Abstract
Since the concept of enhanced recovery after surgery (ERAS) was introduced in the late 1990s the idea of implementing specific interventions throughout the peri-operative period to improve patient recovery has been proven to be beneficial. Minimally invasive surgery is an integral component to ERAS and has dramatically improved post-operative outcomes. ERAS can be applicable to all surgical specialties with the core generic principles used together with added specialty specific interventions to allow for a comprehensive protocol, leading to improved clinical outcomes. Diffusion of ERAS into mainstream practice has been hindered due to minimal evidence to support individual facets and lack of method for monitoring and encouraging compliance. No single outcome measure fully captures recovery after surgery, rather multiple measures are necessary at each stage. More recently the pre-operative period has been the target of a number of strategies to improve clinical outcomes, described as prehabilitation. Innovation of technology in the surgical setting is also providing opportunities to overcome the challenges within ERAS, e.g., the use of wearable activity monitors to record information and provide feedback and motivation to patients peri-operatively. Both modernising ERAS and providing evidence for key strategies across specialties will ultimately lead to better, more reliable patient outcomes.
Keywords: Enhanced recovery after surgery, Laparoscopic surgery, Prehabilitation, Outcome measures, Technology
Core tip: Enhanced recovery after surgery (ERAS) together with laparoscopic surgery improves clinical outcomes in patients post-operatively. Prehabilitation is gaining evidence as a further method of enhancing post-operative recovery. Pre-operative programmes to improve physical function have been used and we review this early literature as well as some current issues within ERAS. Technology, which is already in use in the peri-operative period for interventions and monitoring could be used to further complement ERAS. Small, non-invasive devices which can monitor activity levels could help monitor compliance and post-operative patient activity levels as well as act as an intervention to encourage patients to increase their physical activity and thereby their post-operative outcomes.
INTRODUCTION
The concept of enhanced recovery after surgery (ERAS) was initially proposed by Kehlet[1] who explored the possible determinants of post-operative morbidity in the late 1990s. He identified potential risk factors that needed to be recognised and treated peri-operatively to minimise the effects of surgical stress on the patient. He also championed the idea of working within a multidisciplinary framework. Together these have led to a series of interventions which have been formulated into standardised protocols to span a patient’s entire journey through the surgical process with distinct elements in the pre-operative, intra-operative and post-operative phase (Table 1).
Table 1.
An example of a generic enhanced recovery after surgery protocol
| Pre-operative | Intra-operative | Post-operative |
| Pre-admission counselling | Short acting anaesthetic agents | Mid-thoracic epidural anaesthesia |
| Fluid and carbohydrate loading | Mid thoracic epidural anaesthesia | No Nasogastric tubes |
| No prolonged fasting | No drains | Prevention of nausea and vomiting |
| No/selective bowel preparation | Avoidance of salt and water overload | Avoidance of salt and water overload |
| Antibiotic prophylaxis | Maintenance of normothermia | Early removal of catheter |
| Thromboprophylaxis | Early oral nutrition | |
| No Premedication | Early mobilisation | |
| Non-opioid oral analgesia | ||
| Stimulation of gut motility | ||
| Audit of compliance and outcomes |
Colorectal surgery was the first specialty to implement ERAS in the early 2000s. Early studies proved feasibility and demonstrated that patients benefited from shorter length of hospital stay and reduced post-operative ileus and cardiopulmonary complications, compared with standard care[2-4]. ERAS has also been shown to be feasible and safe in the emergency colorectal setting, leading to shorter length of stay and faster recovery of bowel function[5].
A 2012 consensus review of ERAS guidelines for colonic surgery examined the evidence base for each ERAS intervention and provided graded recommendations[6]. Though given strong recommendation grading, not all the interventions have high levels of evidence for their efficacy (Table 2).
Table 2.
Enhanced recovery after surgery society recommendations for colonic surgery and their evidence level[6]
| ERAS element with high/moderate level evidence | ERAS element with low level evidence |
| Stopping smoking 4 wk prior to surgery | Pre-operative information and counselling |
| No routine use of bowel preparation | Stopping drinking alcohol 4 wk prior to surgery |
| Allowing clear fluids up until 2 h before and solids 6 h before anaesthetic induction | Peri-operative oral nutritional supplements and carbohydrate loading |
| No routine use of sedative premedication | Standard anaesthetic that allows rapid awakening |
| Routine thromboprophylaxis | Post-operative nausea and vomiting prophylaxis |
| Antimicrobial prophylaxis and skin preparation | Routine urinary drainage |
| Balanced intravenous fluids guided by flow measurements | Using stress reducing elements of ERAS to minimise hyperglycaemia |
| Use of mid thoracic epidural blocks in open surgery | Early mobilisation |
| Us of spinal analgesia or PCA in laparoscopic surgery | |
| Laparoscopic surgery | |
| No routine use of nasogastric tubes | |
| Maintenance of normothermia | |
| No routine intra-abdominal drains | |
| Early post-operative enteral feeding | |
| Insulin treatment of severe hyperglycaemia in ICU | |
| Use of chewing gum to prevent post-operative ileus |
ERAS: Enhanced recovery after surgery; PCA: Patient controlled analgesia; ICU: Intensive care unit.
