Abstract
Objective:
Bryonia aspera (Stev. ex Ledeb) is a plant that grows in northeast of Iran. In the present study, cytotoxic and apoptogenic properties of B. aspera root extract was determined against HN-5(head and neck squamous cell carcinoma) and Hela (cervix adenocarcinoma) cell lines.
Materials and Methods:
HN-5 and Hela cell lines were cultured in DMEM medium and incubated with different concentrations of B. aspera root extract. Cell viability was quantitated by MTT assay and the optical absorbance was measured at 570 nm (620 nm as the reference) by an ELISA reader, in each experiment. Apoptotic cells were assessed using PI staining of DNA fragmentation by flow cytometry (sub-G1 peak). The B. aspera inhibited 50% growth (IC50) of Hela and HN-5 cell lines at 100±28 μg/ml and 12.5±4 μg/ml, respectively after 48 hr of incubation.
Results:
Cell viability assay showed that inhibitory effects of B. aspera were time and dose-dependent in both cell lines, which were consistent with morphological changes, observed under light microscope. Apoptosis was investigated by flow cytometry in which percentage of apoptotic cells increased in a dose and time-dependent manner.
Conclusion:
Based on our data, B. aspera has cytotoxic effects in which apoptosis played an important role. Further evaluations are needed to assess the possible anti-tumor properties of this plant.
Key Words: Bryonia aspera, Cytotoxicity, Apoptosis, Cancer
Introduction
Cancer, which has restricted efficient therapies, is one of the leading causes of death (Hsiao and Liu 2010 ▶). As the most of morbid of human cancer, head and neck squamous cell carcinoma is the eighth common cancer disease in the world (Stewart and Kleihues 2003 ▶; Forastiere et al 2001 ▶). HN-5 cells was derived from head and neck squamous carcinoma. Annually, this type of cancer affects approximately 600,000 patients worldwide. Standard treatment strategies include surgery, radiotherapy and chemotherapy. For treatment, several chemotherapeutic agents have been used and in 30–40% of patient-chemotherapy regimens have shown positive responses (Forastiere et al 2001 ▶; Parkin et al 2002 ▶). Worldwide, the second most frequent malignant tumor in women is cervical adenocarcinoma cancer. The cell line was derived from cervical cancer cells taken from Henrietta Lacks, in 1951. Hela cells are human epithelial cells from a fatal cervical carcinoma (Tabrizi et al 2006 ▶; Farjadian et al 2003 ▶). Considering significant levels of toxicity and drug resistance of current anticancer regimens, development of effective drugs with little or no adverse effects is crucial. Naturally occurring chemicals including plants derivatives provide a source of novel and potent bioactive compounds with minimal side effects (Cooper 2004 ▶; Tsao and Zeltzer 2005 ▶).
Herbal therapies and natural remedies are utilized all over the world and several drugs have been originated from herbs (Cooper 2004 ▶; Cooper 2005 ▶).
Bryonia aspera Stev. ex Ledeb (Cucurbitaceae family) is native to Iran. This family has anti-inflammatory, anti-tumor, hepatoprotective, and immunomodulatory activities (Efferth et al 2001 ▶; Tsao and Zeltzer 2005 ▶). Cucurbit plants were recognized to have significant biological values. Ethnopharmacological information show that roots of B. aspera Stev. ex Ledeb, also known as “andaz”, have been traditionally used for treatment of gastrointestinal and cardiac diseases and cancer in the Turkmen Sahra region, north-east of Iran (Ghorbani 2005 ▶).
Despite of these findings, the role of apoptosis in B. aspera induced toxicity has not been understood. Therefore, in this study the cytotoxic and apoptogenic effects of hydro-alcoholic extract of B. aspera against HN-5 and Hela cells were studied.
