Abstract
Disordered calcium balance and homeostasis are common in patients with chronic kidney disease. Such alterations are commonly associated with abnormal bone remodeling, directly and indirectly. Similarly, positive calcium balance may also be a factor in the pathogenesis of extra skeletal soft tissue and arterial calcification. Calcium may directly affect cardiac structure and function through direct effects to alter cell signaling due to abnormal intracellular calcium homeostasis 2) extra-skeletal deposition of calcium and phosphate in the myocardium and small cardiac arterioles, 3) inducing cardiomyocyte hypertrophy through calcium and hormone activation of NFAT signaling mechanisms, and 4) increased aorta calcification resulting in chronic increased afterload leading to hypertrophy. Similarly, calcium may alter vascular smooth muscle cell function and affect cell signaling which may predispose to a proliferative phenotype important in arteriosclerosis and arterial calcification. Thus, disorders of calcium balance and homeostasis due to CKD-MBD may play a role in the high cardiovascular burden observed in patients with CKD.
Keywords: calcium, chronic kidney disease, mineral bone disorder, heart, vascular smooth muscle cells, calcification
Introduction
Chronic kidney disease (CKD) affects 1 in 10 adults and is recognized as an independent risk factor for cardiovascular disease (CVD)[1-4]. In patients with CKD, traditional Framingham risk factors and laboratory based diagnostic cardiovascular biomarkers do not fully explain or predict the increased risk of LVH, diastolic dysfunction, or sudden cardiac death in patients with CKD[5, 6]. The risk of sudden cardiac death increases with progressive loss of kidney function prior to dialysis, and 25% of patients on dialysis die of sudden cardiac death- a 100 fold risk compared to the general population[7-10]. The Multicenter Automatic Defibrillator Implantation Trial-II (MADIT-II) showed the risk for sudden cardiac death was 17% higher for every 10 ml/min/1.73 m2 decrease in the estimated GFR.[9] The result was comparable even when patients considered low risk for sudden cardiac death were analyzed[11]. Biopsy and autopsy studies demonstrate inter-cardiomyocyte fibrosis and cardiomyocyte hypertrophy, and the magnitude of the pathology was predictive of subsequent death and or sudden cardiac death[12-16] leading to a common belief that the cause of sudden cardiac death was left ventricular hypertrophy and fibrosis. In CKD, other major risk factors for LVH are hypertension, anemia, and volume overload. However, aggressive correction of these abnormalities leads to regression of LVH in less than 50% of dialysis patients[17], supporting the importance of factors unique to CKD are likely at least in part causative of the high cardiovascular burden.
CKD-MBD is a systemic disorder of abnormal mineral metabolism biochemistries, bone remodeling, and extra-skeletal (arterial) calcification[18]. The disorder of CKD-MBD is unique to CKD and is a major pathogenic factor for cardiovascular disease (CVD) of CKD in observational data and limited clinical trials in CKD patients[19-24]. As discussed elsewhere in this issue, fibroblast growth factor 23 (FGF23) and klotho may have direct roles in the LVH of CKD. Hyperphosphatemia is a major pathogenic factor in arterial calcification and large vessel arterial calcification increases in prevalence with progression of CKD and is associated with systolic hypertension, amputation, and LVH (via increased afterload)[25]. In addition, small vessel arteriole calcification between cardiomyocytes has been observed and may also contribute to arrhythmias and cardiac hypertrophy[26]. Other issues in this series examine the role of phosphorus, parathyroid hormone (PTH), FGF23, and klotho in the pathogenesis of cardiovascular disease. This article focuses on the importance of calcium in the pathogenesis of cardiovascular disease in CKD.
Calcium overload versus hypercalcemia
Less than 1% of total body calcium is present in measurable extracellular space, and the total calcium measured in blood samples reflects both the ionized calcium and that bound to other anions and albumin. Unfortunately, ionized calcium levels are difficult to reliably measure in outpatient dialysis units and the formulas for ‘correcting’ calcium are notoriously inaccurate in CKD[27]. Thus, studies identifying associations of serum calcium levels with outcomes in advanced CKD are limited by this issue of measurement. Nonetheless, some, but not all, studies have identified both low and high calcium levels associated with mortality in patients with CKD[28-30]. In patients on dialysis, levels of calcium greater than 10.2 mg/dl are associated with increased mortality[31]. However, calcium balance or calcium ‘load’ may be more reflective of disordered calcium homeostasis in CKD.
