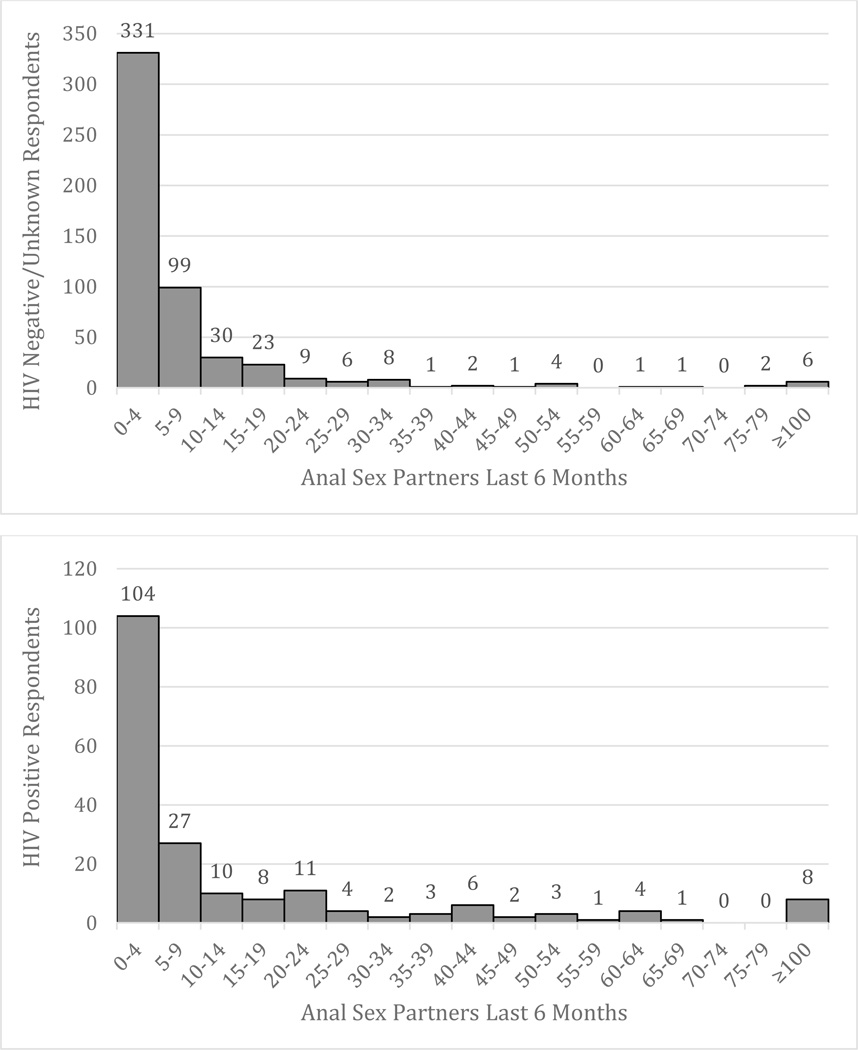
Between February 2012 and February 2014, we recruited a total of 719 GBM from 119 (16.6%) seeds or initial recruits. Table 1 provides descriptive socio-demographic and behavioral statistics of the cross-sectional study population, which was predominantly composed of white (68.0%), gay (80.3%) men with a median age of 33 years (Q1–Q3: 26–47). Approximately three-quarters (76.7%) of the sample were HIV-negative/unknown. Behavioral reports indicated that 65.1% had participated in insertive anal sex in the past six months, and 63.5% participated in receptive anal sex. One in three men reported a preference for receptive anal sex (35.9%) and 27.6% reported a versatile preference. The distribution of the number of sexual partners is shown in Figure 1. The median number of anal sex partners in the past 6 months reported by respondents was 3 (Q1–Q3: 1–7). Popper use in the past six months was reported by 34.3% of men. Other substance use reports included crystal methamphetamine (19.6%), EDD (17.3%), and Ecstasy (18.9%).
Table I.
Selected Descriptive Statistics for Overall Sample
| Categorical Variables | n | % | RDSa % | RDSa % 95% CIb |
||
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Ethnicity | ||||||
| White | 539 | 75.0 | 68.0 | 60.6 | 74.4 | |
| Asian | 72 | 10.0 | 9.9 | 6.2 | 14.8 | |
| Aboriginal | 50 | 7.0 | 10.3 | 5.6 | 16.2 | |
| Other | 58 | 8.1 | 11.8 | 7.0 | 17.1 | |
| Sexual Identity | ||||||
| Gay | 612 | 85.1 | 80.3 | 75.6 | 85.2 | |
| Bisexual/Other | 107 | 14.9 | 19.7 | 14.8 | 24.4 | |
| Education | ||||||
| Less than high school | 61 | 8.7 | 14.2 | 9.5 | 19.5 | |
| Completed/more than high school | 644 | 91.4 | 85.8 | 80.5 | 90.5 | |
| Neighborhood | ||||||
| Downtown | 356 | 49.5 | 51.8 | 43.8 | 59.2 | |
| Vancouver | 223 | 31.0 | 30.5 | 24.0 | 36.7 | |
| Outside Vancouver | 140 | 19.5 | 17.7 | 13.0 | 24.1 | |
| Annual Income | ||||||
| < 30K | 457 | 63.6 | 74.5 | 69.8 | 79.9 | |
| 30–60K | 182 | 25.3 | 17.2 | 13.2 | 20.9 | |
| ≥ 60K | 80 | 11.1 | 8.3 | 5.2 | 11.8 | |
| Self-reported HIV Status | ||||||
| Negative/Unknown | 524 | 72.9 | 76.9 | 68.8 | 84.4 | |
| Positive | 195 | 27.1 | 23.1 | 15.6 | 31.2 | |
| Sexual Behaviors | ||||||
| Unprotected Anal Sex in P6Md | ||||||
| No | 256 | 36.4 | 38.2 | 32.8 | 45.4 | |
| Yes | 185 | 26.3 | 26.0 | 20.6 | 31.6 | |
| High Risk Sex | 262 | 37.3 | 35.8 | 29.1 | 41.4 | |
| Insertive Anal Sex in P6Md | ||||||
| No | 207 | 28.8 | 34.9 | 28.8 | 41.7 | |
| Yes | 512 | 71.2 | 65.1 | 58.3 | 71.2 | |
| Receptive Anal Sexin P6Md | ||||||
| No | 235 | 32.7 | 36.5 | 30.7 | 42.6 | |
| Yes | 484 | 67.3 | 63.5 | 57.4 | 69.3 | |
| Anal Sex Preference | ||||||
| Bottom | 241 | 35.1 | 35.9 | 29.5 | 41.3 | |
| Versatile | 193 | 28.1 | 27.6 | 22.3 | 33.4 | |
| Top | 253 | 36.8 | 36.5 | 31.5 | 42.8 | |
| No anal | ||||||
| Attended Group Sex Event in P6Md | ||||||
| No | 539 | 75.0 | 78.6 | 73.8 | 83.7 | |
| Yes | 180 | 25.0 | 21.4 | 16.3 | 26.2 | |
| Received Money in Exchange for Sex in P6Md | ||||||
| No | 655 | 91.2 | 89.9 | 85.2 | 94.3 | |
| Yes | 63 | 8.8 | 10.1 | 5.7 | 14.8 | |
| Substance Use | ||||||
| AUDITe Harmful Drinker | ||||||
| Yes | 243 | 34.0 | 31.2 | 25.2 | 37.0 | |
| Used EDDf in P6Md | ||||||
| Yes | 162 | 22.5 | 17.3 | 12.2 | 21.6 | |
| Used methamphetamine in P6Md | ||||||
| Yes | 136 | 18.9 | 19.6 | 13.7 | 25.4 | |
| Used Poppers in P6Md | ||||||
| Yes | 266 | 37.0 | 34.3 | 28.7 | 40.3 | |
| Used Ecstasy in P6Md | ||||||
| Yes | 176 | 24.5 | 18.9 | 14.2 | 24.0 | |
| Continuous Variables | ||||||
| Median | Q1, Q3g | |||||
| Age | 33 | 26, 47 | ||||
| HAARTc Treatment Optimism Scale (n=716) | 25 | 21, 28 | ||||
| Sexual Sensations Scale (n=698) | 31 | 28, 34 | ||||
| Cognitive Escape Scale (n=705) | 29 | 25, 33 | ||||
Respondent Driven Sampling
Confidence Interval
Highly Active Antiretroviral Therapy
Past Six Months
Alcohol Use Disorder Identification Test
Erectile Dysfunction Drugs
Quartile 1, Quartile 3
Figure 2.