Materials and Methods
Reagents and Chemicals
Hela and HN-5 cell lines were obtained from Pasteur Institute (Tehran, Iran). Dulbecco’s modified Eagle’s Medium (DMEM), Penicillin-streptomycin solution and fetal calf serums (FCS) were purchased from Gibco (Grand Island, USA). The fluorescent probe propidium iodide (PI), sodium citrate, Triton X-100 and 3-(4, 5-dimethylthiazol-2- yl)-2, 5-diphenyl tetrazolium (MTT) were purchased from Sigma (St Louis, MO, USA). Dimethyl sulfoxide (DMSO) was bought from Merck (Darmstadt, Germany).
Plant material
The root of B. aspera Steven ex Ledeb. was collected from Tirgan Watershed, Razavi Khorasan Province, Iran. The Voucher specimen (No.21733) was deposited in Dargaz Payame Noor University Herbarium.
Preparation of Extract
B. aspera was identified by Pharmacological Research Center of Medicinal Plants. The roots extract was prepared from 20 g of dried and milledroots. The extract was prepared using 90 ml ethanol (70%) by Soxhlet apparatus. After that, the solvent was removed by evaporation at 36-37º C.
Cell culture
Cell lines were kept at 37°C in a humidified atmosphere (90%) containing 5% CO2. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were seeded overnight, and then incubated with various concentrations of B. aspera root extract (12.5 to 500μg/ml) for 24, 48 and 72 hr. For MTT assay, cells were seeded at 5×103 cell/well onto 96-well culture plates. For analysis of apoptosis, cells were seeded at 1×105 cell/well onto a 24-well plate. For each concentration and time course study, there was a control sample, which stayed without extract and received the equal volume of medium. All experiments were performed in triplicate.
Cell viability
Cell viability was determined using a modified 3-(4, 5 dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium (MTT) assay (Mosmann, 1983 ▶; Mousavi et al, 2008 ▶; Mousavi et al, 2009 ▶; Sharifi et al, 2005 ▶).
In brief, cells were seeded at 5×103/well onto flat-bottomed 96-well culture plates and allowed to grow for 24 hr followed by treatment with B. aspera root extract (12.5 to 500μg/ml). After removing the medium, MTT solution was added to cells (5 mg/ml in PBS) for 3 hr. The absorption was quantitated at 570 nm (620 nm as the reference) using an ELISA reader.
Apoptosis
Apoptotic cells were identified using PI staining (Mousavi et al 2008 ▶; Mousavi et al 2009 ▶; Sharifi et al 2005 ▶; Zhang et al 1999 ▶). Briefly, cell lines were cultured overnight in a 24-well plate and treated with B. aspera for 48 hr. Floating and adherent cells were cultured and incubated overnight at 4 °C in the dark with 750 μl of a hypotonic buffer (50 μg/ml PI in 0.1% sodium citrate plus 0.1% triton X-100). Then, apoptosis rate was measured by a FACScan flow cytometer (Becton Dickinson).
Statistical analysis
One way analysis of variance (ANOVA) and Bonferroni’s post hoc were applied for data analysis. All results were expressed as mean±SEM and p<0.05 was considered as statistically significant.