Minimally invasive surgery is one element that has been strongly recommended with a high level of evidence for oncological outcomes and moderate evidence in terms of patient recovery.
ERAS and laparoscopic surgery
Minimally invasive surgery has been shown to reduce post-operative pain, length of hospital stay and complications[7-9]. Recent studies have examined the use of laparoscopic techniques within an enhanced recovery programme. For example, the LAFA-study[10] showed that laparoscopic surgery, as part of an enhanced recovery programme, significantly shortened length of hospital stay compared with open surgery. Other outcomes including morbidity, readmission rates and quality of life were similar between the groups. The EnROL Trial[11] found a statistically significant difference between length of hospital stay and 30 d readmissions favouring the laparoscopic group compared with the open surgery group, but no differences between groups for physical fatigue or other secondary outcomes.
Newer minimally invasive techniques in the form of single incision laparoscopic surgery (SILS), robotic surgery and natural orifice transluminal endoscopic surgery have recently emerged. Although still in the early stages with ongoing research in progress, SILS has been shown to reduce conversion rate to laparotomy and reduce length of hospital stay[12]. Robotic surgery has advantages over purely laparoscopic surgery including the ability for seven degrees of freedom and tremor filtration which could benefit more demanding surgery, e.g., rectal resections. Robotic surgery has been shown to be both safe and feasible with short term outcomes comparable to conventional laparoscopic surgery but longer operative time and higher costs[13,14]. ROLARR (Robotic vs Laparoscopic Resection for Rectal cancer) is an RCT which aims to compare the benefits of robotic vs laparoscopic surgery, the results of which have not yet been published.
The ultimate benefits of laparoscopic surgery and ERAS are essentially the same; improved outcomes and faster recovery. Given that laparoscopic surgery has been shown to improve outcomes both separately from, and as a part of ERAS, it can be seen as a significant and integral component to any ERAS protocol where minimally invasive surgery is applicable.
Specialty specific ERAS
The principles of ERAS have been adopted by most specialties, each formulating their own specific protocols and guidelines. The generic overarching ideas of pre-operative, intra-operative and post-operative elements are included, but the actual interventions and evidence base are specialty specific. Specialties with similar operative procedures, i.e., those within the lower abdominal/pelvic cavity, tend to have similar elements within their protocols, for example colonic surgery[6] and gynaecological oncology surgery[15,16] recommend no pre-operative bowel preparation, avoidance of nasogastric tube insertion and use of minimally invasive surgical techniques when expertise is available. Similar recommendations exist for urological surgery[17], however long-term oncological results following use of minimally invasive techniques are still awaited.
A review of enhanced recovery in pancreatic surgery highlighted placement of intraperitoneal drains as a controversial and highly debated element within ERAS protocols for pancreatectomy[18]. Intraperitoneal drains have been used historically to help in the recognition of a pancreatic fistula or anastomotic leak. This leak of pancreatic fluid can cause erosion of vessels, haemorrhage and sepsis. A recent meta-analysis concluded that those patients without drains had higher mortality but lower overall complications[19]. Current ERAS guidelines recommend systemic post-operative drainage with early removal in patients at low risk of pancreatic fistula, but these recommendations could change as further evidence is highlighted in future studies[20]. Within bariatric surgery pre-operative factors have been suggested to have important post-operative benefits, these include pre-operative weight loss, pre-operative exercise and adequate nutritional supplementation[21]. Studies have shown that pre-operative weight loss is a positive predictor of post-operative weight loss[22]. Together with adequately improving known nutritional deficiencies, which are common in obese patients, these elements seem essential additions to any bariatric ERAS protocol.
Other specialty specific elements include pre-operative respiratory physiotherapy prior to thoracic surgery[23]. This improves exercise capacity and lung function in patients who will lose lung volume after surgery. Use of pre-emptive analgesia and local anaesthetics infiltration within orthopaedic surgery is thought to allow early mobilisation and increased limb movement secondary to decreased somatic sensation[24,25].
Using generic elements as a basis for specialty guidelines with added specific interventions allows for a more comprehensive ERAS protocol with improved outcomes and recovery for each specialty.
CURRENT RESEARCH INSIGHTS AND CHALLENGES
Barriers to the implementation of ERAS
Despite the evidence of improved post-operative outcomes and recovery, ERAS implementation varies in different centres. McLeod et al[26] reported that of the 18 specific ERAS guideline recommendations, only two reached a compliance rate of greater than 75%. Pędziwiatr et al[27] implemented an ERAS protocol over a period of time and found that although only 65% compliance was reached for the first cohort, compliance rose to 89.6% by the third cohort, i.e., a gradual improvement was shown over time. Recently the ERAS Compliance Group found that ERAS protocol compliance in elective colorectal cancer resections were around 75%, but there was variation between centres and elements[28]. Compliance with ERAS protocols was associated with better outcomes and exhibited a form of “dose-dependency” whereby, as compliance increased, complications decreased. Laparoscopic surgery and balanced intravenous fluid therapy were specifically shown to be associated with a reduced risk of complications.