Distribution of the number of reported male anal sex partners in the past 6 months for HIV-negative (top) and HIV-positive (bottom) men.
Descriptive statistics and univariable results for HIV-negative/unknown GBM are provided in Table 2. Among HIV-negative/unknown men with the highest quartile of male anal sex partners, 70.6% reported asking HIV status before sex (versus 56.3% for lower three quartiles), 62.8% reported always using condoms (versus 67.2%), 36.5% reported strategic positioning (versus 18.7%), 32.3% reported serosorting (versus 30.2%), 27.2% reported withdrawal (versus 22.2%), 25.2% reported abstaining from anal sex (versus 47.8%), and 17.6% reported treatment/viral load sorting (versus 5.4%). In univariable analysis, HIV-negative men with the highest quartile number of male anal sex partners were more likely to report these strategies: asking partners their status (OR=1.86, 95% CI [1.25 2.87]), strategic positioning (OR= 2.55, 95%CI [1.61, 3.90]), and treatment/viral load sorting (OR=3.78, 95%CI [2.17,7.69]), but were less likely to report abstention from anal sex (OR=0.37, 95%CI [0.23,0.58]) compared with those in the lower three quartiles of male anal sex partners (See Table 2).
Table II.
Univariable associations with the highest quartile of male anal sex partners among HIV-negative or unknown status men
| <7 Anal Sex Partners (n=398) |
≥7 Anal Sex Partners (n=126) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Categorical Variables | n | % | RDSa% | n | % | RDSa% | ORb | 95% CIc | p-value | ||
| Demographics | |||||||||||
| Ethnicity | |||||||||||
| White | 295 | 74.1 | 69.6 | 95 | 75.4 | 70.5 | 1.00 | 0.482 | |||
| Asian | 51 | 12.8 | 11.8 | 9 | 7.1 | 8.5 | 0.71 | 0.35 | 1.45 | ||
| Aboriginal | 20 | 5.0 | 6.3 | 9 | 7.1 | 9.6 | 1.51 | 0.73 | 3.98 | ||
| Other | 32 | 8.0 | 12.3 | 13 | 10.3 | 11.4 | 0.92 | 0.48 | 1.73 | ||
| Sexual Identity | |||||||||||
| Gay | 331 | 83.2 | 79.0 | 114 | 90.5 | 92.9 | 1.00 | <0.001 | |||
| Bisexual/Other | 67 | 16.8 | 21.0 | 12 | 9.5 | 7.1 | 0.29 | 0.14 | 0.60 | ||
| Education | |||||||||||
| Greater than high school | 23 | 5.9 | 9.0 | 15 | 12.3 | 21.5 | 1.00 | <0.001 | |||
| Some/completed high school | 369 | 94.1 | 91.0 | 107 | 87.7 | 78.5 | 0.36 | 0.29 | 0.63 | ||
| Neighborhood | |||||||||||
| Downtown | 155 | 38.9 | 42.4 | 67 | 53.2 | 54.1 | 1.00 | 0.010 | |||
| Vancouver | 153 | 38.4 | 34.5 | 34 | 27.0 | 20.4 | 0.46 | 0.28 | 0.77 | ||
| Outside Vancouver | 90 | 22.6 | 23.1 | 25 | 19.8 | 25.5 | 0.86 | 0.53 | 1.49 | ||
| Annual Income | |||||||||||
| < 30K | 246 | 61.8 | 71.6 | 67 | 53.2 | 60.7 | 1.00 | 0.051 | |||
| 30–60K | 102 | 25.6 | 17.5 | 40 | 31.8 | 26.6 | 1.79 | 1.16 | 2.95 | ||
| ≥ 60K | 50 | 12.6 | 10.9 | 19 | 15.1 | 12.7 | 1.37 | 0.73 | 2.57 | ||
| Seroadaptive Sexual Behaviors | |||||||||||
| Strategy: Always Using Condoms | |||||||||||
| No | 130 | 32.9 | 32.8 | 55 | 44.0 | 37.2 | 1.00 | 0.358 | |||
| Yes | 265 | 67.1 | 67.2 | 70 | 56.0 | 62.8 | 0.82 | 0.54 | 1.25 | ||
| Strategy: Strategic Positioning | |||||||||||
| No | 311 | 78.7 | 81.3 | 71 | 56.8 | 63.5 | 1.00 | <0.001 | |||
| Yes | 84 | 21.3 | 18.7 | 54 | 43.2 | 36.5 | 2.55 | 1.61 | 3.90 | ||
| Strategy: Anal Sex Avoidance | |||||||||||
| No | 183 | 46.3 | 52.2 | 79 | 63.2 | 74.8 | 1.00 | <0.001 | |||
| Yes | 212 | 53.7 | 47.8 | 46 | 36.8 | 25.2 | 0.37 | 0.23 | 0.58 | ||
| Strategy: Serosorting | |||||||||||
| No | 262 | 66.3 | 69.8 | 76 | 60.8 | 67.7 | 1.00 | 0.650 | |||
| Yes | 133 | 33.7 | 30.2 | 49 | 39.2 | 32.3 | 1.15 | 0.72 | 1.73 | ||
| Strategy: Viral-load Sorting | |||||||||||
| No | 372 | 94.2 | 94.6 | 97 | 77.6 | 82.4 | 1.00 | <0.001 | |||
| Yes | 23 | 5.8 | 5.4 | 28 | 22.4 | 17.6 | 3.78 | 2.17 | 7.69 | ||
| Strategy: Withdrawal | |||||||||||
| No | 295 | 74.7 | 77.8 | 79 | 63.2 | 72.8 | 1.00 | 0.258 | |||
| Yes | 100 | 25.3 | 22.2 | 46 | 36.8 | 27.2 | 1.34 | 0.82 | 2.65 | ||
| Strategy: Ask HIV Status | |||||||||||
| No | 159 | 40.3 | 43.7 | 35 | 28.0 | 29.4 | 1.00 | 0.005 | |||
| Yes | 236 | 59.8 | 56.3 | 90 | 72.0 | 70.6 | 1.86 | 1.25 | 2.87 | ||
| Other Sexual Behaviors | |||||||||||
| Anal Sex Preference | |||||||||||
| Bottom | 136 | 36.3 | 35.2 | 37 | 29.6 | 28.8 | 1.00 | 0.002 | |||
| Versatile | 94 | 25.1 | 21.2 | 38 | 30.4 | 36.7 | 2.12 | 1.27 | 3.55 | ||
| Top | 145 | 38.7 | 43.6 | 50 | 40.0 | 34.4 | 0.97 | 0.59 | 1.59 | ||
|
Condomless Anal Sex in P6Me |
|||||||||||
| No | 174 | 44.7 | 46.9 | 28 | 22.8 | 25.6 | 1.00 | <0.001 | |||
| Yes, but only with seroconcordant partners | 117 | 30.1 | 30.3 | 16 | 13.0 | 15.1 | 0.92 | 0.50 | 1.70 | ||
| Yes, with serodiscordant/unknown partners | 98 | 25.2 | 22.8 | 79 | 64.2 | 59.3 | 4.76 | 2.93 | 7.74 | ||
| Watersports | |||||||||||
| No | 363 | 91.2 | 93.7 | 94 | 74.6 | 80.3 | 1.00 | <0.001 | |||
| Yes | 35 | 8.8 | 6.3 | 32 | 25.4 | 19.7 | 3.64 | 2.02 | 6.57 | ||
| Insertive Anal Sex in P6Me | |||||||||||
| No | 133 | 33.4 | 35.9 | 13 | 10.3 | 17.5 | 1.00 | <0.001 | |||
| Yes | 265 | 66.6 | 64.1 | 113 | 89.7 | 82.5 | 2.65 | 1.60 | 4.40 | ||
| Receptive Anal Sex in P6Me | |||||||||||