Results
Effect of B. aspera on cell viability
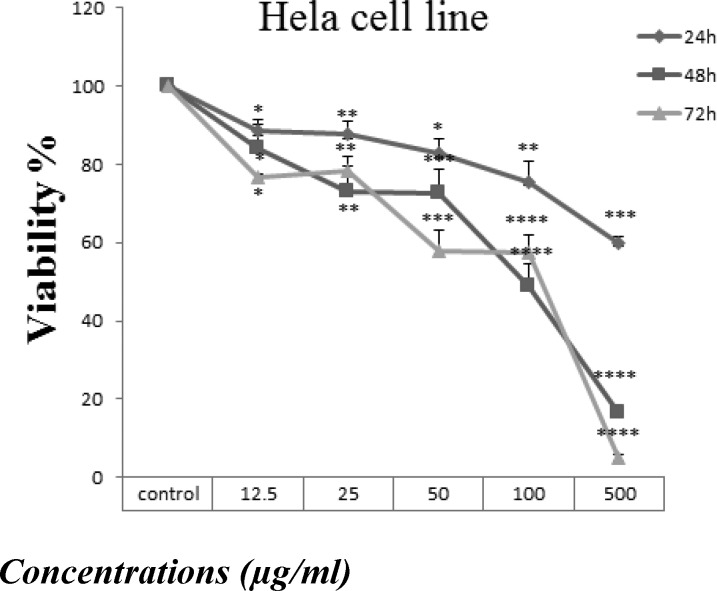
Hela and HN-5 cell lines were incubated with various concentrations of B. aspera root extract (12.5-500 μg/ml) for 24, 48 and 72 hr. Treatment with 50, 100 and 500 µg/ml of B. aspera root extract for 24hr, significantly decreased the percentage of viable Hela cells to 82.6±2.8 (p<0.05), 75.3±3.6 (p<0.01) and 60±1 (p<0.001), respectively. After 48hr incubation with concentrations of 12.5, 25, 50, 100, and 500 µg/ml of B. aspera root extract decreased viability to 99.7±4.2 (p<0.01), 72.8±7 (p<0.01), 72.76±4.05 (p<0.001), 48.9±3.8 (p<0.0001), 16.5±0.04 (p<0.0001), respectively.
After 72 hr of treatment with 12.5, 25, 50, 100 and 500 µg/ml of B. aspera root extract, the percentage of viable Hela cells declined to 76.77 ± 0.5 (p<0.05), 78.3 ± 0.9 (p<0.01), 58 ± 3.7 (p<0.001), 57.5± 3.1 (p<0.001) and 5.1 ± 0.38 (p<0.0001), respectively (Figure 1a).
Figure 1.
Effect of hydro-alcoholic B. aspera extract on viability of Hela (a), HN-5 (b) cells and normal cells (c). Cells were treated with different concentrations of B. aspera root extract for 24, 48 and 72 hr. Viability was quantitated by MTT assay. Results are expressed as mean ± SEM (n = 3). The asterisks are indicator of statistical differences obtained separately at different time points as compared to their control values (*p< 0.05, **p< 0.01, ***p< 0.001, ****p< 0.0001
For HN-5 cell line, 24 hr of treatment with 12.5, 25, 50, 100 and 500 µg/ml of B. aspera root extract significantly decreased the percentage of viable cells to 99.6±4.08 (p<0.0001), 73.6±1.4 (p<0.0001), 68.2±3.2 (p<0.0001), 73.2±0.7 (p<0.0001) and 63±1 (p<0.0001), respectively and after 48 hr of incubation, viability decreased to 50±0.8 (p<0.0001), 40.5±3.2 (p<0.0001), 36.4±1.03 (p<0.0001), 40.2±0.18 (p<0.0001) and 29.5±1.4 (p<0.0001), respectively. After 72 hr treatment with 12.5, 25, 50, 100 and 500 µg/ml of B. aspera root extract the percentage of viable cells decreased to 56.8±0.66 (p<0.0001), 11.2±0.31 (p<0.0001), 7.3±0.17 (p<0.0001), 8±0.4 (p<0.0001) and 5.8±0.12 (p<0.0001), respectively, (Figure 1.b).
In normal cells, treatment with 12, 25, 50,100 and 500 µg/ml of B. aspera root extract for 24 hr did not decrease the percentage of viable cells. Treatment with 50, 100 and 500 µg/ml of B. aspera root extract for 48 hr decreased the percentage of viable cells to 69.21±3.38 (p<0.0001), 64.41±2.57 (p<0.0001) and 60.21±1.66 (p<0.0001), respectively. After 72 hr treatment with 25, 50, 100 and 500 µg/ml of B.aspera root extract the percentage of viable cells decreased to 78.92±7.47 (p<0.05), 42.45±2.77 (p<0.001), 40.56±2.28 (p<0.001) and 43.25±0.78 (p<0.001), respectively (Figure1c).