Certain elements are easier to implement than others, for example if they already form part of routine practice, e.g., prophylactic antibiotics, thromboprophylaxis and using minimally-invasive techniques. Some elements are more difficult to implement despite increased efforts[27], including: No bowel preparation, early urinary catheter removal, no opioids and restrictive fluid therapy. An early study into ERAS protocol compliance indicates that compliance with post-operative factors significantly influenced outcomes[29], but it was difficult to determine which specific elements had an independent influence on outcomes. Conversely, a review by Ahmed et al[30] found that studies achieved similar outcomes despite not including all components of recommended ERAS protocols. Furthermore, a systematic review[31] looking at RCTs of ERAS vs standard care was unable to show that ERAS protocols with more elements were more successful than those with fewer elements.
Given the barriers to implementation and the difficulty in determining the relative importance of each individual component within the ERAS protocol the idea of a flexible and individualised method rather than a rigid protocol has been postulated, with each centre and hospital determining which elements to include for their specific protocols[29,31,32]. Factors thought to encourage the implementation of ERAS and improve compliance include; appointment of specific ERAS coordinators, use of engaged multidisciplinary teams, specific ERAS units/wards, specific teaching sessions about the benefits of ERAS and regular auditing[27,29,30].
Whichever elements are included, auditing compliance with the ERAS protocol, as well as measuring patient outcomes, form an essential part of the ERAS audit cycle[6].
Outcome measures
The impetus behind ERAS is improving post-operative recovery therefore it is necessary to measure recovery objectively. Many outcome measures have been used, yet the most frequently reported is length of hospital stay[33]. However, this surrogate measure of recovery can be influenced by external circumstances, for example patients’ expectations of discharge date, social or support networks not being in place or even hospital administration issues with inability to process discharge summaries or dispense necessary medications. Furthermore, despite meeting the necessary clinical markers required for discharge, e.g., blood tests and physiological observations, the patient is unlikely to be back to their functional baseline, since hospital discharge is based on the patient being safe to convalesce in the community. Other clinical outcomes studied include thirty-day mortality, thirty-day re-admission and post-operative complications[34,35]. These outcomes are often recorded as part of the clinical notes and can be used in conjunction with length of hospital stay. However, they only offer insight into the major complications or post-operative issues in patients who are readmitted or treated. There is little information to represent how patients are recovering at home in the long term.
Since 2009 the NHS in the United Kingdom has invited patients to fill in a patient reported outcomes questionnaire after hip replacement, knee replacement, groin hernia and varicose vein surgery. Such questionnaires measure a patient’s health status and health related quality of life at a single point in time is collected before and after the procedure. This has been introduced to provide an indication of the quality of care being delivered. These outcome measures are more patient-focused, relating to daily living within their own environment and their return to normal function. King et al[33] assessed the influence of an ERAS protocol on quality of life. A validated QOL questionnaire (EORTC QLQ-C30) was used by patients undergoing surgery with an ERAS protocol compared to a historic control group. No statistically significant difference between the two groups in terms of quality of life was found. Another study measured post-operative fatigue as a long-term outcome to compare ERAS vs conventional care[36]. It was shown that post-operative fatigue levels increased in both groups significantly, which reached a maximum level just before discharge. However, the peak level reached was significantly smaller in the ERAS group. They also exhibited a significantly smaller Fatigue Consequence Score during the first thirty post-operative days. More recently proponents of ERAS have started to focus research on the theme of patient experience[37], and qualitative studies undertaken have highlighted areas for improvement including post-discharge support and follow-up[38].
Another consideration is the economic potential of ERAS. Studies have shown that implementing an ERAS protocol is cost effective[39]. Recent systematic reviews by Lemanu et al[40] and Lee et al[41] note however, that there are few RCTs documenting cost data, there are inconsistencies in the reporting of cost data, and suggest the need for well-designed trials in order to fully determine the true cost-effectiveness of ERAS.
A recent systematic review by Neville et al[42] aimed to identify useful recovery parameters within ERAS, noting that validated outcome measures were lacking for this complex recovery process. It was found that multiple different outcome measures are in use and that they tend to reflect short term recovery focusing on biological and physiological outcomes. The paucity of outcomes in the longer term was highlighted, for example few studies actually report any outcomes after thirty days post-surgery. A suggestion has been made for longer-term follow-up for post-surgical patients with a focus on patients’ functional status including physical activity measurement and exercise capacity to help quantify recovery more fully. Another review by Feldman et al[43] postulates that phases of recovery overlap and cannot be defined as a single event within a specific time frame. This means that different outcome measures are relevant at different time periods, but that no single outcome measure is perfect to quantify total recovery. Instead, a core set of outcome measures for each stage of recovery is proposed which reflect the perspectives of each member of the multi-disciplinary team as well as the patient.
It is now clear that different outcomes are relevant at different stages of the recovery process. One measure of recovery that is poorly represented by current outcome measures is physical activity. This is an important indicator of functional recovery both in hospital and back at home whilst convalescing. There is a potential to fill this gap by providing means of continual measurement in a non-invasive and objective manner.
Prehabilitation
Physiotherapy and mobilisation recommendations are frequently given in the post-operative period with a view to improving recovery and function. However, physical “conditioning” prior to operative stresses have been considered with the idea of enhancing patients’ functional capacity and thus improving outcomes post-operatively[44,45]. For example, studies have implemented pre-operative exercise regimens and assessed subsequent post-operative functional activity and outcomes[46].