| No | 153 | 38.4 | 42.6 | 23 | 18.3 | 16.3 | 1.00 | <0.001 | |||
| Yes | 245 | 61.6 | 57.4 | 103 | 81.8 | 83.7 | 3.81 | 2.28 | 6.39 | ||
| Attended Sex Party/Darkroom/Blackout in P6Me | |||||||||||
| No | 342 | 85.9 | 87.8 | 68 | 54.0 | 50.7 | 1.00 | <0.001 | |||
| Yes | 56 | 14.1 | 12.2 | 58 | 46.0 | 49.3 | 7.02 | 4.44 | 11.08 | ||
| Received Money in Exchange for Sex in P6Me | |||||||||||
| No | 380 | 95.7 | 95.3 | 103 | 81.8 | 73.3 | 1.00 | <0.001 | |||
| Yes | 17 | 4.3 | 4.7 | 23 | 18.3 | 26.7 | 7.46 | 4.09 | 13.61 | ||
| Substance Use | |||||||||||
| AUDITfHarmful Drinker | |||||||||||
| No | 246 | 61.8 | 65.3 | 72 | 57.6 | 57.7 | 1.00 | 0.125 | |||
| Yes | 152 | 38.2 | 34.7 | 53 | 42.4 | 42.3 | 1.38 | 0.91 | 2.78 | ||
| Used EDDgin P6Me | |||||||||||
| No | 357 | 89.7 | 91.2 | 88 | 69.8 | 70.2 | 1.00 | <0.001 | |||
| Yes | 41 | 10.3 | 8.8 | 38 | 30.2 | 29.8 | 4.38 | 2.63 | 7.29 | ||
| Used methamphetamine in P6Me | |||||||||||
| No | 364 | 91.5 | 89.7 | 101 | 80.2 | 80.5 | 1.00 | 0.006 | |||
| Yes | 34 | 8.5 | 10.3 | 25 | 19.8 | 19.5 | 2.12 | 1.23 | 3.65 | ||
| Used Poppers in P6Me | |||||||||||
| No | 296 | 74.4 | 77.6 | 59 | 46.8 | 51.8 | 1.00 | <0.001 | |||
| Yes | 102 | 25.6 | 22.4 | 67 | 53.2 | 48.2 | 3.23 | 2.12 | 4.92 | ||
| Used Ecstasy in P6Me | |||||||||||
| No | 312 | 78.4 | 81.2 | 77 | 61.1 | 60.4 | 1.00 | <0.001 | |||
| Yes | 86 | 21.6 | 18.8 | 49 | 38.9 | 39.6 | 2.82 | 1.83 | 4.36 | ||
| Continuous Variables | |||||||||||
| Median | Q1, Q3h | Median | Q1, Q3h | ORb | 95% CIc | p-value | |||||
| Age | 30 | 24, 39 | 29 | 25, 38 | 0.98 | 0.96 | 1.00 | 0.929 | |||
| HAARTd Treatment Optimism Scale (n=716) | 24 | 20, 26 | 24 | 20, 28 | 1.03 | 0.99 | 1.08 | 0.167 | |||
| Sexual Sensations Scale (n=698) | 30 | 27, 33 | 32 | 30, 35 | 1.13 | 1.08 | 1.19 | <0.001 | |||
| Cognitive Escape Scale (n=705) | 28 | 24, 31 | 30 | 25, 33 | 1.03 | 0.99 | 1.06 | 0.014 | |||
Respondent Driven Sampling
Odds Ratio
Confidence Interval
Highly Active Antiretroviral Therapy
Past Six Months
Alcohol Use Disorder Identification Test
Erectile Dysfunction Drugs
Quartile 1, Quartile 3
Table 3 provides the descriptive statistics and univariable results for HIV-positive GBM. Among HIV-positive men with the highest quartile number of male anal sex partners, 68.8% reported serosorting (versus 35.7% for the lower three quartiles), 49.9% reported asking HIV status before sex (versus 31.1%), 48.8% reported treatment/viral load sorting (versus 22.2%), 47.6% reported strategic positioning (versus 24.4%), 38.7% reported avoiding anal sex (versus 38.0%), 34.2% reported withdrawal (versus 25.1%), and 26.6% reported always using condoms (versus 37.6%). In univariate analysis, HIV-positive men with the highest quartile number of male anal sex partners had an increased odds of reporting strategies such as asking partners their HIV status (OR=2.29, 95%CI [1.13,4.32]), strategic positioning (OR=2.82, 95%CI [1.41,5.62]), serosorting (OR=3.97, 95%CI [1.98,7.96]), and treatment/viral load sorting (OR=3.33, 95%CI [1.66,6.71]) compared with those in the lower three quartiles of male anal sex partners (See Table 3).
Table III.
Univariable associations with the highest quartile of male anal sex partners among HIV-positive men.
| <7 Anal Sex Partners (n=125) |
≥7 Anal Sex Partners (n=69) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Categorical Variables | n | % | RDSa % | n | % | RDSa % | ORb | 95% CIc | p-value | ||
| Demographics | |||||||||||
| Ethnicity | 0.349 | ||||||||||
| White | 96 | 76.8 | 72.6 | 53 | 76.8 | 59.4 | 1.00 | ||||
| Asian | 7 | 5.6 | 6.5 | 5 | 7.3 | 6.8 | 1.28 | 0.34 | 4.84 | ||
| Aboriginal | 15 | 12.0 | 13.8 | 6 | 8.7 | 22.2 | 1.97 | 0.83 | 4.68 | ||
| Other | 7 | 5.6 | 7.2 | 5 | 7.3 | 11.6 | 1.98 | 0.64 | 6.13 | ||
| Sexual Identity | 0.231 | ||||||||||
| Gay | 103 | 82.4 | 80.2 | 63 | 91.3 | 87.7 | 1.00 | ||||
| Bisexual/Other | 22 | 17.6 | 19.8 | 6 | 8.7 | 12.3 | 0.57 | 0.22 | 1.45 | ||
| Education | 0.591 | ||||||||||
| Greater than high school | 19 | 15.6 | 14.1 | 4 | 5.9 | 11.1 | 1.00 | ||||
| Some/completed high school | 103 | 84.4 | 85.9 | 64 | 94.1 | 88.9 | 1.32 | 0.48 | 3.64 | ||
| Neighborhood | 0.550 | ||||||||||
| Downtown | 87 | 69.6 | 66.3 | 46 | 66.7 | 74.1 | 1.00 | ||||
| Vancouver | 23 | 18.4 | 21.1 | 13 | 18.8 | 14.5 | 0.62 | 0.25 | 1.51 | ||
| Outside Vancouver | 15 | 12.0 | 12.6 | 10 | 14.5 | 11.4 | 0.87 | 0.29 | 2.25 | ||
| Annual Income | 0.270 | ||||||||||
| < 30K | 101 | 80.8 | 81.6 | 42 | 60.9 | 74.8 | 1.00 | ||||
| 30–60K | 19 | 15.2 | 16.4 | 21 | 30.4 | 18.4 | 1.22 | 0.52 | 2.89 | ||
| ≥ 60K | 5 | 4.0 | 2.0 | 6 | 8.7 | 6.8 | 3.76 | 0.66 | 21.28 | 0.349 | |
| Seroadaptive Sexual Behaviors | |||||||||||
| Strategy: Always Using Condoms | |||||||||||
| No | 72 | 57.6 | 62.4 | 54 | 78.3 | 73.4 | 1.00 | 0.162 | |||
| Yes | 53 | 42.4 | 37.6 | 15 | 21.7 | 26.6 | 0.61 | 0.29 | 1.23 | ||
| Strategy: Strategic Positioning | |||||||||||
| No | 84 | 67.2 | 75.6 | 40 | 58.0 | 52.4 | 1.00 | 0.003 | |||
| Yes | 41 | 32.8 | 24.4 | 29 | 42.0 | 47.6 | 2.82 | 1.41 | 5.62 | ||
| Strategy: Anal Sex Avoidance | |||||||||||