Calcium balance is the net intake minus the output in feces and urine in patients in steady state. As CKD progresses, the urine calcium excretion drops dramatically; in theory this may be an appropriate compensation to maintain balance in the setting of decreased intestinal calcium absorption due to decreased 1,25-vitamin D. However, many patients receive calcitriol and/or calcium in the form of a calcium based phosphate binder. Intestinal calcium absorption is both active (calcitriol mediated) and passive, and thus excess calcium binder will undoubtedly be absorbed. Our group performed formal balance studies in humans with CKD stage 4 (average eGFR 36 ml/min), and demonstrated positive calcium balance in excess of almost 500 mg per day with the administration of 1500 mg of calcium carbonate on top of a normal (1000 mg calcium diet)[32]. Using radiolabeled calcium, the study further demonstrated that the positive calcium balance resulted in net retention of calcium outside the extracellular space, but could not discern if this was in bone or in soft tissues[33].
In our Cy/+ rat model of progressive kidney disease, we examined the role of calcium ‘load’ (positive calcium balance) versus calcium levels on arterial and cardiac calcification. We treated rats with advanced CKD with calcium administered in drinking water, the calcimimetic R-568, and R-568 plus calcium compared versus no treatment[34]. The biochemical profile and magnitude of arterial and cardiac calcification are shown in Table 1. Treatment with calcium in the drinking water led to increased thoracic aorta, heart, and aortic valve calcification regardless of the serum level of calcium. The calcium treatment led to an even greater calcification than observed with hyperphosphatemia and normal calcium levels[34]. These data suggest that positive calcium balance, regardless of whether hypercalcemia or hyperphosphatemia is present may alter calcification.
Table 1.
Is it hypercalcemia or increased calcium intake that matters?
| Treatment | “clinical” Scenario |
Thoracic calcification |
Heart calcification |
Valve calcification |
|---|---|---|---|---|
| None (control CKD animals) |
SHPT High phosph, nl Ca |
↔ | ↔ | ↔ |
| R-568 | Controlled PTH High phosph Low calcium |
↔ | ↓ ↓ | ↓ ↓ |
| R-568 + calcium | Controlled PTH Normal phosph Normal calcium *calcium load |
↔ ↑ | ↑ ↑ | ↔ ↑ |
| Calcium alone | Controlled PTH Normal phosph High calcium *hypercalcemia and calcium load |
↔ ↑ | ↑ ↑ | ↔ ↑ |
Studies utilizing advanced stage CKD rats treated as in the first column. SHPT = secondary hyperparathyroidism; PTH = parathyroid, phosph = phosphorus. Arrows represent increase or decrease compared to control CKD animals. Adapted from [34]
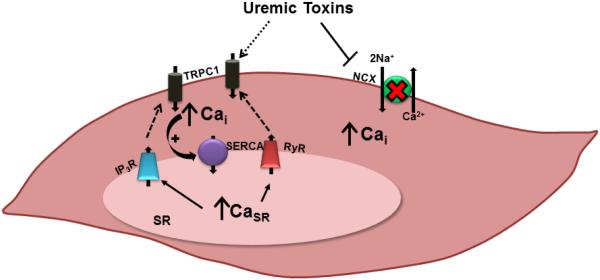
These human and animal data suggest that positive calcium balance may be one mechanism by which cardiovascular disease occurs through extra-skeletal calcification. What are the consequences of positive calcium balance in CKD? Obviously this increases the substrate for calcification of arteries including intra-myocardial arterioles. In addition, excess calcium intake may alter normal bone remodeling and responsiveness to PTH. Hypercalcemia also appears to stimulate FGF23, and elevated FGF23 [23] may predispose to cardiomyoctye hypertrophy. In addition, prolonged exposure to calcium may also alter the expression calcium transporters/channels. Calcium is a critical cell signaling pathway involved in nearly every cell process. As such, the intracellular calcium ([Ca2+]i) level is tightly controlled. In both heart and vascular smooth muscle cells, there are many transporters/channels on the cell membrane and the sarcoplasmic (or endoplasmic) reticulum as shown in Figure 1.
Figure 1. A Schematic of Vascular Smooth Muscle Cell and Cardiomyocyte Calcium Regulating Transporters and Channels.