However, the benefit of prehabilitation is uncertain with systematic reviews reporting contradictory evidence. The review by Valkenet et al[47] included twelve studies [orthopaedic surgery, cardiac surgery and open abdominal aortic aneurysm (AAA) repair]. The risk of developing post-operative pulmonary complications was lower in those patients receiving inspiratory muscle training prior to cardiac and AAA surgery (RR = 0.40, 95%CI: 0.23-0.72). Conversely, there was no significant difference between post-operative complication rates or length of stay in joint replacement surgery. Lemanu et al[48] included eight studies in their review (cardiothoracic surgery, abdominal surgery and orthopaedic surgery), which found that there was poor adherence with the prehabilitation interventions with little evidence of physiological and clinical outcome improvements. One review focused more specifically on total body exercise as a prehabilitation intervention[49]. In this review of twenty one studies, improvements were seen in post-operative pain, length of stay and physical function in those undergoing the prehabilitation intervention. These differing conclusions may be due to the heterogeneity of the included studies with different physiological outcomes recorded and different prehabilitation interventions being used.
A tri-modal prehabilitation intervention was used in a randomised controlled trial with patients undergoing colorectal resection[44]. The intervention consisted of fifty minutes’ total body exercise, alternating between aerobic and resistance training three times a week, nutrition counselling with protein supplementation and provision of stress reducing strategies. The trial found that the prehabilitation group had increased functional walking capacity both pre-operatively and at eight weeks post-operatively compared with the rehabilitation group. There was no difference in self-reported physical activity, health related quality of life, thirty day complications, anxiety or depression between groups.
The evidence for prehabilitation is in its preliminary stages, with mainly low powered, observational studies. It is difficult to quantify or characterise the benefits of a prehabilitation programme, or indeed which interventions should be included. Randomised controlled trials looking at prehabilitation in colorectal cancer patients[50] and in vascular patients undergoing elective abdominal aortic aneurysm repair[51] are currently underway, which will help towards informing the decision of whether or not prehabilitation should become part of the ERAS protocol.
FUTURE DIRECTIONS
Use of technology
A variety of technologies have been used within the peri-operative period as helpful adjuncts within ERAS, for example oesophageal Doppler for monitoring fluid balance[52], pneumatic calf compression to provide thromboprophylaxis[53] and the use of forced air warming units to maintain normothermia[54]. Furthermore, recent advances in technology have led to the emergence of small, wearable sensors that can measure, store and transmit large amounts of patient and environmental data[55,56]. These sensors have been used to objectively and continuously monitor physical activity in the home environment following discharge from hospital[57] and within the hospital setting[58].
Studies in the early post-operative period have offered insight on patient mobility and functional recovery[59]. Cook et al[60] monitored patient steps after elective cardiac surgery. An association was found between number of steps taken by a patient and their length of hospital stay and post-operative discharge destination. Wasowicz-Kemps et al[61] measured daily physical activity following laparoscopic cholecystectomy in a controlled study where advice was given to resume normal activity quickly following their operation. Recovery to baseline daily activity took more than one week in 64% of patients but women in the intervention group resumed normal daily activity quicker than those in the control group. One study comparing laparoscopic vs open distal gastrectomy used an objective physical activity monitor to evaluate post-operative recovery[62]. Recovery of activity on each post-operative day was higher in the laparoscopic group. Studies assessing longer term physical activity monitoring[63,64] have shown this is both feasible and beneficial for collecting data on longer-term outcomes.
Providing feedback on activity levels to participants has been shown to increase physical activity in a randomised controlled trial in young healthy Finnish men[65]. A randomised controlled trial assessing interventions for patients with intermittent claudication[66] showed that wearing a feedback-enabled physical activity monitor improved claudication and walking distance as well as quality of life scores at three months.
There is therefore the potential to use sensor technology to complement and augment ERAS, leading to improved patient experience and outcomes. Knowing patients’ pre-operative activity levels might correlate to their baseline function and wellbeing, which could provide an indication of anticipated support the patient may require post-operatively. Monitoring physical activity in the hospital post-operatively can help monitor compliance with post-operative mobilisation recommendations as well as measure inpatient activity providing an indication of functional recovery and screening for complications. Over time, monitoring physical activity unobtrusively can give useful long-term outcome measures that truly reflects a patient’s recovery in the community[67]. Activity feedback to patients both in hospital and in the community may help to encourage an increase in their activity levels, as well as motivate them to be more engaged in their own recovery and care (Figure 1).
Figure 1.
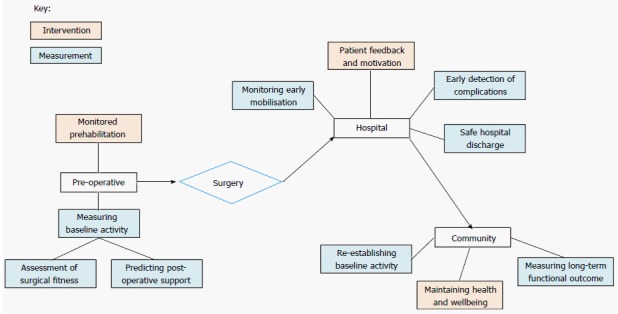
Uses of physical activity monitoring in the peri-operative period. Multiple opportunities exist for implementation of activity monitors in the peri-operative period. Pre-operatively, this includes the assessment of surgical fitness, and guiding a prehabilitation programme. Post-operatively there are multiple options for intervention and measurement in the hospital setting, as well as longer term assessments of functional outcome and encouraging an active lifestyle for overall physical and mental wellbeing.