| No | 84 | 67.2 | 62.0 | 43 | 62.3 | 61.3 | 1.00 | 0.932 | |||
| Yes | 41 | 32.8 | 38.0 | 26 | 37.7 | 38.7 | 1.30 | 0.53 | 2.14 | ||
| Strategy: Serosorting | |||||||||||
| No | 69 | 55.2 | 64.3 | 24 | 34.8 | 31.2 | 1.00 | <0.001 | |||
| Yes | 56 | 44.8 | 35.7 | 45 | 65.2 | 68.8 | 3.97 | 1.98 | 7.96 | ||
| Strategy: Viral-load Sorting | |||||||||||
| No | 92 | 73.6 | 77.8 | 34 | 49.3 | 51.2 | 1.00 | 0.001 | |||
| Yes | 33 | 26.4 | 22.2 | 35 | 50.7 | 48.8 | 3.33 | 1.66 | 6.71 | ||
| Strategy: Withdrawal | |||||||||||
| No | 94 | 75.2 | 74.9 | 45 | 65.2 | 65.8 | 1.00 | 0.229 | |||
| Yes | 31 | 24.8 | 25.1 | 24 | 34.8 | 34.2 | 1.54 | 0.76 | 3.14 | ||
| Strategy: Ask HIV Status | |||||||||||
| No | 71 | 56.8 | 68.9 | 28 | 40.6 | 50.1 | 1.00 | 0.019 | |||
| Yes | 54 | 43.2 | 31.1 | 41 | 59.4 | 49.9 | 2.29 | 1.13 | 4.32 | ||
| Other Sexual Behaviors | |||||||||||
| Anal Sex Preference | |||||||||||
| Bottom | 40 | 34.2 | 29.2 | 28 | 40.6 | 63.9 | 1.00 | <0.001 | |||
| Versatile | 39 | 33.3 | 41.2 | 21 | 30.4 | 13.7 | 0.15 | 0.59 | 0.39 | ||
| Top | 38 | 32.5 | 29.6 | 20 | 29.0 | 22.4 | 0.35 | 0.15 | 0.85 | ||
| Condomless Anal Sex in P6Me | |||||||||||
| No | 49 | 39.8 | 39.5 | 5 | 7.4 | 5.7 | 1.00 | <0.001 | |||
| Yes, but only with seroconcordant partners | 34 | 27.6 | 30.4 | 18 | 26.5 | 19.3 | 4.44 | 1.09 | 18.16 | ||
| Yes, with serodiscordant/unknown partners | 40 | 32.5 | 30.1 | 45 | 66.2 | 75.0 | 17.36 | 4.74 | 63.59 | ||
| Watersports | |||||||||||
| No | 116 | 92.8 | 96.7 | 46 | 66.7 | 77.6 | 1.00 | <0.001 | |||
| Yes | 9 | 7.2 | 3.3 | 23 | 33.3 | 22.4 | 8.60 | 2.49 | 29.72 | ||
| Insertive Anal Sex in P6Me | |||||||||||
| No | 51 | 40.8 | 47.0 | 10 | 14.5 | 18.4 | 1.00 | <0.001 | |||
| Yes | 74 | 59.2 | 53.0 | 59 | 85.5 | 81.6 | 3.93 | 1.80 | 8.62 | ||
| Receptive Anal Sex in P6Me | |||||||||||
| No | 50 | 40.0 | 45.6 | 9 | 13.0 | 7.4 | 1.00 | <0.001 | |||
| Yes | 75 | 60.0 | 54.4 | 60 | 87.0 | 92.6 | 10.55 | 3.56 | 31.31 | ||
| Attended Group Sex Event, P6M e | |||||||||||
| No | 101 | 80.8 | 84.4 | 27 | 39.1 | 52.9 | 1.00 | <0.001 | |||
| Yes | 24 | 19.2 | 15.6 | 42 | 60.9 | 47.1 | 4.80 | 2.28 | 10.11 | ||
| Received Money in Exchange for Sex in P6Me | |||||||||||
| No | 116 | 92.8 | 93.3 | 56 | 81.2 | 85.8 | 1.00 | 0.121 | |||
| Yes | 9 | 7.2 | 6.7 | 13 | 18.8 | 14.2 | 2.29 | 0.79 | 6.69 | ||
| Substance Use | |||||||||||
| AUDITf Harmful Drinker | |||||||||||
| No | 97 | 78.9 | 76.9 | 56 | 82.4 | 86.2 | 1.00 | 0.164 | |||
| Yes | 26 | 21.1 | 23.1 | 12 | 17.7 | 13.8 | 0.53 | 0.22 | 1.34 | ||
| Used EDDg in P6Me | |||||||||||
| No | 87 | 69.6 | 78.1 | 24 | 34.8 | 47.0 | 1.00 | <0.001 | |||
| Yes | 38 | 30.4 | 21.9 | 45 | 65.2 | 53.0 | 4.25 | 2.00 | 8.12 | ||
| Used methamphetamine in P6Me | |||||||||||
| No | 92 | 73.6 | 77.7 | 25 | 36.2 | 46.3 | 1.00 | <0.001 | |||
| Yes | 33 | 26.4 | 22.3 | 44 | 63.8 | 53.7 | 4.43 | 2.70 | 8.14 | ||
| Used Poppers in P6Me | |||||||||||
| No | 75 | 60.0 | 59.2 | 22 | 31.9 | 36.0 | 1.00 | 0.005 | |||
| Yes | 50 | 40.0 | 40.8 | 47 | 68.1 | 64.0 | 2.59 | 1.32 | 5.79 | ||
| Used Ecstasy in P6Me | |||||||||||
| No | 110 | 88.0 | 91.9 | 43 | 62.3 | 73.4 | 1.00 | 0.002 | |||
| Yes | 15 | 12.0 | 8.1 | 26 | 37.7 | 26.6 | 4.13 | 1.65 | 1.32 | ||
| Continuous Variables | |||||||||||
| Median | Q1, Q3h | Median | Q1, Q3h | ORb | 95% CIc | p-value | |||||
| Age (Median Q1, Q3) | 47 | 41, 51 | 47 | 42, 52 | 1.02 | 0.99 | 1.06 | 0.564 | |||
| HAARTd Treatment Optimism Scale (n=716) | 28 | 25, 31 | 29 | 26, 32 | 1.02 | 0.97 | 1.08 | 0.044 | |||
| Sexual Sensations Scale (n=698) | 30 | 27, 33 | 34 | 31, 37 | 1.15 | 1.06 | 1.24 | <0.001 | |||
| Cognitive Escape Scale (n=705) | 30 | 26, 35 | 33 | 28, 36 | 1.10 | 1.04 | 1.15 | 0.028 | |||
Respondent Driven Sampling
Odds Ratio
Confidence Interval
Highly Active Antiretroviral Therapy
Past Six Months
Alcohol Use Disorder Identification Test
Erectile Dysfunction Drugs
Quartile 1, Quartile 3
Multivariable models for HIV-negative/unknown GBM are presented in Table 4. Among HIV-negative/unknown men, being in the highest quartile of male anal sex partners was associated with being more likely to engage in strategic positioning (AOR=3.81, 95%CI [1.79,8.11]), ask HIV status prior to sex (AOR=2.15, 95%CI [1.18,3.93]), prefer a versatile versus bottom anal sex position (AOR=2.75, 95%CI [1.31,5.75]), identify as gay compared with bisexual or other (AOR=3.88, 95%CI [1.52,9.90]), live in downtown Vancouver compared with the metro Vancouver area (AOR=3.13, 95%CI [1.47,6.67]), achieve education greater than the high school level (AOR=3.13, 95%CI [1.20,8.13]), use ecstasy in the past six months (AOR=2.96, 95%CI [1.49, 5.89]), engage in “watersports” (46) (AOR=2.78, 95%CI [1.23,6.28]), attend a group sex event in the past six months (AOR=6.07, 95%CI [3.08,11.98]), and receive money in exchange for sex in the past six months (AOR=5.35, 95% CI [1.79,15.98]) compared with men in the lower three quartiles of male anal sex partners; they were also less likely to avoid anal sex (AOR=0.22, 95%CI [1.11,0.42]) (See Table 4).