The presence of a variety of uremic toxins in the extracellular environment may either directly or indirectly contribute to the described perturbations in intracellular Ca2+ regulation. Elevated SERCA function drives increases in SR Ca2+ store capacity. To compensate for SR Ca2+ store overload, SR Ca2+ is released either by the RyR or by the IP3R, initiating signaling mechanisms triggering store-operated Ca2+ entry through TRPC1 channels. This phenomenon, together with reductions in NCX expression, cause resting [Ca2+]i to become elevated in CKD. TRPC1 = transient receptor potential canonical 1 channel; NCX = Na+/Ca2+ exchanger; Cai = intracellular free Ca2+; IP3R = inositol 1,4,5-trisphosphate receptor; SERCA = sarco/endoplasmic reticulum Ca2+ ATPase; RyR = ryanodine receptor; SR = sarcoplasmic reticulum; CaSR = SR Ca2+. (From [47])
Calcium and Cardiac Abnormalities
Calcium regulates cardiomyocyte contraction, growth and remodeling. Abormalities in calcium dependent cardiac ion channel remodeling has been reported in various animal models of hypertrophic cardiomyopathy and predisposes to arrhythmias[35]. Cardiac conduction is dependent on a well-orchestrated set of action potentials. Following an initial sodium influx (Phase 0), there is a transient potassium efflux (Phase 1). Phase 2 of the action potential requires an influx of Ca+2 through L-type channels, and Phase 3 involves appropriate closure of such channels. The sarcoplasmic reticulum acutely ‘buffers’ intracellular calcium to help avoid prolonged changes in [Ca2+]i. A disconnect between sodium and calcium influx, and potassium efflux, from the cell membrane results in prolonged [Ca2+]i and prolonged action potential; this increases the effective and relative refractory period in the cardiac cycle allowing for non-propagated depolarization and arrhythmias. A disconnect between sarcoplasmic reticulum calcium efflux and cell membrane calcium influx can result in prolonged calcium transients. Increased [Ca2+]i will activate cell signaling pathways such as the transcription factor NFAT that activates ‘fetal’ pathways of growth in cardiomyocytes via upregulation of atrial natriuretic peptide and brain natriuretic peptide (ANP and BNP) leading to hypertrophy. Thus, the activity and expression of transporters and channels that regulate all aspects of cellular calcium homeostasis may be involved in the pathogenesis of both left ventricular hypertrophy and arrhythmias[35].
Many factors will increase intracellular calcium including hormones that are involved in CKD-MBD. Rat cardiomyocytes incubated with PTH have a rapid increase in intracellular calcium, leading to apoptosis. Incubation of the cells with uremic serum from animals reproduced these findings but not if the animal had undergone parathyroidectomy[36]. In pre-dialysis patients, PTH is a major risk factor for heart failure and myocardial infarction, even after adjustment for age and diabetes[37]. In dialysis patients, elevated PTH is associated with cardiomyopathy, and parathyroidectomy improves left ventricular function[38, 39]. Elevations in FGF23 in CKD patients is associated with progression of LVH, and FGF23 can directly induce LVH and cardiomyocyte hypertrophy in mice through the phospholipase-C-calcineurin-NFAT axis[23]. In addition to direct effects of these hormones on intracellular signaling pathways, elevations of both PTH and FGF23 lead to increased circulating serum calcium-phosphorus product. Such elevation is kept in check through a circulating protein that binds excess mineral (calcium and phosphorus) called fetuin-A; low levels of which associated with increased cardiovascular mortality in CKD[40]. In an animal model of fetuin-A deficiency there is diffuse cardiac calcification, fibrosis and diastolic dysfunction but no large arterial calcification[41] proving that disordered mineral metabolism can induce cardiomyopathy even in the absence of arterial calcification.
Thus, CKD-MBD may induce cardiac abnormalities through 1) direct effects to alter cell signaling due to increased [Ca2+]i from altered function and expression of calcium transport exchangers and channels 2) extra-skeletal deposition of calcium and phosphate in the myocardium and small cardiac arterioles, 3) inducing cardiomyocyte hypertrophy through calcium and hormone activation of NFAT signaling mechanisms , and 4) increased aorta calcification resulting in chronic increased afterload leading to cardiac hypertrophy.
Calcium and vascular smooth muscle phenotype
Vascular smooth muscle cells (VSMC) have a remarkable ability to change phenotype from a contractile to synthetic phenotype in response to a number of stimuli. This change facilitates normal remodeling, but if sustained, can lead to abnormal remodeling and further cell differentiation to a chondro-osteogenic phenotype. In CKD models, VSMC exhibit phenotypic plasticity and transform from a healthy contractile phenotype to synthetic and osteogenic phenotypes, ultimately resulting in vascular calcification, a major contributor to adverse CV outcomes [42].