Sensor technology could, therefore, help overcome the current barriers to ERAS and help assess and improve patient outcomes and experience throughout the surgical period, in keeping with Kehlet’s initial ERAS concept. Additional elements to add to specialty specific protocols could include pre-operative activity monitoring, prehabilitation and post-operative activity monitoring with feedback (Table 3).
Table 3.
Additional enhanced recovery after surgery elements using sensor technology
| Additional ERAS element | What this adds |
| Pre-operative physical activity monitoring | Measuring patient's baseline function to assess for surgical fitness and to predict support required post operatively |
| Prehabilitation | Exercise training prescribed to patients to improve their baseline functional capacity, together with nutritional advice and psychological support |
| Post-operative physical activity monitoring | Providing feedback to clinicians of patient recovery, monitoring compliance with mobilisation recommendations and picking up complications/allowing safer hospital discharge |
| Activity feedback | Providing motivation to patient to encourage them to mobilise in the initial post-operative phase, thereby reducing complications and enhancing recovery |
ERAS: Enhanced recovery after surgery.
CONCLUSION
Enhanced recovery after surgery is an evolving principle that aims to improve patient outcomes following surgery, with minimally-invasive surgery as an integral core. Current problems that are being discussed by ERAS proponents include barriers of implementation of ERAS protocols and the difficulty of measuring post-operative outcomes and improvements. Evidence for prehabilitation is being explored in randomised controlled trials, as initial studies are contradictory and based on observational studies with few participants. Technological advances have enabled wearable devices to continuously and objectively collect data about the wearer’s well-being. This could provide an opportunity to assess ERAS compliance, monitor patient outcomes and offer a variety of promising therapeutic interventions.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest for this article.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: July 12, 2016
First decision: August 11, 2016
Article in press: November 2, 2016
P- Reviewer: Doll D, Fogli L, Mayol J, Nakayama Y, Pavlidis TE S- Editor: Qiu S L- Editor: A E- Editor: Wu HL
References
- 1.Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- 2.Basse L, Raskov HH, Hjort Jakobsen D, Sonne E, Billesbølle P, Hendel HW, Rosenberg J, Kehlet H. Accelerated postoperative recovery programme after colonic resection improves physical performance, pulmonary function and body composition. Br J Surg. 2002;89:446–453. doi: 10.1046/j.0007-1323.2001.02044.x. [DOI] [PubMed] [Google Scholar]
- 3.Muller S, Zalunardo MP, Hubner M, Clavien PA, Demartines N. A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology. 2009;136:842–847. doi: 10.1053/j.gastro.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 4.Basse L, Hjort Jakobsen D, Billesbølle P, Werner M, Kehlet H. A clinical pathway to accelerate recovery after colonic resection. Ann Surg. 2000;232:51–57. doi: 10.1097/00000658-200007000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohsiriwat V. Enhanced recovery after surgery vs conventional care in emergency colorectal surgery. World J Gastroenterol. 2014;20:13950–13955. doi: 10.3748/wjg.v20.i38.13950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, MacFie J, Liberman AS, Soop M, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy GD, Heise C, Rajamanickam V, Harms B, Foley EF. Laparoscopy decreases postoperative complication rates after abdominal colectomy: results from the national surgical quality improvement program. Ann Surg. 2009;249:596–601. doi: 10.1097/SLA.0b013e31819ec903. [DOI] [PubMed] [Google Scholar]
- 8.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 9.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 10.Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, Gerhards MF, van Wagensveld BA, van der Zaag ES, van Geloven AA, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study) Ann Surg. 2011;254:868–875. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy RH, Francis EA, Wharton R, Blazeby JM, Quirke P, West NP, Dutton SJ. Multicenter randomized controlled trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme: EnROL. J Clin Oncol. 2014;32:1804–1811. doi: 10.1200/JCO.2013.54.3694. [DOI] [PubMed] [Google Scholar]
- 12.Hirano Y, Hattori M, Douden K, Ishiyama Y, Hashizume Y. Single-incision laparoscopic surgery for colorectal cancer. World J Gastrointest Surg. 2016;8:95–100. doi: 10.4240/wjgs.v8.i1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aly EH. Robotic colorectal surgery: summary of the current evidence. Int J Colorectal Dis. 2014;29:1–8. doi: 10.1007/s00384-013-1764-z. [DOI] [PubMed] [Google Scholar]