Table IV.
Multivariable results with the highest quartile of male anal sex partners among HIV-negative or unknown men.
| AORa | 95% CIb | p-value | |||
|---|---|---|---|---|---|
| Sexual Identity | |||||
| Gay | 1.00 | ||||
| Bisexual/Other | 0.26 | 0.10 | 0.66 | 0.005 | |
| Education | |||||
| Greater than high school | 1.00 | ||||
| Some/completed high school | 0.32 | 0.12 | 0.83 | 0.019 | |
| Neighborhood | |||||
| Downtown | 1.00 | ||||
| Vancouver | 0.32 | 0.15 | 0.68 | 0.003 | |
| Outside Vancouver | 0.69 | 0.35 | 1.37 | 0.290 | |
| Strategy: Strategic Positioning | |||||
| No | 1.00 | ||||
| Yes | 3.81 | 1.79 | 8.11 | <.001 | |
| Strategy: Anal Sex Avoidance | |||||
| No | 1.00 | ||||
| Yes | 0.22 | 0.11 | 0.42 | <.001 | |
| Strategy: Ask HIV Status | |||||
| No | 1.00 | ||||
| Yes | 2.15 | 1.18 | 3.93 | 0.013 | |
| Sexual Sensations Scale (n=698) | 1.07 | 0.99 | 1.16 | 0.087 | |
| Anal Sex Preference | |||||
| Bottom | 1.00 | ||||
| Versatile | 2.75 | 1.31 | 5.75 | 0.007 | |
| Top | 0.62 | 0.28 | 1.36 | 0.229 | |
| Unprotected Anal Sex, P6Mc | |||||
| No | 1.00 | ||||
| Yes, but only with seroconcordant partners | 0.72 | 0.32 | 1.60 | 0.417 | |
| Yes, with serodiscordant/unknown partners | 1.78 | 0.87 | 3.65 | 0.115 | |
| Watersports | |||||
| No | 1.00 | ||||
| Yes | 2.78 | 1.23 | 6.28 | 0.014 | |
| Attended Group Sex Event, P6Mc | |||||
| No | 1.00 | ||||
| Yes | 6.07 | 3.08 | 11.98 | <.001 | |
| Received Money for Sex, P6M c | |||||
| No | 1.00 | ||||
| Yes | 5.35 | 1.79 | 15.98 | 0.003 | |
| Used Ecstasy, P6Mc | |||||
| No | 1.00 | ||||
| Yes | 2.96 | 1.49 | 5.89 | 0.002 | |
Adjusted Odds Ratio
CI - Confidence Interval
P6M - Past Six Months.
Multivariable models for HIV-positive GBM are presented in Table 5. These results showed that the majority of seroadaptive strategies were not selected into the model. Being in the highest quartile of male anal sex partners for HIV-positive men was associated with a greater likelihood of asking about HIV status prior to sex (AOR=3.10, 95%CI [1.17,8.26], having higher scores on the Sexual Sensation Seeking scale (AOR=1.17, 95%CI [1.05,1.31], per 1 unit increase), having used crystal methamphetamine in the past six months (AOR=3.06 95%CI [1.16,8.10]), having attended a group sex event in the past six months (AOR=3.41 95%CI [1.21,9.66]), having received money in exchange for sex in the past six months (AOR=5.07, 95%CI [1.09,23.61]), and having less preference for a versatile versus bottom anal sex position (AOR=0.16, 95%CI [0.05,0.53]) compared with men in the lower three quartiles of male anal sex partners (See Table 5).
Table V.
Multivariable associations with the highest quartile of male anal sex partners among HIV-positive men.
| AORa | 95% CIb | p-value | |||
|---|---|---|---|---|---|
| Strategy: Ask HIV status | |||||
| No | 1.00 | ||||
| Yes | 3.10 | 1.17 | 8.26 | 0.024 | |
| Sexual Sensations Scale (n=698) | 1.17 | 1.05 | 1.31 | 0.004 | |
| Anal Sex Preference | |||||
| Bottom | 1.00 | ||||
| Versatile | 0.16 | 0.05 | 0.53 | 0.003 | |
| Top | 0.44 | 0.15 | 1.25 | 0.122 | |
| Attended Group Sex Event, P6Mc | |||||
| No | 1.00 | ||||
| Yes | 3.41 | 1.21 | 9.66 | 0.021 | |
| Received Money for Sex, P6Mc | |||||
| No | 1.00 | ||||
| Yes | 5.07 | 1.09 | 23.61 | 0.039 | |
| Used Methamphetamine, P6Mc | |||||
| No | 1.00 | ||||
| Yes | 3.06 | 1.16 | 8.10 | 0.024 | |
| Used EDD, P6Mc | |||||
| No | 1.00 | ||||
| Yes | 2.52 | 0.93 | 6.83 | 0.068 | |
Adjusted Odds Ratio
Confidence Interval
Past Six Months.
The sensitivity analysis, which was conducted to assess how differing dichotomous cut-off points at the global median (≥3 versus <3) and the HIV status specific third quartile cut-offs (HIV-Positive: ≥15 versus <15, HIV-Negative: ≥6 versus <6), showed that several variables acted in a dose response relationship to having more sexual partners. However, the overall results of the final model did not appear to be highly sensitive to changes in the cut-off point employed.
DISCUSSION
We observed a higher prevalence of seroadaptive behaviors among GBM with more sexual partners, using a global data-driven definition of the highest quartile number of male anal sex partners (i.e., more than 7 in the past six months). Although both HIV-positive and HIV-negative men with ≥7 partners were more likely to engage in condomless anal sex on the univariable level, this factor was not retained in either of the multivariable models. However, in both groups, men in with the highest quartile number of male anal sex partners were more likely to employ other seroadaptive behaviors and prevention practices. HIV-negative men were significantly more likely to engage in strategic positioning, and both groups were more likely to engage in serostatus inquiry, which is a necessary precursor to effective use of other seroadaptive strategies. Contrary to this trend we observed that HIV-negative men were less likely to avoid anal sex as a means of seroadaptation.
We also note that, in general, HIV-positive men with the highest quartile number of male anal sex partners reported higher prevalence of seroadaptive behaviors than HIV-negative men with the highest quartile number of male anal sex partners. This latter finding may reflect a high level of either concern (e.g., HIV prevention altruism) and/or awareness (e.g., HIV risk perception) among HIV-positive men regarding HIV transmission (47,48).This supports other findings suggesting that HIV-positive men aware of their HIV-infection are less likely to infect sexual partners than those who are not aware (48,49).
Together these findings highlight seroadaptitve behaviors as common harm reduction strategies for GBM in the highest quartile number of male anal sex partners, which parallels other documented harm reduction tactics in other populations. For example, in their analysis of injection drug users who remain HIV and HCV infection-free, Friedman and colleagues (50,51) adapted the concept of Positive Deviance, originating in infant/child nutrition studies to identify parental strategies associated with well-nourished children living in communities with high malnutrition rates (52) to explain how “subjects control their personal risk, even though they have engaged in high-risk activities for lengthy periods”. Seroadaptive strategies may therefore provide insights into how GBM with the highest quartile number of male anal sex partners manage their sexual health and prevent seroconversion despite having higher than average rates of partnering.