Myocardin is considered the master regulator of the VSMC phenotypic switch from a contractile to synthetic state; many circulating and locally produced factors that are associated with arteriosclerosis regulate myocardin[43]. The majority of these stimuli signal through intracellular free calcium ([Ca2+]i) mediated pathways. In animal models, altered [Ca2+]i homeostasis has been found in atherosclerosis [44] and arteriosclerosis[45]. We recently demonstrated decreased expression of myocardin in ex vivo arterial tissue and cultured VSMC from CKD animals compared to normal littermates [46] and an increase in resting [Ca2+]i in freshly isolated VSMC in CKD compared to normal animal[47].
Similar to cardiomyocytes, [Ca2+]i is tightly regulated by proteins that facilitate Ca2+ transport across the plasma membrane and other proteins that transport Ca2+ across the sarcoplasmic reticulum (SR), where the majority of [Ca2+]i is sequestered (Figure 1). When VSMCs transform from a contractile to proliferative phenotype, there are changes in Ca2+ regulatory proteins, such as decreased expression of the ryanodine receptor (RyR) and up-regulation of inositol 1,4,5-triphosphate receptor (IP3R), and sarcoplasmic reticulum Ca++-ATPase (SERCA2a), with overall increasing resting [Ca2+]i [48]. Changes in [Ca2+]i regulate transcription factors including cAMP response element binding protein, nuclear factor of activated T lymphocytes (NFAT), and serum response factor [49]. As [Ca2+]i increases with disease there is a complicated series of events that triggers the phenotype of the VSMC to change from a quiescent contractile cell to a proliferative synthetic cell through down-regulation of genes such as α-SM actin and up-regulation of genes involved in proliferation.
Lessons learned from a progressive model of CKD-MBD
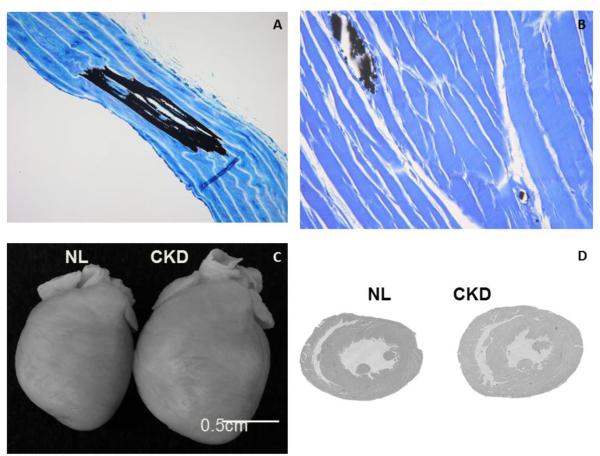
Our laboratory has characterized a slowly progressive model of CKD, the Cy/+ rat[34, 50-53]. This is an autosomal dominant cystic kidney disease model without defects in the cilia. The slow progression allows for interventions that mimic the timing and prolonged course of human disease. The model progressively develops all three manifestations of CKD-MBD including biochemical abnormalities, bone abnormalities (remodeling and strength), and medial calcification in the aorta (Fig 2A). In addition, we see cardiovascular disease that parallels that observed in humans: intra-myocardial arteriole calcification (Fig 2B), LVH (Figure 2C and D)[53] spontaneous ventricular arrhythmias on surface ECG[53], and sudden cardiac death[54]. As noted above, the administration of calcium to these animals exacerbated the medial arterial calcification[34] and increases FGF23[55].
Figure 2. Vascular and Cardiac Abnormalities in the Cy/+ CKD Rat Model of Progressive CKD.
As CKD progresses in the Cy/+ rat model of kidney disease, the animals develop medial calcification of the aorta (black in A, von Kossa stain; from[50]) and small arteriole calcification in the heart (black in B, von Kossa stain, unpublished). In addition, the animals develop left ventricular hypertrophy (C, D from[53]).
Calcium and Cardiac Disease
To study arrhythmias in our CKD animal model, we used a Langendorff heart preparation that allowed us to assess a ‘pseudo ECG’ via widely spaced electrodes on ex vivo perfused hearts[26]. Optical mapping was done with a calcium sensitive dye to acquire calcium fluorescence with widely spaced bipolar electrodes. A pacing lead was also placed and the pacing cycle length (PCL) was progressively shortened to induce arrhythmias. The electrophysiology studies demonstrated that in the CKD rats, compared to normal control rats, there was prolonged action potential duration, increased susceptibility to alternans (electrical instability), occurrence of early depolarization, and increased ventricular fibrillation inducibility [26]. Moreover, spontaneous heart rhythm disorders occurred without any external stimulus in CKD rats. CKD rats exhibited higher frequency of premature ventricular contractions (PVCs), which has been shown to be associated with increased risk for sudden cardiac death. One CKD rat showed spontaneous atrial fibrillation during electrocardiography examination.