- 14.Kim CW, Kim CH, Baik SH. Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg. 2014;18:816–830. doi: 10.1007/s11605-014-2469-5. [DOI] [PubMed] [Google Scholar]
- 15.Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, Antrobus J, Huang J, Scott M, Wijk L, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations--Part I. Gynecol Oncol. 2016;140:313–322. doi: 10.1016/j.ygyno.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Nelson G, Altman AD, Nick A, Meyer LA, Ramirez PT, Achtari C, Antrobus J, Huang J, Scott M, Wijk L, et al. Guidelines for postoperative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations--Part II. Gynecol Oncol. 2016;140:323–332. doi: 10.1016/j.ygyno.2015.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerantola Y, Valerio M, Persson B, Jichlinski P, Ljungqvist O, Hubner M, Kassouf W, Muller S, Baldini G, Carli F, et al. Guidelines for perioperative care after radical cystectomy for bladder cancer: Enhanced Recovery After Surgery (ERAS(®)) society recommendations. Clin Nutr. 2013;32:879–887. doi: 10.1016/j.clnu.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Kagedan DJ, Ahmed M, Devitt KS, Wei AC. Enhanced recovery after pancreatic surgery: a systematic review of the evidence. HPB (Oxford) 2015;17:11–16. doi: 10.1111/hpb.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YC, Szatmary P, Zhu JQ, Xiong JJ, Huang W, Gomatos I, Nunes QM, Sutton R, Liu XB. Prophylactic intra-peritoneal drain placement following pancreaticoduodenectomy: a systematic review and meta-analysis. World J Gastroenterol. 2015;21:2510–2521. doi: 10.3748/wjg.v21.i8.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, Parks RW, Fearon KC, Lobo DN, Demartines N, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg. 2013;37:240–258. doi: 10.1007/s00268-012-1771-1. [DOI] [PubMed] [Google Scholar]
- 21.Lemanu DP, Srinivasa S, Singh PP, Johannsen S, MacCormick AD, Hill AG. Optimizing perioperative care in bariatric surgery patients. Obes Surg. 2012;22:979–990. doi: 10.1007/s11695-012-0648-6. [DOI] [PubMed] [Google Scholar]
- 22.Livhits M, Mercado C, Yermilov I, Parikh JA, Dutson E, Mehran A, Ko CY, Gibbons MM. Preoperative predictors of weight loss following bariatric surgery: systematic review. Obes Surg. 2012;22:70–89. doi: 10.1007/s11695-011-0472-4. [DOI] [PubMed] [Google Scholar]
- 23.Jones NL, Edmonds L, Ghosh S, Klein AA. A review of enhanced recovery for thoracic anaesthesia and surgery. Anaesthesia. 2013;68:179–189. doi: 10.1111/anae.12067. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim MS, Twaij H, Giebaly DE, Nizam I, Haddad FS. Enhanced recovery in total hip replacement: a clinical review. Bone Joint J. 2013;95-B:1587–1594. doi: 10.1302/0301-620X.95B12.31303. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim MS, Alazzawi S, Nizam I, Haddad FS. An evidence-based review of enhanced recovery interventions in knee replacement surgery. Ann R Coll Surg Engl. 2013;95:386–389. doi: 10.1308/003588413X13629960046435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLeod RS, Aarts MA, Chung F, Eskicioglu C, Forbes SS, Conn LG, McCluskey S, McKenzie M, Morningstar B, Nadler A, et al. Development of an Enhanced Recovery After Surgery Guideline and Implementation Strategy Based on the Knowledge-to-action Cycle. Ann Surg. 2015;262:1016–1025. doi: 10.1097/SLA.0000000000001067. [DOI] [PubMed] [Google Scholar]
- 27.Pędziwiatr M, Kisialeuski M, Wierdak M, Stanek M, Natkaniec M, Matłok M, Major P, Małczak P, Budzyński A. Early implementation of Enhanced Recovery After Surgery (ERAS®) protocol - Compliance improves outcomes: A prospective cohort study. Int J Surg. 2015;21:75–81. doi: 10.1016/j.ijsu.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 28.ERAS Compliance Group. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results From an International Registry. Ann Surg. 2015;261:1153–1159. doi: 10.1097/SLA.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 29.Maessen J, Dejong CH, Hausel J, Nygren J, Lassen K, Andersen J, Kessels AG, Revhaug A, Kehlet H, Ljungqvist O, et al. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg. 2007;94:224–231. doi: 10.1002/bjs.5468. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed J, Khan S, Lim M, Chandrasekaran TV, MacFie J. Enhanced recovery after surgery protocols - compliance and variations in practice during routine colorectal surgery. Colorectal Dis. 2012;14:1045–1051. doi: 10.1111/j.1463-1318.2011.02856.x. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson A, Lowe MC, Parker J, Lewis SR, Alderson P, Smith AF. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg. 2014;101:172–188. doi: 10.1002/bjs.9394. [DOI] [PubMed] [Google Scholar]
