Abstract
Objective:
It has been well documented that oxidative stress is involved in the pathogenesis of cardiac diseases. Previous studies have shown that pomegranate seed oil (PSO) has antioxidant properties. This study was designed to investigate probable protective effects of PSO against hydrogen peroxide (H2O2)-induced damage in H9c2 cardiomyocytes.
Materials and Methods:
The cells were pretreated 24 hr with PSO 1 hr before exposure to 200 µM H2O2. Cell viability was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) assay. The level of reactive oxygen species (ROS) and lipid peroxidation were measured by fluorimetric methods.
Results:
H2O2 significantly decreased cell viability which was accompanied by an increase in ROS production and lipid peroxidation and a decline in superoxide dismutase activity. Pretreatment with PSO increased viability of cardiomyocytes and decrease the elevated ROS production and lipid peroxidation. Also, PSO was able to restore superoxide dismutase activity.
Conclusion:
PSO has protective effect against oxidative stress-induced damage in cardiomyocytes and can be considered as a natural cardioprotective agent to prevent cardiovascular diseases.
Key Words: Pomegranate seed, Cardiomyocytes, Reactive oxygen species, Super oxide dismutase, Oxidative stress
Introduction
Oxidative stress plays a critical role in the pathophysiology of several major cardiovascular diseases such as atherosclerosis, hypertension, heart failure, and myocardial ischemic reperfusion injury (Higashi et al., 2009 ▶). Oxidative stress causes excessive production of reactive oxygen species (ROS), which is an important event in the development of cardiovascular diseases. ROS accumulation may contribute to a number of cardiovascular disorders (Dhalla et al., 2000 ▶). The cellular sources of ROS generation in the heart are including cardiac myocytes, endothelial cells and neutrophils. Within cardiac myocytes, ROS can be derived from mitochondria, xanthine oxidase, NADPH oxidase and uncoupled nitric oxide synthases (Tsutsui et al., 2011 ▶). Increased ROS result in considerable damage to myocardial cells. Antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) play significant role in neutralization of elevated ROS. ROS damage cellular membrane by inducing lipid peroxidation. Malondialdehyde (MDA) is a major lipid peroxidation product and may reflect the degree of cellular injury (Dhalla et al., 2000 ▶).
Hydrogen peroxide (H2O2), a major source of ROS can lead to cell membrane injury and cause lipid peroxidation and DNA damage in cells. However, antioxidants can protect cells against H2O2-induced cell damage via decreasing ROS production (Winstead et al., 2005 ▶; Sun et al., 2012 ▶). Previous studies have shown that some herbal medicine can protect H9C2 cells against H2O2.
Pomegranate fruit (Punica granatum L.) is used as a fruit and has therapeutic effects in traditional medicine. In vivo and in vitro studies have demonstrated the beneficial effects of pomegranate including anti-microbial, antioxidant, anti-diabetic, and hypolipidemic activities as well as its effect on improving of cardiovascular health (Sadeghian et al., 2011 ▶; Forouzanfar et al., 2013 ▶; Viuda-Martos et al., 2010 ▶). Pomegranate fruit include 78% juice and 22% seed (Kullkarni and Aradhya, 2005 ▶). Pomegranate seeds contain sugars, vitamins, polysaccharides, polyphenols, minerals and low oil (Miguel et al., 2004). Recent studies have found that pomegranate seed is a potential source of nutrients and antioxidants which can be used as a dietary supplement. Recently, studies have shown that pomegranate has several pharmacological activities, such as anti-microbial (El-Sherbini et al., 2010 ▶; Braga et al., 2005 ▶), antioxidant, anti-inflammatory, and anticarcinogenic effects (Lansky and Newman, 2007 ▶). Pomegranate-derived products have shown beneficial effects in the treatment and prevention of cancers, cardiovascular diseases, neurological disorders, diabetes, etc. (Hartman et al., 2006 ▶). Previous investigations have reported that pomegranate seed oil (PSO) causes regeneration of epidermal tissue (Sassano et al., 2009 ▶), boosts the immune system in vivo, reduces hepatic triglycerides and has chemopreventive activity against prostate, breast and colon cancers (Caligiani et al., 2010 ▶). Also, it reduces weight gain, and type 2 diabetes risk (Brian et al., 2009 ▶) and improves menopausal symptoms (Lansky and Newmana, 2007 ▶).