These studies demonstrate the occurrence of cardiac electrical remodeling abnormalities that favor onset and maintenance of ventricular fibrillation and thus predisposes to sudden cardiac death. These remodeling abnormalities indicate altered cellular calcium homeostasis with reduced SR calcium content[53]. Our data identified Cav1.2, one of the major cardiac calcium channels was down-regulated and the sodium-calcium exchanger 1 ( NCX1) was up-regulated and the sarcoplasmic reticulum Ca++-ATPase (SERCA2a) was down-regulated[53]. These abnormalities of intracellular calcium handling may play a role in ventricular arrhythmogenesis. Our animals had increased PTH and FGF23 which can increase [Ca2+]i in isolated cardiomyocytes and may explain our findings. In addition, increased angiotensin II levels can also induce an increase in [Ca2+]i [56]. However, we performed electrophysiology studies ex vivo without circulating PTH, FGF23 or angiotensin II. In a previous report profound fibrosis was found in hearts from a 5/6th nephrectomy model of CKD[57] . In contrast, in our study, we found only limited fibrosis, quantified using picrosirus red staining[53]. Histological studies showed myocardial calcification in CKD rats that involved the conduction system including the sinus node (unpublished data). Thus, at present, studies in humans and animals suggest multiple pathways as a cause of arrhythmias including altered expression/activity of calcium transport proteins which may lead to changes in [Ca2+]I, small vessel and coronary artery calcification, and cardiomyocyte hypertrophy and fibrosis.
We also noted a high incidence of sudden death of the CKD rats after 35 weeks of age (about 10% of normal kidney function) prompting evaluation of possible cardiac etiologies. Thus, we tested the hypotheses that detection of subcutaneous nerve activity (SCNA) can be used to estimate sympathetic tone and that reduction of sympathetic nerve activation (SCNA) precedes the spontaneous arrhythmic death of Cy/+ rats[58]. Radio transmitters were implanted in ambulatory normal (N=6) and Cy/+ (CKD; N=6) rats to record electrocardiogram and subcutaneous nerve activity (SCNA). Compared to normal rats, the CKD rats had reduced baseline heart rate and sympathetic nerve activation. In addition, when sympathetic activity was increased, there was an inappropriate low heart rate increase in CKD but not normal animals. All of the CKD rats died suddenly, proceeded by sinus bradycardia, advanced (2nd and 3rd degree) atrioventricular (AV) block (N=6) and/or ventricular tachycardia or fibrillation (N=3). Sudden death was preceded by a further reduction of quantified sympathetic nerve activity and sinus bradycardia. Thus, abrupt reduction of sympathetic tone precedes AV block, ventricular arrhythmia and sudden death of the CKD rats[58]. These studies in rats parallel observations from studies in patients on dialysis that demonstrate severe bradycardia precedes sudden cardiac death [59]. The etiology of the abnormal sympathetic response may be neuropathy, and or calcification and fibrosis at innervation sites in the heart where the nerves travel along the small arterioles.
Calcium signaling and vascular smooth muscle cell phenotype
Using freshly isolated VSMC from our CKD rats, we tested the hypothesis that there is increased [Ca2+]i in VSMC with progressive loss of kidney function [47]. In this study, we freshly isolated aorta VSMC to better evaluate the in vivo state, as cultured cells immediately take on a proliferative phenotype[48]. Our results demonstrated that compared to normal animals, resting [Ca2+]i levels were lower in early CKD, but were elevated in advanced CKD indicating that progressive uremia leads to a rise in VSMC [Ca2+]i [47]. This increase in resting [Ca2+]I was due, at least in part, to altered SERC2a function and impaired extrusion of calcium and decreased Na+/Ca2+ exchanger-1 (NCX1) expression.
Given the known alterations of PTH and FGF23 in CKD-MBD, we treated animals with calcium in the drinking water to simulate a calcium based phosphate binder. These animals had slightly lower phosphorus and higher calcium and a marked reduction in calcium and FGF23. With these derangements there was no difference in resting [Ca2+]i. However, SERCA activity NCX1 expression were lower in the CKD+Ca animals compared to CKD without calcium[47]. Based on these findings, one would have expected the baseline [Ca2+]i to be greater in CKD+Ca animals than in the CKD animals, but we did not observe that. This may have been due to small sample size and normal animal variability or other factors that counteracted the SR defect by increasing extracellular calcium influx such as elevated FGF23, angiotensin and/or oxidative stress. More work is required to understand the almost certain multi-factor etiology.
