Abstract
In the early 2000’s in Europe and shortly thereafter in the USA, it was reported that “legal” forms of marijuana were being sold under the name K2 and/or Spice. Active ingredients in K2/Spice products were determined to be synthetic cannabinoids (SCBs), producing psychotropic actions via CB1 cannabinoid receptors, similar to those of Δ9-THC, the primary active constituent in marijuana. Often abused by adolescents and military personnel to elude detection in drug tests due to their lack of structural similarity to Δ9-THC, SCBs are falsely marketed as safe marijuana substitutes. Instead, SCBs are a highly structural diverse group of compounds, easily synthesized, which produce very dangerous adverse effects occurring by, as of yet, unknown mechanisms. Therefore, available evidence indicates that K2/Spice products are clearly not safe marijuana alternatives.
Keywords: Synthetic Cannabinoids, K2, Spice, CB1 Cannabinoid Receptor, Marijuana Substitute
Synthetic cannabinoids (SCBs): Not simply fake marijuana
Synthetic cannabinoids (SCBs) are a growing class of highly potent, highly efficacious cannabinoid agonists that, until recently, have been falsely marketed as “safe” and “legal” alternatives to marijuana [1]. As early as 2004, SCBs were promoted by Internet retailers and European “head shops” as meditation potpourris and tropical incense products under names such as K2 and Spice [2]. It was not until late 2008 that K2/Spice products were investigated by the European Monitoring Center for Drugs and Drug Addiction for their psychoactive properties [3, 4]. Upon analysis of these herbal mixtures, the synthetic cannabimimetics JWH-018 and CP 47,497-C8 were identified as the primary psychoactive components [1]. Since then, several structural classes of SCBs have quickly evolved and diversified to avoid forensic detection and legislative scheduling [1, 5]. Individual users have sought after SCBs to avoid detection in standardized drug testing as well as to achieve a more intense high than that associated with marijuana [3]. The purpose of this review is to summarize reported literature demonstrating that SCBs are neither similar nor suitable substitutes for marijuana and that use of these compounds can result in tolerance and dependence, as well as numerous other documented adverse, toxic and potentially fatal effects.
Evolution of Cannabinoid Nomenclature
The term “cannabinoid” originally referred to a number of structurally related C21 aromatic hydrocarbon compounds isolated from the Cannabis Sativa plant [6]. However, following characterization of Δ9-THC, the principal psychoactive constituent in cannabis [7], and cloning of cannabinoid receptors [8, 9], the term “cannabinoid” instead came to be associated with drugs sharing pharmacological profiles similar to Δ9-THC and exhibiting affinity for cannabinoid receptors, apart from any structurally similarity to compounds originally isolated from the cannabis plant [10]. Therefore, currently accepted nomenclature for “cannabinoids” are ligands that bind to and modulate the activity of cannabinoids receptors [11]. Cannabinoids are structurally diverse and range from compounds that are endogenously produced (endocannabinoids) [12], to plant-derived (phytocannabinoids) [13] and synthesized compounds (synthetic cannabinoids) [14]. This review will focus on the growing epidemic of synthetic cannabinoid abuse, sought primarily for agonist actions of these compounds at CB1 cannabinoid receptors [15].
History of emerging SCB abuse and progression of SCB structural scaffolds
Cannabis sativa, commonly known as marijuana, has had an extensive history of medicinal and recreational use dating back to 2600 BC [16]. It was not until 1965 that Dr. Raphael Mechoulam and colleagues discovered the primary psychoactive compound in marijuana, Δ9-tetrahydrocannabinol (Δ9-THC) [17]. Upon this finding, significant advancements were made in the cannabinoid field including the characterization of the endocannabinoid system and the identification and cloning of the CB1 and CB2 cannabinoid receptors [8, 9]. As cannabinoid research progressed, production of high affinity synthetic CB1 and CB2 cannabinoid receptor ligands began to emerge. Alongside classic plant-derived phytocannabinoids, novel synthetic cannabinoid classes such as the aminoaklylindoles (e.g., WIN-55,212-2) and bicyclic cannabinoids (e.g., CP-55,940) contributed to the structural diversity of cannabinoid pharmacology [18, 19]. Questions of how structurally distinct molecules like Δ9-THC and WIN-55,212-2 bind to CB1 and CB2 cannbinoid receptors with high affinity led to the development of novel cannabimimetics by substituting the morpholino group of aminoalkylindoles with the C3 pentyl side chain of Δ9-THC [20]. Synthesis of pyrrole and indole-derived cannabinoids with the substituted n-pentyl group lead to the discovery of the high affinity, full CB1 cannabinoid receptor agonist JWH-018 [1-pentyl-3-(1-naphthoyl)indole] [21, 22].