The therapeutic effects of pomegranate seeds may be related to the presence of a variety of active compounds, particularly polyphenols, which have antioxidant properties (Balasundram et al., 2006 ▶). Moreover, PSO is a rich source of polyunsaturated fatty acids (PUFAs) including conjugated linolenic acid (CLA) which is an important therapeutic agent that can be used for improvement of human health (Fadavi et al., 2006 ▶)
Based on these findings, we hypothesized that PSO may have protective effect against H2O2-induced cardiomyopathy. Therefore, the present study was designed to investigate effects of PSO on activity of antioxidant enzyme superoxide dismutase (SOD), lipid peroxidation level and ROS content in H9c2 cardiomyocyte.
Materials and Methods
Reagents and chemicals
H2O2, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT), thiobarbituric acid (TBA), 2,7-dichlorofluorescin diacetate (DCFH-DA), High-glucose Dulbecco’s Modified Eagles Medium (DMEM), penicillin-streptomycin and fetal bovine serum were purchased from Gibco. Trichloro acetic acid (TCA) and malondialdehydebis-(dimethyl acetal) (MDA) were obtained from Merck (Darmstadt, Germany). SOD assay kit was purchased from Randox (Autrim, UK). H9C2 cells were obtained from Pasteur Institute (Tehran, Iran).
Cell Culture and Treatment
H9C2 Cells were maintained at 37oC in a humidified atmosphere containing 5% CO2. The cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin. For the experiments, cells were seeded in 96-well and 24-well culture plates for MTT/ROS assay and MDA assay, respectively. After 24 hr, the cells were pretreated (for 24 hr) with PSO (1-100 µM) and then, incubated with 200 µM H2O2 for 1 hr. Also, cells were exposed to PSO (12-800µg/ml) alone for 24 hr for evaluation of PSO toxicity.
Cell viability assay
Cell viability was determined using a modified 3-(4,5- dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) assay as described previously (Mortazavian et al., 2012; Ghorbani et al., 2014). Briefly, MTT solution in phosphate-buffered saline (5 mg/ml) was added to each well at a final concentration of 0.05%. After 3 hr, formazan precipitate was dissolved in DMSO. The absorbance at 570 and 620 nm (background) was measured using a StatFAX303 plate reader.
Measurement of reactive oxygen species
Intracellular ROS levels were evaluated using a fluorescent probe, DCF-DA. At the end of incubation, the cells were treated (30 min) with DCFH-DA (10µM) at 4 ˚C in the dark. Then, the fluorescence intensity was detected at excitation/emission of 485/530 nm. The experiment was performed in triplicate.
Lipid peroxidation assay
The level of lipid peroxidation was estimated by measuring MDA which is the end product of lipid peroxidation (Sadeghnia et al., 2013 ▶). At the end of incubation, the cells were scraped and centrifuged for 30 min. Then, 400 µl of TCA (15%) and 800 µl of TBA (0.7%) were added to 500 µl of cell samples. The mixture was vortexed and then, heated for 40 min in a boiling water bath. Then, 200 µl of the sample was transferred to 96-well plate and the fluorescence intensity was read at excitation/emission of 480/530 nm. The experiment was carried out in triplicate.
Determination of SOD
The level of SOD activity was determined using a kit which measures SOD activity based on the formation of red formazan from 2-(4-iodophenyl)-3-(4-nitrophe-nol)-5-phenyltetrazolium chloride after reaction with superoxide radicals. At the end of incubation, the cells were harvested with a scraper and centrifuged at 2000 g for 10 min at 4ºC. The supernatant was discarded and the cells were broken using the freeze-thaw method (-20ºC for 20 min, then 37ºC bath for 10 min). Phosphate-buffered saline (1 ml) was added to the cell lysate and the sample was sonicated on an ice bath (60 W with 0.5 sec interval for 15 min). In the final step, cells were centrifuged at 10000 g for 15 min at 4ºC and the optical density of supernatant was read spectrophotometrically at 505 nm.