Conclusion
Patients with CKD have increased cardiovascular disease and association studies demonstrate a clear link between CKD-MBD and cardiovascular disease. The complex biochemical milieu and the treatments used for CKD-MBD alter calcium balance and calcium homeostasis. By so doing, there may be adaptive changes in calcium transporters/channels, and calcification of cardiac conduction system and arteries and arterioles increasing the risk of arrhythmias and sudden cardiac death. Similarly, changes in calcium transporters/channels may also lead to altered intracellular calcium/cell signaling, predisposing to a proliferative VSMC state. Such a state not only leads to arteriosclerosis, but also to vascular calcification.
Our initial studies using our CKD rat model demonstrate calcification in the arterioles of the hearts and abnormal cardiac conduction leading to sudden cardiac death. In addition, we identified increased [Ca2+]I in freshly isolated VSMC from our rats, a finding that increased with progression of CKD. Although the resting [Ca2+]I was not different when the animals received oral calcium, there were alterations in calcium transport proteins suggesting that either the calcium itself, or the hormonal changes that positive calcium balance induces, may be partly responsible. Unraveling the role of calcium and the interaction of calcium with the many other abnormalities present in CKD-MBD and uremia in the pathogenesis of these changes will require further studies.
Acknowledgements
Dr. Moe was supported by National Institutes of Health [grants R01-AR058005 and DK100306] and a VA Merit Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soliman EZ, Prineas RJ, Go AS, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC) Am Heart J. 2010;159(6):1102–7. doi: 10.1016/j.ahj.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naruse Y, Tada H, Sekiguchi Y, et al. Concomitant chronic kidney disease increases the recurrence of atrial fibrillation after catheter ablation of atrial fibrillation: a mid-term follow-up. Heart Rhythm. 2011;8(3):335–41. doi: 10.1016/j.hrthm.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 3.Herzog CA, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80(6):572–86. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 4.Baber U, Howard VJ, Halperin JL, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011;4(1):26–32. doi: 10.1161/CIRCEP.110.957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney International. 2000;58(1):353–62. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 6.Desai AA, Nissenson A, Chertow GM, et al. The relationship between laboratory-based outcome measures and mortality in end-stage renal disease: a systematic review. Hemodial Int. 2009;13(3):347–59. doi: 10.1111/j.1542-4758.2009.00377.x. [DOI] [PubMed] [Google Scholar]
- 7.Green D, Roberts PR, New DI, et al. Sudden cardiac death in hemodialysis patients: an in-depth review. Am J Kidney Dis. 2011;57(6):921–9. doi: 10.1053/j.ajkd.2011.02.376. [DOI] [PubMed] [Google Scholar]
- 8.Mann JF, Gerstein HC, Pogue J, et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134(8):629–36. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Goldenberg I, Moss AJ, McNitt S, et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2006;98(4):485–90. doi: 10.1016/j.amjcard.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 10.Shamseddin MK, Parfrey PS. Sudden cardiac death in chronic kidney disease: epidemiology and prevention. Nat Rev Nephrol. 2011;7(3):145–54. doi: 10.1038/nrneph.2010.191. [DOI] [PubMed] [Google Scholar]
- 11.Saxon LA, Bristow MR, Boehmer J, et al. Predictors of sudden cardiac death and appropriate shock in the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Trial. Circulation. 2006;114(25):2766–72. doi: 10.1161/CIRCULATIONAHA.106.642892. [DOI] [PubMed] [Google Scholar]
- 12.Aoki J, Ikari Y, Nakajima H, et al. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int. 2005;67(1):333–40. doi: 10.1111/j.1523-1755.2005.00086.x. [DOI] [PubMed] [Google Scholar]
- 13.Mall G, Huther W, Schneider J, et al. Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant. 1990;5(1):39–44. doi: 10.1093/ndt/5.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Ansari A, Kaupke CJ, Vaziri ND, et al. Cardiac pathology in patients with end-stage renal disease maintained on hemodialysis. Int J Artif Organs. 1993;16(1):31–6. [PubMed] [Google Scholar]
- 15.Amann K, Kronenberg G, Gehlen F, et al. Cardiac remodelling in experimental renal failure--an immunohistochemical study. Nephrol Dial Transplant. 1998;13(8):1958–66. doi: 10.1093/ndt/13.8.1958. [DOI] [PubMed] [Google Scholar]