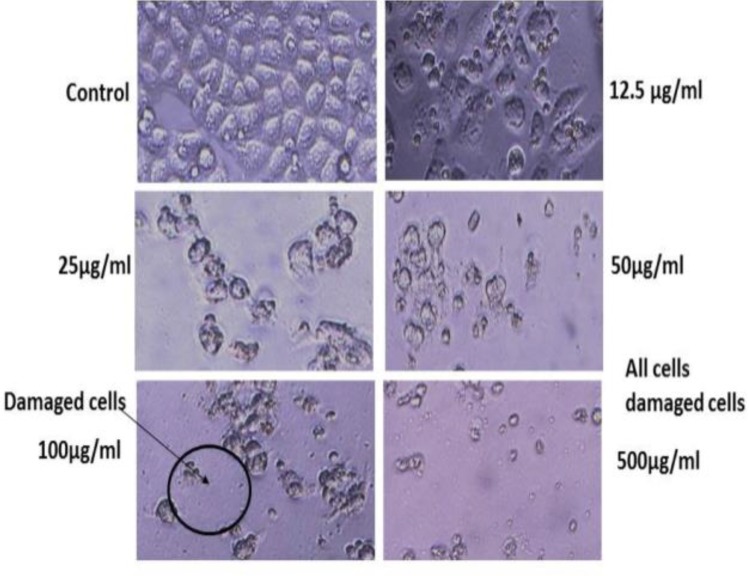
After incubation with B. aspera root extract, morphologic changes (reduction in volume and rounding until the nucleus constituted the majority of the cell volume) were observed in Hela cells (Figure 2).
Figure 2.
Effect of B. aspera (12.5-500µg/ml) on morphological changes in cultured cervix cancer (Hela cells) after incubation. Hela cells were completely damaged and round at 500µg/ml and partly at 100µg/ml. Morphologic changes included reduction in volume and rounding until the nucleus constituted the majority of the cellular volume
As shown in Figures 1 and 2 B. aspera root extract decreased cell viability in Hela and HN-5 cell lines in a concentration and time-dependent manner.
Role of apoptosis
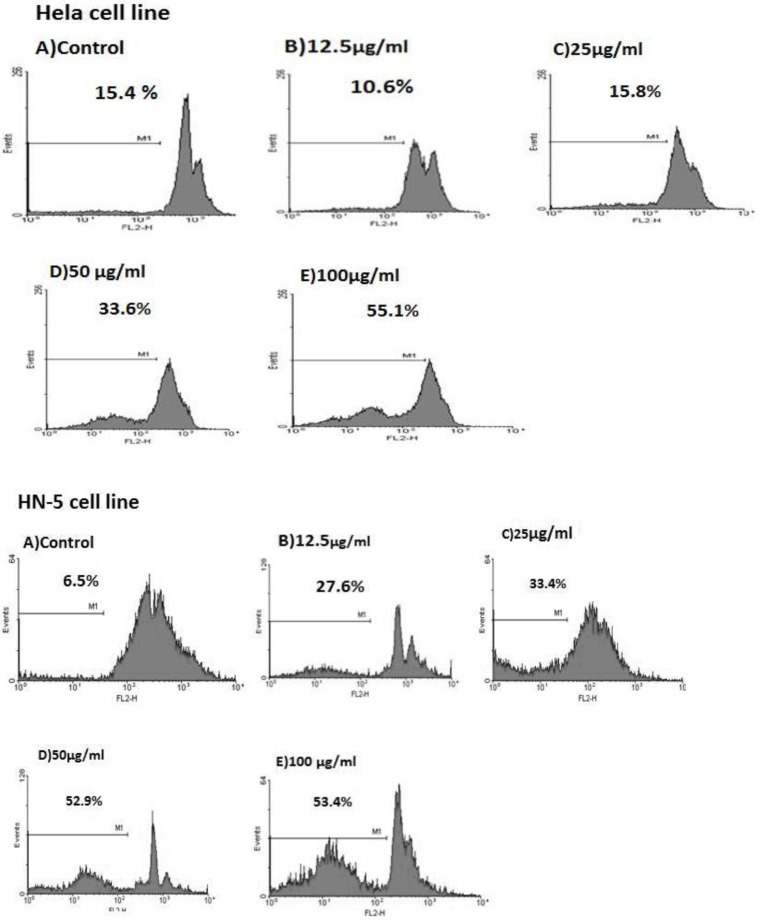
Sub-G1 peak is one of the reliable biochemical markers of apoptosis. There was a sub-G1 peak in flowcytometry histogram of B. aspera-treated but not in control cells indicating apoptotic cell death is involved in B. aspera-induced toxicity in Hela and HN-5 cell lines (Figure 3).