- 32.Lyon A, Payne CJ, Mackay GJ. Enhanced recovery programme in colorectal surgery: does one size fit all? World J Gastroenterol. 2012;18:5661–5663. doi: 10.3748/wjg.v18.i40.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King PM, Blazeby JM, Ewings P, Longman RJ, Kipling RM, Franks PJ, Sheffield JP, Evans LB, Soulsby M, Bulley SH, et al. The influence of an enhanced recovery programme on clinical outcomes, costs and quality of life after surgery for colorectal cancer. Colorectal Dis. 2006;8:506–513. doi: 10.1111/j.1463-1318.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 34.Hendry PO, Hausel J, Nygren J, Lassen K, Dejong CH, Ljungqvist O, Fearon KC. Determinants of outcome after colorectal resection within an enhanced recovery programme. Br J Surg. 2009;96:197–205. doi: 10.1002/bjs.6445. [DOI] [PubMed] [Google Scholar]
- 35.Faiz O, Brown T, Colucci G, Kennedy RH. A cohort study of results following elective colonic and rectal resection within an enhanced recovery programme. Colorectal Dis. 2009;11:366–372. doi: 10.1111/j.1463-1318.2008.01604.x. [DOI] [PubMed] [Google Scholar]
- 36.Zargar-Shoshtari K, Paddison JS, Booth RJ, Hill AG. A prospective study on the influence of a fast-track program on postoperative fatigue and functional recovery after major colonic surgery. J Surg Res. 2009;154:330–335. doi: 10.1016/j.jss.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Knott A, Pathak S, McGrath JS, Kennedy R, Horgan A, Mythen M, Carter F, Francis NK. Consensus views on implementation and measurement of enhanced recovery after surgery in England: Delphi study. BMJ Open. 2012;2:pii: e001878. doi: 10.1136/bmjopen-2012-001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernard H, Foss M. Patient experiences of enhanced recovery after surgery (ERAS) Br J Nurs. 2014;23:100–102, 104-106. doi: 10.12968/bjon.2014.23.2.100. [DOI] [PubMed] [Google Scholar]
- 39.Roulin D, Donadini A, Gander S, Griesser AC, Blanc C, Hübner M, Schäfer M, Demartines N. Cost-effectiveness of the implementation of an enhanced recovery protocol for colorectal surgery. Br J Surg. 2013;100:1108–1114. doi: 10.1002/bjs.9184. [DOI] [PubMed] [Google Scholar]
- 40.Lemanu DP, Singh PP, Stowers MD, Hill AG. A systematic review to assess cost effectiveness of enhanced recovery after surgery programmes in colorectal surgery. Colorectal Dis. 2014;16:338–346. doi: 10.1111/codi.12505. [DOI] [PubMed] [Google Scholar]
- 41.Lee L, Li C, Landry T, Latimer E, Carli F, Fried GM, Feldman LS. A systematic review of economic evaluations of enhanced recovery pathways for colorectal surgery. Ann Surg. 2014;259:670–676. doi: 10.1097/SLA.0b013e318295fef8. [DOI] [PubMed] [Google Scholar]
- 42.Neville A, Lee L, Antonescu I, Mayo NE, Vassiliou MC, Fried GM, Feldman LS. Systematic review of outcomes used to evaluate enhanced recovery after surgery. Br J Surg. 2014;101:159–170. doi: 10.1002/bjs.9324. [DOI] [PubMed] [Google Scholar]
- 43.Feldman LS, Lee L, Fiore J. What outcomes are important in the assessment of Enhanced Recovery After Surgery (ERAS) pathways? Can J Anaesth. 2015;62:120–130. doi: 10.1007/s12630-014-0263-1. [DOI] [PubMed] [Google Scholar]
- 44.Gillis C, Li C, Lee L, Awasthi R, Augustin B, Gamsa A, Liberman AS, Stein B, Charlebois P, Feldman LS, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology. 2014;121:937–947. doi: 10.1097/ALN.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 45.Carli F, Scheede-Bergdahl C. Prehabilitation to enhance perioperative care. Anesthesiol Clin. 2015;33:17–33. doi: 10.1016/j.anclin.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Carli F, Zavorsky GS. Optimizing functional exercise capacity in the elderly surgical population. Curr Opin Clin Nutr Metab Care. 2005;8:23–32. doi: 10.1097/00075197-200501000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Valkenet K, van de Port IG, Dronkers JJ, de Vries WR, Lindeman E, Backx FJ. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clin Rehabil. 2011;25:99–111. doi: 10.1177/0269215510380830. [DOI] [PubMed] [Google Scholar]
- 48.Lemanu DP, Singh PP, MacCormick AD, Arroll B, Hill AG. Effect of preoperative exercise on cardiorespiratory function and recovery after surgery: a systematic review. World J Surg. 2013;37:711–720. doi: 10.1007/s00268-012-1886-4. [DOI] [PubMed] [Google Scholar]
- 49.Santa Mina D, Clarke H, Ritvo P, Leung YW, Matthew AG, Katz J, Trachtenberg J, Alibhai SM. Effect of total-body prehabilitation on postoperative outcomes: a systematic review and meta-analysis. Physiotherapy. 2014;100:196–207. doi: 10.1016/j.physio.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Li C, Carli F, Lee L, Charlebois P, Stein B, Liberman AS, Kaneva P, Augustin B, Wongyingsinn M, Gamsa A, et al. Impact of a trimodal prehabilitation program on functional recovery after colorectal cancer surgery: a pilot study. Surg Endosc. 2013;27:1072–1082. doi: 10.1007/s00464-012-2560-5. [DOI] [PubMed] [Google Scholar]