- 16.Losi MA, Memoli B, Contaldi C, et al. Myocardial fibrosis and diastolic dysfunction in patients on chronic haemodialysis. Nephrol Dial Transplant. 2010;25(6):1950–4. doi: 10.1093/ndt/gfp747. [DOI] [PubMed] [Google Scholar]
- 17.Glassock RJ, Pecoits-Filho R, Barberato SH. Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol. 2009;4(Suppl 1):S79–91. doi: 10.2215/CJN.04860709. [DOI] [PubMed] [Google Scholar]
- 18.Moe S, Drueke T, Cunningham J, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69(11):1945–53. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 19.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–18. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 20.Akmal M, Barndt RR, Ansari AN, et al. Excess PTH in CRF induces pulmonary calcification, pulmonary hypertension and right ventricular hypertrophy. Kidney International. 1995;47(1):158–63. doi: 10.1038/ki.1995.18. [DOI] [PubMed] [Google Scholar]
- 21.Gutierrez OM, Januzzi JL, Isakova T, et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutierrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–92. doi: 10.1056/NEJMoa0706130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faul C, Amaral AP, Oskouei B, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Investigators ET, Chertow GM, Block GA, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–94. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 25.London GM. Cardiovascular calcifications in uremic patients: clinical impact on cardiovascular function. J Am Soc Nephrol. 2003;14(9 Suppl 4):S305–9. doi: 10.1097/01.asn.0000081664.65772.eb. [DOI] [PubMed] [Google Scholar]
- 26.Hsueh CH, Chen NX, Lin SF, et al. Pathogenesis of arrhythmias in a model of CKD. J Am Soc Nephrol. 2014;25(12):2812–21. doi: 10.1681/ASN.2013121343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clase CM, Norman GL, Beecroft ML, et al. Albumin-corrected calcium and ionized calcium in stable haemodialysis patients. Nephrol Dial Transplant. 2000;15(11):1841–6. doi: 10.1093/ndt/15.11.1841. [DOI] [PubMed] [Google Scholar]
- 28.Block GA, Hulbert-Shearon TE, Levin NW, et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. American Journal of Kidney Diseases. 1998;31(4):607–17. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 29.Floege J, Kim J, Ireland E, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26(6):1948–55. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller JE, Kovesdy CP, Norris KC, et al. Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. Am J Nephrol. 2010;32(5):403–13. doi: 10.1159/000319861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivara MB, Ravel V, Kalantar-Zadeh K, et al. Uncorrected and Albumin-Corrected Calcium, Phosphorus, and Mortality in Patients Undergoing Maintenance Dialysis. J Am Soc Nephrol. 2015;26(7):1671–81. doi: 10.1681/ASN.2014050472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill KM, Martin BR, Wastney ME, et al. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int. 2013;83(5):959–66. doi: 10.1038/ki.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hill KM, Martin BR, Wastney ME, et al. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int. 2012 doi: 10.1038/ki.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moe SM, Seifert MF, Chen NX, et al. R-568 reduces ectopic calcification in a rat model of chronic kidney disease-mineral bone disorder (CKD-MBD) Nephrol Dial Transplant. 2009 doi: 10.1093/ndt/gfp078. [DOI] [PubMed] [Google Scholar]
- 35.Nuss HB, Kaab S, Kass DA, et al. Cellular basis of ventricular arrhythmias and abnormal automaticity in heart failure. Am J Physiol. 1999;277(1):H80–91. doi: 10.1152/ajpheart.1999.277.1.H80. Pt 2. [DOI] [PubMed] [Google Scholar]
- 36.Bogin E, Massry SG, Harary I. Effect of parathyroid hormone on rat heart cells. J Clin Invest. 1981;67(4):1215–27. doi: 10.1172/JCI110137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganesh SK, Stack AG, Levin NW, et al. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol. 2001;12(10):2131–8. doi: 10.1681/ASN.V12102131. [DOI] [PubMed] [Google Scholar]
- 38.Goldsmith DJ, Covic AA, Venning MC, et al. Blood pressure reduction after parathyroidectomy for secondary hyperparathyroidism: further evidence implicating calcium homeostasis in blood pressure regulation. American Journal of Kidney Diseases. 1996;27(6):819–25. [Google Scholar]
- 39.Drueke T, Fauchet M, Fleury J, et al. Effect of parathyroidectomy on left-ventricular function in haemodialysis patients. Lancet. 1980;1(8160):112–4. doi: 10.1016/s0140-6736(80)90602-9. [DOI] [PubMed] [Google Scholar]