Figure 3.
Flowcytometry histograms of apoptosis analysis following PI staining in Hela and HN-5 cell lines. Cells were treated with 12.5-100 μg/mL B. aspera root extract for 48 hr. Sub-G1 peak as an indicative of apoptotic cells, was induced in B. aspera root extract-treated cells
The rate of apoptosis induced by B. aspera in Hela and HN-5 cells is shown in Table 1.
Table 1.
The rate of apoptosis in HN-5 and Hela cells following 48-hr treatment with B. aspera root extract
| Apoptosis% | Control | 12.5 µg/ml |
25 µg/ml |
50 µg/ml |
100 µg/ml |
|---|---|---|---|---|---|
| Hela cell line | 15.4 | 10.6 | 15.8 | 33.6 | 55.1 |
| HN-5 cell line | 6.5 | 27.6 | 33.4 | 52.9 | 53.4 |
Discussion
Natural products have been utilized to prevent and treat many diseases including cancer, so they are good candidates for the development of anticancer drugs (Song et al, 2005 ▶). In vitro cell proliferation inhibition test using MTT viability assay confirmed that hydro-alcoholic root extract of B. aspera has cytotoxic activity against Hela and HN-5 cell lines. This data is consistent with previous study in which anti- proliferative effects of this extract were evaluated on MCF-7 (human breast adenocarcinoma), HepG2 (human hepato cellular carcinoma) and WEHI (mouse fibrosarcoma) cell lines (Sahranavard et al, 2010 ▶).
Here, the cytotoxic effects of B. aspera root extract and the role of apoptosis in this effect were studied for the first time. The results showed B. aspera–induced apoptosis was involved in induction of cell death. Apoptosis is a gene-regulated phenomenon, which is induced by many chemotherapeutic agents in cancer treatment (Song et al 2005 ▶). It is distinguished by distinct morphological features including chromatin condensation, cell and nuclear shrinkage, membrane blebbing, and oligonucleosomal DNA fragmentation (Hersey and Zhang, 2001 ▶). The induction of apoptosis in tumor cells is considered extremely beneficial in cancer therapy as well in the prevention of cancer. A variety of natural substances has been shown to have the ability to induce apoptosis in different types of cancer cells (Green and Reed, 1998 ▶).
The present study is the first to reveal cytotoxic effects of B. aspera on Hela and HN-5 cell lines in which apoptosis was involved. Further studies are required to determine the mechanisms involved in the cytotoxic activities of HN-5 and Hela cell line. B. aspera root extract could be considered as a potential chemotherapeutic agent in cancer treatment after further studies.