Statistical analyses
Statistical differences among groups were assessed by one-way ANOVA, followed by the Tukey’s post hoc test. The results are shown as mean ± SEM. Values of p less than 0.05 were considered statistically significant.
Results
Effect of PSO alone on cell viability
As shown in Figure 1, incubation with PSO for 24 hr significantly decreased the viability of cells at concentration of 800 g/ml (84.5 ± 1.58% of control, p<0.05). Other concentrations did not reduce cell viability.
Figure 1.
Effect of PSO alone on cell viability in H9c2 cells. The cells were treated (for 24 hr) with different concentrations of PSO. Data are expressed as mean ± SEM of three separate experiments. *p<0.001 vs control
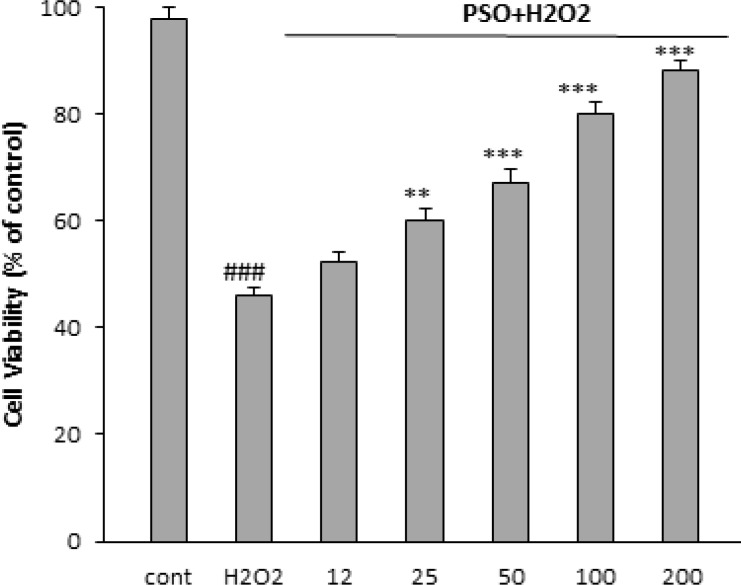
Effect of PSO on cell viability against H 2 O 2
Incubation with H2O2 significantly decreased cell viability to 47 ± 1.5% of control (p<0.001) (Figure 2). Pretreatment with 25, 50, 100 and 200 µg/ml of PSO could increase the viability of H9c2 cells to 60 ± 2.1% (p<0.01), 67± 2.7% (p<0.001), 80.25 ± 2% (p<0.001) and 88± 1.9% (p<0.001), respectively (Figure1). The increase in cell viability at the dose of 12 µg/ml was not significant.
Figure 2.
Effect of PSO on H2O2-induced cytotoxicity in H9c2 cells. The cells were pretreated (for 24 hr) with different concentrations of PSO before to exposure (for 1 hr) to 200 𝜇M of H2O2. Data are expressed as mean ± SEM of three separate experiments. ### p<0.001 vs control, ** p<0.001 and *** p<0.001 versus H2O2
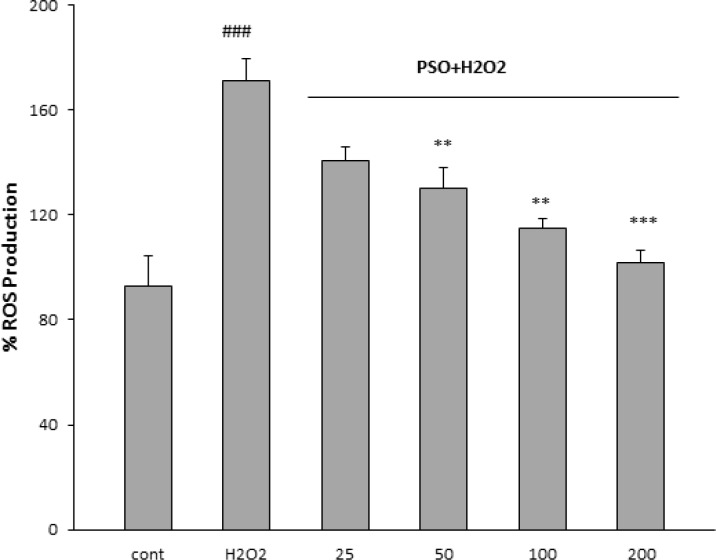
Effect of PSO on ROS content
As expected, H2O2 caused a significant increase in the level of ROS in H9c2 cells as compared to the control (171±8.3%; p<0.001). PSO at concentrations of 50 (130±7.5%, p<0.01); 100 (115±3.6%, p<0.01) and 200 µM (105±4.6%, p<0.001) decreased intracellular ROS level (Figure 3). At concentration of 25 µM did not reduce ROS significantly.