- 40.Ketteler M, Bongartz P, Westenfeld R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361(9360):827–33. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 41.Merx MW, Schafer C, Westenfeld R, et al. Myocardial stiffness, cardiac remodeling, and diastolic dysfunction in calcification-prone fetuin-A-deficient mice. J Am Soc Nephrol. 2005;16(11):3357–64. doi: 10.1681/ASN.2005040365. [DOI] [PubMed] [Google Scholar]
- 42.Chen NX, Moe SM. Vascular calcification: pathophysiology and risk factors. Curr Hypertens Rep. 2012;14(3):228–37. doi: 10.1007/s11906-012-0265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Z, Wang DZ, Pipes GC, et al. Myocardin is a master regulator of smooth muscle gene expression. Proc Natl Acad Sci U S A. 2003;100(12):7129–34. doi: 10.1073/pnas.1232341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 45.Kisters K, Wessels F, Kuper H, et al. Increased calcium and decreased magnesium concentrations and an increased calcium/magnesium ratio in spontaneously hypertensive rats versus Wistar-Kyoto rats: relation to arteriosclerosis. Am J Hypertens. 2004;17(1):59–62. doi: 10.1016/j.amjhyper.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Chen NX, Kiattisunthorn K, O'Neill KD, et al. Decreased MicroRNA Is Involved in the Vascular Remodeling Abnormalities in Chronic Kidney Disease (CKD) PLoS One. 2013;8(5):e64558. doi: 10.1371/journal.pone.0064558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rodenbeck SD, Zarse CA, McKenney-Drake ML, et al. Intracellular calcium increases in vascular smooth muscle cells with progression of CKD in a rat model. Nephrol Dial Transplant. 2016 doi: 10.1093/ndt/gfw274. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, et al. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol. 2008;295(3):C779–90. doi: 10.1152/ajpcell.00173.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kudryavtseva O, Aalkjaer C, Matchkov VV. Vascular smooth muscle cell phenotype is defined by Ca2+-dependent transcription factors. FEBS J. 2013;280(21):5488–99. doi: 10.1111/febs.12414. [DOI] [PubMed] [Google Scholar]
- 50.Moe SM, Chen NX, Seifert MF, et al. A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009;75(2):176–84. doi: 10.1038/ki.2008.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moe SM, Radcliffe JS, White KE, et al. The pathophysiology of early-stage chronic kidney disease-mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J Bone Miner Res. 2011;26(11):2672–81. doi: 10.1002/jbmr.485. [DOI] [PubMed] [Google Scholar]
- 52.Allen MR, Chen NX, Gattone VH, 2nd, et al. Skeletal effects of zoledronic acid in an animal model of chronic kidney disease. Osteoporos Int. 2013;24(4):1471–81. doi: 10.1007/s00198-012-2103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsueh CH, Chen NX, Lin SF, et al. Pathogenesis of Arrhythmias in a Model of CKD. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013121343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, Chen NX, Shirazi JT, et al. Subcutaneous nerve activity and mechanisms of sudden death in a rat model of chronic kidney disease. Heart Rhythm. 2016;13(5):1105–12. doi: 10.1016/j.hrthm.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moe SM, Chen NX, Newman CL, et al. A Comparison of Calcium to Zoledronic Acid for Improvement of Cortical Bone in an Animal Model of CKD. J Bone Miner Res. 2014;29(4):902–10. doi: 10.1002/jbmr.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang LN, Wang C, Lin Y, et al. Involvement of calcium-sensing receptor in cardiac hypertrophy-induced by angiotensinII through calcineurin pathway in cultured neonatal rat cardiomyocytes. Biochem Biophys Res Commun. 2008;369(2):584–9. doi: 10.1016/j.bbrc.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 57.Koleganova N, Piecha G, Ritz E, et al. Calcitriol ameliorates capillary deficit and fibrosis of the heart in subtotally nephrectomized rats. Nephrol Dial Transplant. 2009;24(3):778–87. doi: 10.1093/ndt/gfn549. [DOI] [PubMed] [Google Scholar]
- 58.Zhao Y, Chen NX, Shirazi JT, et al. Subcutaneous nerve activity and mechanisms of sudden death in a rat model of chronic kidney disease. Heart Rhythm. 2015 doi: 10.1016/j.hrthm.2015.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong MC, Kalman JM, Pedagogos E, et al. Temporal distribution of arrhythmic events in chronic kidney disease: Highest incidence in the long interdialytic period. Heart Rhythm. 2015;12(10):2047–55. doi: 10.1016/j.hrthm.2015.06.033. [DOI] [PubMed] [Google Scholar]