This interpretation of our findings raises the question of the effectiveness of various seroadaptive behaviors. For example, Vallabhaneni and colleagues (15) studied the risks associated with condomless anal sex, serosorting, strategic positioning, and partner concurrency and found that while condomless seroadaptive behaviors were more risky than sex with condoms, these behaviors did seem to offer some benefits compared with condomless sex without seroadaptation. Other research by Kurtz and colleagues (19) doubted that sero-sorting, while widely practiced, could be effective in major cities because: 1) HIV-negative men in their study averaged 10 partners and almost 20 episodes of condomless anal intercourse during a three-month period, 2) 45% of HIV-positive GBM were unaware of their status and, 3) 31% of HIV-negative GBM seroconverted within 5 years of moving to South Florida. In contrast, only 0.6% of Momentum Health Study participants did not know they were HIV-positive, 83% of HIV-negative men had an HIV test within at least two years, and HIV incidence in our study was at least 1 per 100 person-years within 1.5 years of follow-up (53,54). Of course, some participants lost to follow-up may also have seroconverted, and we note that the number of new diagnoses in British Columbia remains stable (3). Under these conditions, some seroadaptive behaviors may have the potential to reduce HIV transmission risks, though further research is needed to establish the efficacy of each behavior with regards to preventing HIV in different epidemic settings. To accomplish this, we recommend a more purposive sexual health needs assessment to determine which seroadatpvie behaviors or which combination of these behaviors might effectively reduce HIV-transmission and how condom use might be better utilized with consideration of these practices.
Finally, our results offer further insight into the behavioral strategies of men with the highest quartile number of male anal sex partners and may therefore provide public health leaders the information necessary to identify different overlapping sub-groups of men (e.g., those who participate in the sex industry as escorts, those who attend group sex events, those who engage in condomless anal sex, and those who use “sex drugs”) who might most benefit from more targeted risk reduction and HIV prevention programming.
Also important, our multivariable results indicated significant associations with substance use (i.e., crystal methamphetamine, EDD, and Ecstasy) for men with the highest quartile number of male anal sex partners. Yet despite this association, the Escape Motivation Scale – which has been used to link substance use and sexual risk (37) – was non-significant in the multivariable model for either HIV-negative or HIV-positive men, suggesting that substance use among these men is not explained by escape behavior but is likely attributable to other motivators, such as pleasure seeking. This association between sexual risk and substance use, especially EDD, is not entirely surprising considering the role sex-drugs play in some venues and cultures (35).
Further, the HAART Optimism scale did not appear to be significant in either the univariable or multivariable results, suggesting that HAART treatment optimism and risk compensation do not explicitly determine patterns of sexual partnering, and, conversely, having more sexual partners does not seem to greatly influence treatment optimism. Considering the risks associated with more densely connected social-sexual networks, this finding speaks positively to the expansion of HAART access. In contrast, the Sexual Sensation Seeking scale, which is a commonly used measure for pleasure seeking and sexual risk (38), was only significant for HIV-positive men with the highest quartile number of male anal sex partners. However, because items on this scale relate to the number of sex partners, it is difficult to assess whether higher scores among this group are attributable to increased pleasure-seeking or are simply a result of the natural correlation between scale items and our primary outcome.
STRENGTHS & LIMITATIONS
Our study offered several important strengths with regard to past research focusing on sexual partnering and seroadaptation. This analysis included the use of RDS methodology to recruit a large number of gay and bisexual men not specifically from sexual health clinics or community venues, and re-weighting their responses to provide more accurate population estimates of seroadaptation and sexual partnering (30). Further, by considering demographic, psychosocial, sexual behavior, and substance use together, our explanatory model provides useful analysis for understanding the diverse covariates associated with having higher numbers of male anal sex partners.
There are also limitations to this study. First, the strategies analyzed were self-reports, not actual events. Therefore, we lack a measurable association between what participants say and what they actually do (55). For example, even event-level sexual histories may not include instances when GBM decide not to have sex, perhaps because of serodiscordance. Similarly, anal sexual positioning may simply represent preferred sex roles, rather than seroadaptation. To determine seroadaptation intentionality requires a longitudinal study design (17). In addition, while data were corrected for RDS-bias, they pertain only to Metro Vancouver, and may not be representative of areas where HAART access/uptake and Treatment as Prevention programs are not as well developed. This is particularly relevant when reviewing the high reported prevalence of viral load sorting, which may not be found in areas with decreased HAART availability. In consideration of the psychosocial scales and other categorical measures used in this analysis it is difficult to assess whether differences between groups are clinically meaningful, despite being statistically significant. However, as this is an exploratory analysis, we feel that the direction of the association may serve as an important indicator for understanding the factors associated with having more sexual partners. Finally, speaking to our dichotomous comparison groups, the use of quartiles, while potentially arbitrary and difficult to apply clinically, better describes the natural distribution of sexual partnering frequency, offers simpler interpretations of effect measures resulting from statistical modeling, and allows us to avoid the linearity assumption implicit in modeling continuous covariates (56). Further, the use of data-driven cut-off points serve to provide a descriptive measure of sexual partner frequency.
CONCLUSION
Our findings show that men with the highest quartile number of male anal sex partners are more likely to employ seroadaptive strategies (other than condom use and anal sex avoidance) than men with fewer sexual partners. This suggests that these men are taking steps that are at least perceived to improve their sexual health and reduce HIV transmission while balancing their needs for sexual intimacy and pleasure. Consistent with these findings we recommend further research to: (1) determine the efficacy of these strategies, (2) assess how GBM perceive the efficacy of these strategies, and (3) to determine the social and health impacts of widespread uptake of these strategies. Each of these suggested areas remain important because, even if these strategies were sufficiently effective to prevent seroconversion, they are by no means universally employed, and confusion regarding their efficacy is almost certain to continue. Further, though these strategies are more common among those with more sexual partners, they may not be used, nor understood, in sufficiently high rates to reduce population level HIV incidence. Yet still, we do not know what widespread uptake of these strategies might mean for condom use, which is still regarded as the most efficacious prevention strategy. Finally, we note that these strategies are not necessarily effective in combating other STIs, which may put GBM at increased risk for HIV transmission or acquisition.
Supplementary Material
Acknowledgments
The authors would like to thank the Momentum Study participants, office staff, and community advisory board, as well as our community partner agencies, Health Initiative for Men, YouthCoHIV and Hep C Society, and Positive Living Society of BC. Momentum is funded through the National Institute on Drug Abuse (R01DA031055-01A1) and the Canadian Institutes for Health Research (MOP-107544). NJL is supported by a CANFAR/CTN Postdoctoral Fellowship Award. DMM is supported by a Scholar Award from the Michael Smith Foundation for Health Research (#5209).
Footnotes
Compliance with Ethical Standards
Ethical Approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committees at Simon Fraser University, The University of British Columbia, and the University of Victoria and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent was obtained from all individual participants included in the study.
This article does not contain any studies with animals performed by any of the authors.
Conflict of Interest:
Kiffer G. Card declares that he has no conflict of interest.
Nathan J. Lachowsky declares that he has no conflict of interest.
Zishan Cui declares that she has no conflict of interest.
Paul Sereda declares that he has no conflict of interest.
Ashleigh Rich declares that she has no conflict of interest.
Jody Jollimore declares that he has no conflict of interest.
Terry Howard declares that he has no conflict of interest.
Robert Birch declares that he has no conflict of interest.
Allison Carter declares that she has no conflict of interest.
Julio Montaner declares that he has no conflict of interest.
David Moore declares that he has no conflict of interest.
Robert S. Hogg declares that he has no conflict of interest.
Eric A. Roth declares that he has no conflict of interest.