Figure 3.
Effect of PSO on H2O2-induced reactive oxygen species (ROS) generation in H9c2 cells. The cells were pretreated (for 24 hr) with different concentrations of PSO, before exposure (1 hr) to 200 𝜇M of H2O2. Data are expressed as mean ± SEM of three separate experiments. ### p<0.001 vs control, and **p<0.001 and ***p<0.001 vs H2O2.
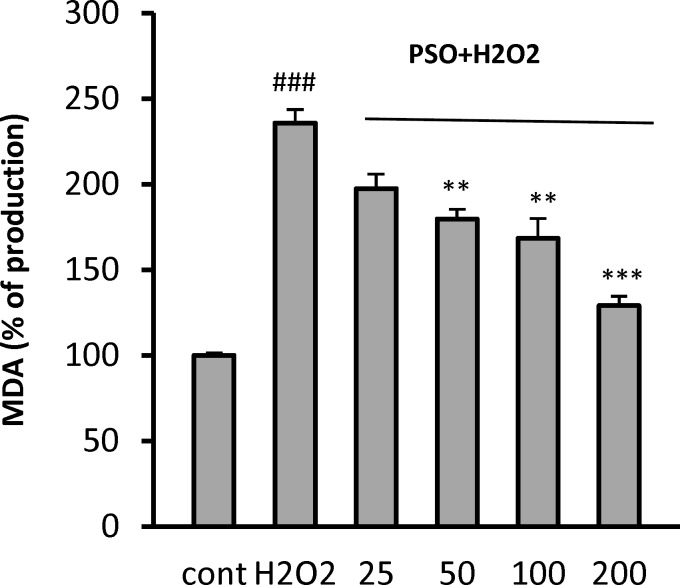
Effect of PSO on Lipid Peroxidation
The level of lipid peroxidation was evaluated by measuring the level of MDA, which is the end product of lipid peroxidation. As shown in Figure 4, exposure of the cells to H2O2 resulted in a significant increase of MDA level (235.7 ± 7.9%, p<0.001) as compared to control cells (100 ± 1.3%). The content of MDA was significantly decreased in the cells pretreated with 50 (179.8 ± 5.6%, p<0.01), 100 (168.4± 11.7, p<0.01) and 200 µg/ml (129± 5, p<0.001) (Figure4).
Figure 4.
Effect of PSO on H2O2-induced MDA production in H9c2 cells. The cells were pretreated (for 24 hr) with different concentrations of PSO before exposure (for 1 hr) to 200 𝜇M of H2O2. Data are expressed as mean ± SEM of three independent experiments. ### p<0.001 vs control, and **p<0.001 and ***p<0.001 vs H2O2
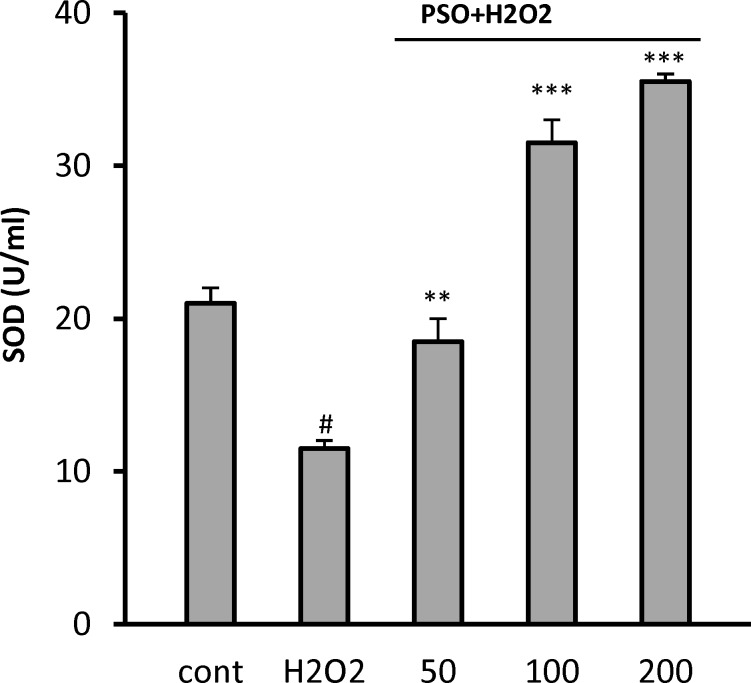
Effect of PSO on Superoxide Dismutase
In order to determine the effect of PSO on cellular antioxidant defenses, the level of SOD was measured (Figure4). H2O2-induced oxidative stress decreased the level of SOD from 21 ± 1 U/ml (control) to 11.5 ± 0.6 U/ml (p<0.05). As compared to the untreated cells, the level of SOD was significantly increased in pretreated cells at PSO concentrations of 50 (18.5 ± 1.5 U/ml, p<0.01), 100 (31 ± 1.8 U/ml, p<0.001), and 200 µg/ml (35 ± 0.6 U/ml, p<0.001) (Figure 5).
Figure 5.
Effect of PSO on superoxide dismutase (SOD) activity following H2O2 treatment in H9c2 cells. The cells were pretreated (for 24 hr) with different concentrations of PSO before exposure (for 1 hr) to 200 𝜇M of H2O2. Data are expressed as mean ± SEM of three separate experiments. # p<0.05 vs control, and **p<0.001 and ***p<0.001 vs H2O2
Discussion
The heart is a target organ for oxidative stress-related injuries. Oxidative stress plays a critical role in the pathophysiology of several major cardiovascular diseases such as atherosclerosis, hypertension, heart failure, and myocardial ischemical reperfusion injury (Higashi et al., 2009 ▶). Poor anti-oxidative capacity of cardiomyocytes subjects them to oxidative damage (Dhalla et al., 2000 ▶). Therefore, a promising approach to cardioprotection is the use of pharmacological means in order to prevent oxidative-damage in the heart. H9c2 is a rat-derived cardiomyoblast cell line that has been used to investigate heart function (L'Ecuyer et al., 2004 ▶). H9c2 cells exhibit morphological characteristics similar to those of immature embryonic cardiomyocytes but preserve several elements of the electrical and hormonal signal pathway found in adult cardiac cells. Evidence have shown that H2O2 induces the same oxidative stress in H9c2 cells as in primary cultured rat cardiomyocytes (Winstead et al., 2005 ▶). Therefore, this cell line may be useful as a model of oxidative stress-induced