- 51.Tew GA, Weston M, Kothmann E, Batterham AM, Gray J, Kerr K, Martin D, Nawaz S, Yates D, Danjoux G. High-intensity interval exercise training before abdominal aortic aneurysm repair (HIT-AAA): protocol for a randomised controlled feasibility trial. BMJ Open. 2014;4:e004094. doi: 10.1136/bmjopen-2013-004094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colquhoun DA, Roche AM. Oesophageal Doppler cardiac output monitoring: a longstanding tool with evolving indications and applications. Best Pract Res Clin Anaesthesiol. 2014;28:353–362. doi: 10.1016/j.bpa.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Pavon JM, Williams JW, Jr . Adam SS, Razouki ZA, McDuffie JR, Lachiewicz PF, Kosinski AS, Beadles CA, Ortel TL, Nagi A. VA Evidence-based Synthesis Program Reports. Effectiveness of Intermittent Pneumatic Compression Devices for Venous Thromboembolism Prophylaxis in High-risk Surgical and Medical Patients. Washington (DC): Department of Veterans Affairs (US); 2015. [PubMed] [Google Scholar]
- 54.John M, Crook D, Dasari K, Eljelani F, El-Haboby A, Harper CM. Comparison of resistive heating and forced-air warming to prevent inadvertent perioperative hypothermia. Br J Anaesth. 2016;116:249–254. doi: 10.1093/bja/aev412. [DOI] [PubMed] [Google Scholar]
- 55.Appelboom G, Camacho E, Abraham ME, Bruce SS, Dumont EL, Zacharia BE, D’Amico R, Slomian J, Reginster JY, Bruyère O, et al. Smart wearable body sensors for patient self-assessment and monitoring. Arch Public Health. 2014;72:28. doi: 10.1186/2049-3258-72-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dobkin BH, Dorsch A. The promise of mHealth: daily activity monitoring and outcome assessments by wearable sensors. Neurorehabil Neural Repair. 2011;25:788–798. doi: 10.1177/1545968311425908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aziz O, Atallah L, Lo B, Gray E, Athanasiou T, Darzi A, Yang GZ. Ear-worn body sensor network device: an objective tool for functional postoperative home recovery monitoring. J Am Med Inform Assoc. 2011;18:156–159. doi: 10.1136/jamia.2010.005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown CJ, Redden DT, Flood KL, Allman RM. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57:1660–1665. doi: 10.1111/j.1532-5415.2009.02393.x. [DOI] [PubMed] [Google Scholar]
- 59.Kwasnicki RM, Hettiaratchy S, Jarchi D, Nightingale C, Wordsworth M, Simmons J, Yang GZ, Darzi A. Assessing functional mobility after lower limb reconstruction: a psychometric evaluation of a sensor-based mobility score. Ann Surg. 2015;261:800–806. doi: 10.1097/SLA.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 60.Cook DJ, Thompson JE, Prinsen SK, Dearani JA, Deschamps C. Functional recovery in the elderly after major surgery: assessment of mobility recovery using wireless technology. Ann Thorac Surg. 2013;96:1057–1061. doi: 10.1016/j.athoracsur.2013.05.092. [DOI] [PubMed] [Google Scholar]
- 61.Wasowicz-Kemps DK, Slootmaker SM, Kemps HM, Borel-Rinkes IH, Biesma DH, van Ramshorst B. Resumption of daily physical activity after day-case laparoscopic cholecystectomy. Surg Endosc. 2009;23:2034–2040. doi: 10.1007/s00464-008-9928-6. [DOI] [PubMed] [Google Scholar]
- 62.Takiguchi S, Fujiwara Y, Yamasaki M, Miyata H, Nakajima K, Sekimoto M, Mori M, Doki Y. Laparoscopy-assisted distal gastrectomy versus open distal gastrectomy. A prospective randomized single-blind study. World J Surg. 2013;37:2379–2386. doi: 10.1007/s00268-013-2121-7. [DOI] [PubMed] [Google Scholar]
- 63.Skender S, Schrotz-King P, Böhm J, Abbenhardt C, Gigic B, Chang-Claude J, Siegel EM, Steindorf K, Ulrich CM. Repeat physical activity measurement by accelerometry among colorectal cancer patients--feasibility and minimal number of days of monitoring. BMC Res Notes. 2015;8:222. doi: 10.1186/s13104-015-1168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reid RE, Carver TE, Andersen KM, Court O, Andersen RE. Physical activity and sedentary behavior in bariatric patients long-term post-surgery. Obes Surg. 2015;25:1073–1077. doi: 10.1007/s11695-015-1624-8. [DOI] [PubMed] [Google Scholar]
- 65.Jauho AM, Pyky R, Ahola R, Kangas M, Virtanen P, Korpelainen R, Jämsä T. Effect of wrist-worn activity monitor feedback on physical activity behavior: A randomized controlled trial in Finnish young men. Prev Med Rep. 2015;2:628–634. doi: 10.1016/j.pmedr.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Normahani P, Bicknell C, Allen L, Kwasnicki R, Jenkins M, Gibbs R, Cheshire N, Darzi A, Riga C. Wearable Sensor Technology Efficacy in Peripheral Vascular Disease (wSTEP): A Randomised Clinical Trial. European Vascular Endova Surg. 2015;50:e15–e16. doi: 10.1097/SLA.0000000000002300. [DOI] [PubMed] [Google Scholar]
- 67.Kwasnicki RM, Ali R, Jordan SJ, Atallah L, Leong JJ, Jones GG, Cobb J, Yang GZ, Darzi A. A wearable mobility assessment device for total knee replacement: A longitudinal feasibility study. Int J Surg. 2015;18:14–20. doi: 10.1016/j.ijsu.2015.04.032. [DOI] [PubMed] [Google Scholar]