REFERENCES
- 1.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012 Jul 28;380(9839):367–377. doi: 10.1016/S0140-6736(12)60821-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halkitis PN, Moeller RW, Siconolfi DE, Storholm ED, Solomon TM, Bub KL. Measurement model exploring a syndemic in emerging adult gay and bisexual men. AIDS Behav. 2013 Feb;17(2):662–673. doi: 10.1007/s10461-012-0273-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.BC PHO. HIV, Stigma and Society: Tackling a Complex Epidemic and Renewing HIV Prevention for Gay and Bisexual Men in British Columbia [Internet] Provincial Health Officer’s 2010 Annual Report. 2014 Available from: http://www2.gov.bc.ca/assets/gov/health/about-bc-s-health-care-system/office-of-the-provincial-health-officer/reports-publications/annual-reports/hiv-stigma-and-society.pdf.
- 4.Aral SO, Patel DA, Holmes KK, Foxman B. Temporal trends in sexual behaviors and sexually transmitted disease history among 18- to 39-year-old Seattle, Washington, residents: results of random digit-dial surveys. Sex Transm Dis. 2005 Nov;32(11):710–717. doi: 10.1097/01.olq.0000175370.08709.72. [DOI] [PubMed] [Google Scholar]
- 5.Blair J. A Probability Sample of Gay Urban Males: The Use of Two-Phase Adaptive Sampling. J Sex Res. 1999 Feb 1;36(1):39–44. [Google Scholar]
- 6.Glick SN, Morris M, Foxman B, Aral SO, Manhart LE, Holmes KK, et al. A comparison of sexual behavior patterns among men who have sex with men and heterosexual men and women. J Acquir Immune Defic Syndr 1999. 2012 May 1;60(1):83–90. doi: 10.1097/QAI.0b013e318247925e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, et al. Risk factors for HIV infection among men who have sex with men. AIDS Lond Engl. 2006 Mar 21;20(5):731–739. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 8.Morris M, Kretzschmar M. Concurrent partnerships and transmission dynamics in networks. Soc Netw. 1995 Jul;17(3–4):299–318. [Google Scholar]
- 9.Grov C, Golub SA, Parsons JT. HIV status differences in venues where highly sexually active gay and bisexual men meet sex partners: results from a pilot study. AIDS Educ Prev Off Publ Int Soc AIDS Educ. 2010 Dec;22(6):496–508. doi: 10.1521/aeap.2010.22.6.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grov C, Whitfield THF, Rendina HJ, Ventuneac A, Parsons JT. Willingness to Take PrEP and Potential for Risk Compensation Among Highly Sexually Active Gay and Bisexual Men. AIDS Behav. 2015 Mar 4; doi: 10.1007/s10461-015-1030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parsons JT, Rendina HJ, Moody RL, Ventuneac A, Grov C. Syndemic Production and Sexual Compulsivity/Hypersexuality in Highly Sexually Active Gay and Bisexual Men: Further Evidence for a Three Group Conceptualization. Arch Sex Behav. 2015 Oct;44(7):1903–1913. doi: 10.1007/s10508-015-0574-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.On Slut-shaming and Gay Men [Internet] [cited 2016 Jan 22];Daily Kos. Available from: http://www.dailykos.com/story/2012/2/11/1063712/-On-Slut-shaming-and-Gay-Men. [Google Scholar]
- 13.McDavitt B, Mutchler MG. “Dude, You’re Such a Slut!” Barriers and Facilitators of Sexual Communication Among Young Gay Men and Their Best Friends. J Adolesc Res. 2014 Jul;29(4):464–498. doi: 10.1177/0743558414528974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baggaley RF, White RG, Boily M-C. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. Int J Epidemiol. 2010 Aug;39(4):1048–1063. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vallabhaneni S, Li X, Vittinghoff E, Donnell D, Pilcher CD, Buchbinder SP. Seroadaptive practices: association with HIV acquisition among HIV-negative men who have sex with men. PloS One. 2012;7(10):e45718. doi: 10.1371/journal.pone.0045718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rönn M, White PJ, Hughes G, Ward H. Developing a Conceptual Framework of Seroadaptive Behaviors in HIV-Diagnosed Men Who Have Sex With Men. J Infect Dis. 2014 Dec 1;210(Suppl 2):S586–S593. doi: 10.1093/infdis/jiu482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McFarland W, Chen Y-H, Nguyen B, Grasso M, Levine D, Stall R, et al. Behavior, intention or chance? A longitudinal study of HIV seroadaptive behaviors, abstinence and condom use. AIDS Behav. 2012 Jan;16(1):121–131. doi: 10.1007/s10461-011-9936-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snowden JM, Wei C, McFarland W, Raymond HF. Prevalence, correlates and trends in seroadaptive behaviours among men who have sex with men from serial cross-sectional surveillance in San Francisco, 2004–2011. Sex Transm Infect. 2014 Sep;90(6):498–504. doi: 10.1136/sextrans-2013-051368. [DOI] [PubMed] [Google Scholar]
- 19.Kurtz SP, Buttram ME, Surratt HL, Stall RD. Resilience, syndemic factors, and serosorting behaviors among HIV-positive and HIV-negative substance-using MSM. AIDS Educ Prev Off Publ Int Soc AIDS Educ. 2012 Jun;24(3):193–205. doi: 10.1521/aeap.2012.24.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 Infection with Early Antiretroviral Therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montaner JSG, Lima VD, Harrigan PR, Lourenço L, Yip B, Nosyk B, et al. Expansion of HAART Coverage Is Associated with Sustained Decreases in HIV/AIDS Morbidity, Mortality and HIV Transmission: The “HIV Treatment as Prevention” Experience in a Canadian Setting. PLoS ONE. 2014 Feb 12;9(2):e87872. doi: 10.1371/journal.pone.0087872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eaton LA, Kalichman SC. Risk Compensation in HIV Prevention: Implications for Vaccines, Microbicides, and Other Biomedical HIV Prevention Technologies. Curr HIV/AIDS Rep. 2007 Dec;4(4):165–172. doi: 10.1007/s11904-007-0024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blower SM, Gershengorn HB, Grant RM. A tale of two futures: HIV and antiretroviral therapy in San Francisco. Science. 2000 Jan 28;287(5453):650–654. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 24.McConnell JJ, Bragg L, Shiboski S, Grant RM. Sexual Seroadaptation: Lessons for Prevention and Sex Research from a Cohort of HIV-Positive Men Who Have Sex with Men. PLoS ONE. 2010 Jan 21;5(1):e8831. doi: 10.1371/journal.pone.0008831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsons JT, Schrimshaw EW, Wolitski RJ, Halkitis PN, Purcell DW, Hoff CC, et al. Sexual harm reduction practices of HIV-seropositive gay and bisexual men: serosorting, strategic positioning, and withdrawal before ejaculation. AIDS Lond Engl. 2005 Apr;19(Suppl 1):S13–S25. doi: 10.1097/01.aids.0000167348.15750.9a. [DOI] [PubMed] [Google Scholar]
- 26.Jin F, Crawford J, Prestage GP, Zablotska I, Imrie J, Kippax SC, et al. HIV risk reduction behaviours in gay men. AIDS Lond Engl. 2009 Jan 14;23(2):243–252. doi: 10.1097/QAD.0b013e32831fb51a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ilaria G, Jacobs JL, Polsky B, Koll B, Baron P, MacLow C, et al. Detection of HIV-1 DNA sequences in pre-ejaculatory fluid. Lancet Lond Engl. 1992 Dec 12;340(8833):1469. doi: 10.1016/0140-6736(92)92658-3. [DOI] [PubMed] [Google Scholar]