cardiomyocyte injury. Antioxidants inhibit or delay oxidation process by preventing the initiation or propagation of oxidizing chain reactions. In the present study, protective effect of PSO on H2O2-induced oxidative stress was investigated. Our results showed H2O2 significantly increased the level of ROS, lipid peroxidation and decreased the SOD activity in H9c2 cells. Pretreatment with PSO decreased ROS and MDA production; also, it restored the SOD enzyme activity. Also, treatment of cells with PSO alone did not reduce cell viability except at 800µg/ml. Recent studies have shown that polyphenoles and flavonoids are effective in prevention of some disease such as cardiovascular and inflammatory disorders (Noda et al., 2002 ▶) by inhibition of oxidative stress (Miguel et al., 2004). Previous studies have shown that juice and seed of pomegranate contain antioxidant compounds (Singh et al., 2002 ▶). Studies have shown that antioxidant activity of flavonoids and anthocyanidins in seed oil and juice is three times greater than green tea extract (Okamoto et al., 2004 ▶). PSO has ellagic acid, an antioxidant agent that reduces lipid peroxidation by removing peroxy radical (Ramanathan and Das, 2005 ▶). Moreover, PSO is composed of unsaturated fatty acids as linolenic acids, oleic acid, palmitic acid, and stearic acid. Evidence have suggested that fatty acids with conjugated double bonds may have beneficial effects in inflammation and some types of cancer (Hwang et al., 2007 ▶; Boussetta et al., 2009 ▶) by inhibition of TNFα and prevention of ROS production (Saha and Ghosh, 2009 ▶). Some researchers showed that linolenic acid has antioxidant activity and punicic acid increased antioxidant activity against sodium arsenite-induced oxidative stress (Bouroshaki et al., 2010 ▶; Boroushaki et al., 2013 ▶). Boroushaki and co-workers have shown that PSO has protective effect against hexachlorobutadiene (Boroushaki et al., 2014 ▶) diazinone (Saha et al., 2012 ▶) and mercuric chloride (El-Sherbini et al., 2010 ▶) toxicity in kidney tissue. Tissue protection of PSO may be a result of the presence of antioxidant compounds. Therefore, the protective effect of PSO may be due to the presence of a variety of biologically active compounds. Antioxidant and free radicals scavenging properties of PSO may be partially responsible for its cardioprotective activity. Our results regarding protective action of PSO on cardiomyocyte suggest that it may also induce protective effect against drugs-induced cardiotoxicity. For example, doxorubicin, as an anthracycline antibiotic, is widely used in cancer therapy. However, its clinical usage is limited due to its toxic effect on cardiomyocytes (Xin et al., 2009 ▶). Doxorubicin affects specific enzymes, transporters and metabolic pathways in the cardiac muscle, and may ultimately result in irreversible cardiac failure (Turakhia et al., 2007 ▶). One of the main mechanisms of doxorubicin-induced cardiotoxicity is increase in ROS production (Xin et al., 2009 ▶). According to the results of the present work, PSO antioxidant effect on cardiac cells may reduce cardiotoxic effect of doxorubicin. However, this should be investigated by future studies.