Acknowledgments
This study was financially supported (grant No.910855) by Research Affairs of Mashhad University of Medical Sciences, Mashhad, Iran. In addition, the authors would like to appreciate Ms. Aghaee for preparation of B. aspera root extract. Also, we are grateful to Mr. Malaeke for his assistance in flow cytometry.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- Cooper EL. Drug discovery, CAM and natural products. Evid Based Complement Alternat Med. 2004;3:215. doi: 10.1093/ecam/neh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coope EL. CAM, eCAM, bioprospecting: the 21st century pyramid. Evid Based Complement Alternat Med. 2005;2:125–127. doi: 10.1093/ecam/neh094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferth T, Dunstan H, Sauerbrey A, Miyachi H, Chitambar CR. The anti-malarial artesunate is also active against cancer. Int J Oncol. 2001;18:767–773. doi: 10.3892/ijo.18.4.767. [DOI] [PubMed] [Google Scholar]
- Farjadian S, Asadi E, Doroudchi M, Dehaghani A, SamsamiTabei SZ, Kumar VP, Ghaderi A. High risk HPV types in southern Iranian patients with cervical cancer. Pathol Oncol Res. 2003;9:121–125. doi: 10.1007/BF03033756. [DOI] [PubMed] [Google Scholar]
- Forastiere A, Koch W, Trotti A, Sidransky D. Head and neck cancer. N Engl J Med. 2001;345:1890–1900. doi: 10.1056/NEJMra001375. [DOI] [PubMed] [Google Scholar]
- Ghorbani A. Studies on pharmaceutical ethnobotany in the region of Turkmen Sahra, north of Iran :( Part 1): General results. J Ethnopharmacol. 2005;102:58–68. doi: 10.1016/j.jep.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- Hersey P, Zhang XD. How melanoma cells evade trail-induced apoptosis. Nat Rev Cancer. 2001;1:142–150. doi: 10.1038/35101078. [DOI] [PubMed] [Google Scholar]
- Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy–from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mousavi SH, Tavakkol Afsharic J, Brook A. Study of cytotoxic effects of saffron in MCF-7 Cells. IJPR. 2008;4:261–268. [Google Scholar]
- Mousavi SH, Tavakkol-Afshari J, Brook A, Jafari-Anarkooli I. Direct toxicity of Rose Bengal in MCF-7 cell line: role of apoptosis. Food Chem Toxicol. 2009;47:855–859. doi: 10.1016/j.fct.2009.01.018. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Sahranavard S, Naghibi F, Ghffari S. Cytotoxic activity of extracts and pure compounds of Bryonia aspera. Int J Pharm Pharmaceut Sci. 2012;4:541–543. [Google Scholar]
- Sharifi AM, Mousavi SH, Bakhshayesh M, Khakinegad Tehrani F, Mahmoudian M, Oryan S. Study of correlation between lead-induced cytotoxicity and nitric oxide production in PC12 cells. Toxicol Lett. 2005;160:43–48. doi: 10.1016/j.toxlet.2005.06.008. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Cancer facts and figures. In: Stewart BW, KleihuesP , editors. World cancer report. London: IACRPress; 2003. [Google Scholar]
- Song SY, Lee I, Park C, Lee H, Hahm JC, Kang W K. Neolignans from Saururuschinens is inhibit PC-3 prostate cancer cell growth via apoptosis and senescence-like mechanisms. Int J Mol Med. 2005;16:517–523. [PubMed] [Google Scholar]
- Tabrizi AD, Alizadeh M, Sayyah Melli M, Jafari M, Madarek E. Incidence rate of cervical cancer and precancerous lesions in East Azarbayjan, Iran. Asia Pac J Clin Oncol. 2006;2:87–90. [Google Scholar]
- Taraphdar AK, Roy M, Bhattacharya RK. Natural products as inducers of apoptosis: Implication for cancer therapy and prevention. Curr Sci. 2001;80:1387–1396. [Google Scholar]
- Tsao JCI, Zeltzer LK. Complementary and alternative medicine approaches for pediatric pain: a review of the state-of-the-science. Evid Based Complement Alternat Med. 2005;2:149–159. doi: 10.1093/ecam/neh092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XD, Franco A, Myers K, Gray C, Nguyen T, Hersey P. Relation of TNF-related apoptosis-inducing ligand (TRAIL) receptor and FLICE-inhibitory protein expression to TRAIL-induced apoptosis of melanoma. Cancer Res. 1999;59:2747–2753. [PubMed] [Google Scholar]