- 28.Pudney J, Oneta M, Mayer K, Seage G, Anderson D. Pre-ejaculatory fluid as potential vector for sexual transmission of HIV-1. Lancet Lond Engl. 1992 Dec 12;340(8833):1470. doi: 10.1016/0140-6736(92)92659-4. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y. Treatment-related optimistic beliefs and risk of HIV transmission: a review of recent findings (2009–2012) in an era of treatment as prevention. Curr HIV/AIDS Rep. 2013 Mar;10(1):79–88. doi: 10.1007/s11904-012-0144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heckathorn D. Respondent-Driven Sampling: A New Approach to the Study of Hidden Populations*. Soc Study Soc Probl. 1997 May;44(2) [Google Scholar]
- 31.Forrest JI, Stevenson B, Rich A, Michelow W, Pai J, Jollimore J, et al. Community mapping and respondent-driven sampling of gay and bisexual men’s communities in Vancouver, Canada. Cult Health Sex. 2014 Feb 10; doi: 10.1080/13691058.2014.881551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore DM, Cui Z, Lachowsky N, Raymond HF, Roth E, Rich A, et al. HIV Community Viral Load and Factors Associated With Elevated Viremia Among a Community-Based Sample of Men Who Have Sex With Men in Vancouver, Canada: JAIDS. J Acquir Immune Defic Syndr. 2016 May;72(1):87–95. doi: 10.1097/QAI.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lachowsky NJ, Lal A, Forrest JI, Card KG, Cui Z, Sereda P, et al. Including Online-Recruited Seeds: A Respondent-Driven Sample of Men Who Have Sex With Men. J Med Internet Res. 2016;18(3):e51. doi: 10.2196/jmir.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addict Abingdon Engl. 1993 Jun;88(6):791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 35.Fisher DG, Reynolds GL, Napper LE. Use of Crystal Meth, Viagra and Sexual Behaviour. Curr Opin Infect Dis. 2010 Feb;23(1):53–56. doi: 10.1097/QCO.0b013e328334de0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rich A, Lachowsky N, Cui Z, Sereda P, Lal A, Moore D, et al. Anal Sex Roles Explain Substance Use Patterns Among a Sample of HIV-Negative and HIV-Positive Gay, Bisexual, and Other Men who have Sex with Men in Vancouver, British Columbia, Canada. Arch Sex Behav. 2015 [Google Scholar]
- 37.McKirnan DJ, Vanable PA, Ostrow DG, Hope B. Expectancies of sexual “escape” and sexual risk among drug and alcohol-involved gay and bisexual men. J Subst Abuse. 2001;13(1–2):137–154. doi: 10.1016/s0899-3289(01)00063-3. [DOI] [PubMed] [Google Scholar]
- 38.Kalichman SC, Rompa D. Sexual sensation seeking and Sexual Compulsivity Scales: reliability, validity, and predicting HIV risk behavior. J Pers Assess. 1995 Dec;65(3):586–601. doi: 10.1207/s15327752jpa6503_16. [DOI] [PubMed] [Google Scholar]
- 39.Van de Ven P, Crawford J, Kippax S, Knox S, Prestage G. A scale of optimism-scepticism in the context of HIV treatments. AIDS Care. 2000 Apr;12(2):171–176. doi: 10.1080/09540120050001841. [DOI] [PubMed] [Google Scholar]
- 40.SAS. Cary, NC, USA: SAS Institute Inc; [Google Scholar]
- 41.Voltz E, Wejnert C, Cameron C, Spiller M, Barash V, Degani I, et al. Respondent-Driven Sampling Analysis Tool (RDSAT) Ithaca, New York: Cornell University; 2012. [Google Scholar]
- 42.Akaike H. A New Look at the Statistical Model Identification. In: Parzen E, Tanabe K, Kitagawa G, editors. Selected Papers of Hirotugu Akaike [Internet] New York: Springer; 1974. [cited 2016 May 26]. pp. 215–222. (Springer Series in Statistics). Available from: http://link.springer.com/chapter/10.1007/978-1-4612-1694-0_16. [Google Scholar]
- 43.PROC GENMOD. [cited 2016 May 26];Type 3 Analysis :: SAS/STAT(R) 9.2 User’s Guide, Second Edition [Internet] Available from: https://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#statug_genmod_sect035.htm. [Google Scholar]
- 44.Lima VD, Geller J, Bangsberg DR, Patterson TL, Daniel M, Kerr T, et al. The effect of adherence on the association between depressive symptoms and mortality among HIV-infected individuals first initiating HAART. AIDS Lond Engl. 2007 May 31;21(9):1175–1183. doi: 10.1097/QAD.0b013e32811ebf57. [DOI] [PubMed] [Google Scholar]
- 45.Heckathorn DD, Semaan S, Broadhead RS, Hughes JJ. Extensions of Respondent-Driven Sampling: A New Approach to the Study of Injection Drug Users Aged 18–25. AIDS Behav. 2002 Mar;6(1):55–67. [Google Scholar]
- 46.Rehor JE. Sensual, Erotic, and Sexual Behaviors of Women from the “Kink” Community. Arch Sex Behav. 2015;44(4):825–836. doi: 10.1007/s10508-015-0524-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolitski RJ, Bailey CJ, O’Leary A, Gómez CA, Parsons JT Seropositive Urban Men’s Study (SUMS) Self-perceived responsibility of HIV-seropositive men who have sex with men for preventing HIV transmission. AIDS Behav. 2003 Dec;7(4):363–372. doi: 10.1023/b:aibe.0000004728.73443.32. [DOI] [PubMed] [Google Scholar]
- 48.O’Dell BL, Rosser BRS, Miner MH, Jacoby SM. HIV prevention altruism and sexual risk behavior in HIV-positive men who have sex with men. AIDS Behav. 2008 Sep;12(5):713–720. doi: 10.1007/s10461-007-9321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS Lond Engl. 2006 Jun 26;20(10):1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 50.Friedman SR, Mateu-Gelabert P, Sandoval M, Hagan H, Des Jarlais DC. Positive deviance control-case life history: a method to develop grounded hypotheses about successful long-term avoidance of infection. BMC Public Health. 2008;8:94. doi: 10.1186/1471-2458-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vazan P, Mateu-Gelabert P, Cleland CM, Sandoval M, Friedman SR. Correlates of staying safe behaviors among long-term injection drug users: psychometric evaluation of the staying safe questionnaire. AIDS Behav. 2012 Aug;16(6):1472–1481. doi: 10.1007/s10461-011-0079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bisits Bullen PA. The positive deviance/hearth approach to reducing child malnutrition: systematic review. Trop Med Int Health TM IH. 2011 Nov;16(11):1354–1366. doi: 10.1111/j.1365-3156.2011.02839.x. [DOI] [PubMed] [Google Scholar]
- 53.Lachowsky N, Stephenson K, Rich A, Lal A, Cui Z, Colley G, et al. Characteristics and Rates of Newly Infected HIV-Positive Gay, Bisexual and other Men Who Have Sex With Men (MSM) in Vancouver, British Columbia: Preliminary Findings of the Momentum Health Study. Can J Infect Dis Med Microbiol. 2015 Spring;26(B):83b. [Google Scholar]
- 54.Moore D, Cui Z, Lachoswky N, Fisher RH, Roth E, RIch A, et al. HIV positive MSM with unsuppressed viral load are more likely to engage in risky sex. Conference on Retroviruses and Opportunistic Infections (CROI); 2015 Feb 23; Seattle, United States. [Google Scholar]
- 55.Cassels S, Katz DA. Seroadaptation among Men Who Have Sex with Men: Emerging Research Themes. Curr HIV/AIDS Rep. 2013 Dec;10(4):305–313. doi: 10.1007/s11904-013-0188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams BA, Mandrekar JN, Mandrekar SJ, Cha SS, Furth AF. Finding Optimal Cutpoints for Continuous Covariates with Binary and Time-to-Event Outcomes [Internet] Rochester, Minnesota: Department of Health Sciences Research Mayo Clinic; 2006. Jun, [cited 2016 May 30]. (Technical Report Series). Report No.: 79. Available from: http://www.mayo.edu/research/documents/biostat-79pdf/doc-10027230. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.