In conclusion, our data demonstrated that PSO has protective effect against hydrogen peroxide-induced cardiomyocyte death. The protective effect was mediated through reducing ROS production and lipid peroxidation. Therefore, PSO may be considered as a natural cardioprotective agent to prevent cardiovascular diseases. But more investigations are needed to elucidate the probable underlying mechanisms of these valuable effects.
Acknowledgment
This work was supported by Pharmacological Research Center of Medicinal Plants, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran (grant number: 910812).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrialby-roducts: antioxidant activity, occurrence and potential uses. Food Chem. 2006;99:191–203. [Google Scholar]
- Boroushaki MT, Arshadi D, Jalili-Rasti H, Asadpour E, Hosseini A. Protective Effect of Pomegranate Seed Oil Against Acute Toxicity of Diazinon in Rat Kidney. Iran J Pharmaceutic Res. 2013;12:821–827. [PMC free article] [PubMed] [Google Scholar]
- Boroushaki MT, Mollazadeh H, Rajabian A, Dolati K, Hoseini A, Paseban M, Farzadnia M. Protective effect of pomegranate seed oil against mercuric chloride-induced nephrotoxicity in rat. Ren Fail. 2014;36:1581–6. doi: 10.3109/0886022X.2014.949770. [DOI] [PubMed] [Google Scholar]
- Bouroshaki MT, Sadeghnia HR, Banihasan M, Yavari S. Protective effect of pomegranate seed oil on hexachlorobutadiene-induced nephrotoxicity in rat kidneys. Ren Fail. 2010;32:612–617. doi: 10.3109/08860221003778056. [DOI] [PubMed] [Google Scholar]
- Boussetta T, Raad H, Lettéron P, Gougerot-Pocidalo MA, Marie JC, Driss F. Punicic acid a conjugated linolenic acid inhibits TNFalpha - induced neutrophil hyperactivation and protects from experimental colon inflammation in rats. PLoS One. 2009;4:e6458. doi: 10.1371/journal.pone.0006458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga LC, Shupp JW, Cummings C. Pomegranate extract inhibits Staphylococcus aureus growth and subsequent enterotoxin production. J Ethnopharmacol. 2005;96:335–339. doi: 10.1016/j.jep.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Brian KM, Strohacker KA, Kueht ML. Pomegranate seed oil consumption during a period of high-fat feeding reduces weight gain and reduces type 2 diabetes risks in CD-1 mice. Br J Nutr. 2009;102:54–59. doi: 10.1017/S0007114508159001. [DOI] [PubMed] [Google Scholar]
- Caligiani A, Bonzanini F, Palla G, Cirlini M, Bruni R. Characterization of a potential nutraceutical ingredient: Pomegranate (Punicagranatum L) seed oil unsaponifiable fraction. Plant Food Hum Nutr. 2010;65:277–283. doi: 10.1007/s11130-010-0173-5. [DOI] [PubMed] [Google Scholar]
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J. Hypertension. 2000;18:655–73. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- El-Sherbini GM, Ibrahim KM, El Sherbiny ET, Abdel-Had NM, Morsy TA. Efficacy of Punicagranatumextract on in-vitro andin-vivo control of Trichomonas vaginalis. J Egypt Soc Parasitol. 2010;40:229–244. [PubMed] [Google Scholar]
- Fadavi A, Barzegar M, Azizi MH. Determination of fatty acids and total lipid content in Oil seed of 25 pomegranates varieties grown in Iran. J Food Comp Anal. 2006;19:676–680. [Google Scholar]
- Ghorbani A, Hadjzadeh MR, Rajaei Z, Zendehbad SB. Effects of fenugreek seeds on adipogenesis and lipolysis in normal and diabetic rat. Pak J Biol Sci. 2014;17:523–528. doi: 10.3923/pjbs.2014.523.528. [DOI] [PubMed] [Google Scholar]
- Forouzanfar F, Goli AA, Assadpour E, Ghorbani A, Sadeghnia HR. Protective effect of Punicagranatum L against serum/glucosedeprivation-induced PC12 cells injury. Evid based Complement Altern Med. 2013;2013:7167730. doi: 10.1155/2013/716730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Shah A, Fagan AM. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411–418. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- Hwang DM, Kundu JK, Shin JW, Lee JC, Lee HJ, Surh YJ. cis-9, trans-11-Conjugated linoleic acid down-regulates phorbol ester-induced NF-𝜅B activation and subsequent COX-2 expression in hairless mouse skin by targeting I𝜅B kinase and PI3K-Akt. Carcinogenesis. 2007;28:363–371. doi: 10.1093/carcin/bgl151. [DOI] [PubMed] [Google Scholar]
- Kullkarni AP, Aradhya SM. Chemical Changes and Antioxidant Activity in Pomegranate Arils during Fruit Development. Food Chem. 2005;93:319–324. [Google Scholar]
- Lansky EP, Newmana RA. Punicagranatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer: Review. J Ethnopharmacol. 2007;109:177–206. doi: 10.1016/j.jep.2006.09.006. [DOI] [PubMed] [Google Scholar]
- L'Ecuyer T, Allebban Z, Thomas R, Vander Heide R. Glutathione S-transferase overexpression protects against anthracycline-induced H9c2 cell death. Am. J Physiol Heart Circ Physiol. 2004;286:H2057–H2064. doi: 10.1152/ajpheart.00778.2003. [DOI] [PubMed] [Google Scholar]
- Miguel G, Dandlen S, Antunes D, Neves A, Martins D. The Effect of Two Methods of Pomegranate (Punicagranatum L) Juice Extraction on Quality during Storage at 4°C. J Biomed Biotech. 2004;5:332–337. doi: 10.1155/S1110724304403064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel G, Fontes C, Antunes D, Neves A, Marthins D. Anthocyanin Concertration of Assaria Pomegranate Fruits during Different Cold Storage Conditions. J Biomed Biotech. 2004;5:338–342. doi: 10.1155/S1110724304403076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavian SM, Ghorbani A, Hesari TG. Effect of hydro-alcoholic extracts of viola tricolor and its fractions on proliferation of cervix carcinoma cells. Iran J Obstet Gynecol Infertility. 2012;15:9–16. [Google Scholar]
- Nagao K, Yanagita T. Conjugated fatty acids in food and their health benefits. J Biosci Bioengineering. 2005;100:152–157. doi: 10.1263/jbb.100.152. [DOI] [PubMed] [Google Scholar]
- Noda Y, Kaneyuki T, Mori A, Packer L. Anitioxdant Activities of Pomegranate Fruit Extract and ItsAnthocyanidins: Delphinidin, Cyanidin, and Pelargonidin. J Agric Food Chem. 2002;50:166–171. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- Okamoto JM, Hamamoto YO, Yamato H, Yoshimura H. Pomegranate Extract Improves a Depressive State and Bone Properties in Menopausal Syndrome Model Ovariectomized Mice. J Ethnopharmacol. 2004;92:93–101. doi: 10.1016/j.jep.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Ramanathan L, Das NP. Inhibitory effects of some natural products on metal-induced lipid oxidation in cooked fish. Biol Trace Elem Res. 1992;34:35–44. doi: 10.1007/BF02783896. [DOI] [PubMed] [Google Scholar]
- Sadeghian A, Ghorbani A, Mohamadi-Nejad A, Rakhshandeh H. Antimicrobial activity of aqueous and methanolic extracts of pomegranate fruit skin. Avicenna J Phytomed. 2011;1:67–73. [Google Scholar]
- Sadeghnia HR, Yousefsani BS, Rashidfar M, Boroushaki MT, Assadpour E, Ghorbani A. Protective effect of rutin on hexachlorobutadiene-induced nephrotoxicity. Ren Fail. 2013;35:1151–1155. doi: 10.3109/0886022X.2013.815546. [DOI] [PubMed] [Google Scholar]
- Saha SS, Ghosh M. Comparative study of antioxidant activity of alpha-eleostearic acid and punicic acid against oxidative stress generated by sodium arsenite. Food Chem Toxicol. 2009;47:2551–2556. doi: 10.1016/j.fct.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Saha SS, Dasgupata P, Sengupata S, Ghosh M. Synergistic effect of conjugated linolenic acid isomers against induced oxidative stress, inflammation and erythrocyte membrane disintegrity in rat model. Biochim Biophys Acta. 2012;1820:1951–1970. doi: 10.1016/j.bbagen.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Sassano G, Sanderson P, Franx J, Groot P, Straalen JV, Bassaganya-Riera J. Analysis of pomegranate seed oil for the presence of jacaric acid. J Sci Food Agr. 2009;89:1046–1052. [Google Scholar]
- Singh RP, Murthy KNC, Jayaprakasha GK. Studies on the Antioxidant Activity of Pomegarante (Punicagranatum) Peel and Seed Extracts Usning in Vitro Models. J Agric Food Chem. 2002;50:81–86. doi: 10.1021/jf010865b. [DOI] [PubMed] [Google Scholar]
- Sun B, Sun GB, Xiao J, Chen RC, Wang X, Wu Y, Cao L, Yang ZH, Sun XB. Isorhamnetin inhibits H₂O₂-induced activation of the intrinsic apoptotic pathway in H9c2 cardiomyocytes through scavenging reactive oxygen species and ERK inactivation. J Cell Biochem. 2012;113:473–485. doi: 10.1002/jcb.23371. [DOI] [PubMed] [Google Scholar]
- Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol. 2011;301:H2181–H90. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- Turakhia S, Venkatakrishnan CD, Dunsmore K, Wong H, Kuppusamy P, Zweier JL, Ilangovan G. Doxorubicin-induced cardiotoxicity: direct correlation of cardiac fibroblast and H9c2 cell survival and aconitase activity with heat shock protein 27. Am J Physiol Heart Circ Physiol. 2007;293:H3111. doi: 10.1152/ajpheart.00328.2007. [DOI] [PubMed] [Google Scholar]
- Viuda-Martos M, Fernandez-L O, Pereza Alvarez JA. Pomegranate and its many functional components as related to human health: a review. Comperhensive Reviews in Food Science and Food Safety. 2010;9:635–654. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- Winstead M, Lucas K, Dennis E. Group IV cytosolic phospholipase A2 mediates arachidonic acid release in H9c2 rat cardiomyocyte cells in response to hydrogen peroxide. Prostagland Other Lipid Med. 2005;78:55–66. doi: 10.1016/j.prostaglandins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Xin H, Liu XH, Zhun Zhu Y. Herbal eonurine attenuates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells. Eur J Pharmacol. 2009;612:75–79. doi: 10.1016/j.ejphar.2009.03.067. [DOI] [PubMed] [Google Scholar]