More than twenty years following the synthesis of JWH-018, over 150 SCBs have been identified [3]. Aside from the major chemical classes of SCBs, including classical cannabinoids, cyclohexyl-substituted phenols, naphthoylindols, and benzoylindoles, newer SCB structures such as tetramethylcyclopropylindoles, adamantoylindoles, indazole carboximides and quinolinyl esters have been popularized in K2/Spice products [5, 23] (Figure 1). While these SCBs have been advertised as “synthetic marijuana,” when compared to Δ9-THC, they are structurally heterologous. Of the five structural components that contribute to the high affinity and partial agonism of Δ9-THC at CB1 and CB2 cannabinoid receptors — including the C3 side chain, phenolic hydroxyl, and three rings: aromatic A-ring, pyran B-ring, and the cyclohexenyl C-ring — only one of the pharmacophores are shared with SCBs, that being the C3 side chain [16]. Although SCBs are highly diverse molecules, primary structural motifs that comprise SCBs reveal common pharmacophores. These common pharmacophores include 1) an indole or indazole core, 2) an amide, ketone, or ester linker, 3) a ring consisting of a naphthyl, quinolinyl, adamantyl, tetramethylcyclopropyl moiety, and 4) a hydrophobic alkyl group attached to the nitrogen atom of the indole or indazole ring [5]. Many SCBs such as JWH-018, UR-144, AKB48, and PB-22 have also been subjected to the addition of molecular substituents like halogenation of corresponding alkyl chains and stacking of aromatic naphthoyl and indole groups in order to increase affinity and maximize in vivo cannabimimetic effects at CB1 cannabinoid receptor [23, 24]. Continuous manipulation and modification of these compounds by clandestine laboratories has accelerated the evolution of unique and potentially toxic SCBs, while legislatures have been working vigorously to ban the active constituents in K2/Spice products [2].
Figure 1. Structural evolution and legislative scheduling of SCBs between 2010 and 2014.
Schematic illustration shows the prevalent SCB structural classes and corresponding compounds available in K2/Spice products. In 2010, naphthoylindoles, such as JWH-018 and JWH-073, and cyclohexylphenols, like CP-47,497, were the primary SCBs found in seized K2/Spice products. Use of these SCBs continued throughout 2011, with the addition of the flouroalkyl derivative of JWH-018, AM-2201. On March 1, 2011 legislation under the 76 FR 11075 act temporarily scheduled numerous SCBs (many not shown) that were structurally similar to naphthoylindole and cyclohexylphenol classes. Although numerous SCB analogues within these two classes were permanently scheduled July 9, 2012 under the 152 FDASIA act, new, structurally diverse classes of SCBs were subsequently identified in K2/Spice products. These novel classes included the tetramethylcyclopropylindoles, e.g., UR-144 and its fluorinated analogue XLR-11, as well as adamantoylindoles, e.g., AKB48. Because these structurally distinct SCBs were not included in section the 1152 of FDASIA scheduling act in 2012, May 6, 2013, legislation temporarily scheduled compounds associated with the tetramethylcyclopropylindole and adamantoylindole classes under the 78 FR 28735 act. As previous trends suggested, before completion of the 78 FR 28735 scheduling act, new SCBs had once again emerged in K2/Spice products that were also not included in section the 1152 of FDASIA scheduling act. The new classes of SCBs were the indazole carboximides, AB-PINACA and AB-FUBNACA, and quinolinyl esters, PB-22 and its fluorinated analogue 5F-PB-22 (not shown). Although, most of the compounds in these classes (excluding AB-PINACA) were temporarily scheduled on February 10, 2014 under the 78 FR 28735 act, it can only be assumed that new classes of SCBs will emerge in the future [2].
Evidence of K2/Spice usage in the USA was first reported in 2009. However, it was not until late 2010 that the National Forensic Laboratory Information System (NFLIS) — under the guidance of the USA Drug Enforcement Administration (DEA) — reported tremendous spikes in K2/Spice product usage. As shown in Figure 1, the major SCBs found in seized K2/Spice products during 2010 were JWH-018, JWH-073 and CP-47,497. While actions were taken to have the naphthoylindole- and cyclohexylphenol-like analogues regulated by the DEA as Schedule I compounds (e.g., having no currently accepted medical use and thus illegal to possess, except for researchers with schedule I licenses), the new, chemically distinct SCBs UR-144, XLR-11 and AKB48 emerged on the market simultaneously and were not captured by these legislative actions. By July 9, 2012, legislation was able to permanently schedule the naphthoylindole- and cyclohexylphenol-like analogues under 1152 FDASIA. Since then, two separate legislative cases, 78 FR 28735 (May 6, 2013) and 79 FR 7577 (February 10, 2014), successfully resulted in the scheduling of numerous novel SCBs (e.g., UR-144, XLR-11, AKB48, AB-FUBINACA and PB-22) found in K2 products. Unfortunately, the development of novel SCBs has remained ahead of the legislative scheduling process and continues to diversify to escape coverage by existing laws and to elude forensic detection, with no apparent end in sight [2].
SCB Toxicity in humans - comparison to Δ9-THC
While often advertised as “safe” and/or “legal” alternatives to marijuana on the internet, SCBs have proved to be dangerous novel chemicals that are structurally distinct from Δ9-THC, and their use results in a constellation of adverse effects that are distinct from, and markedly more toxic than, those produced by marijuana (Table 1). In particular, reports from a number of clinical case studies have documented markedly greater toxicity following acute use of K2/Spice than marijuana, across a broad number of organ/tissue systems, including gastrointestinal [1, 3, 25–27], neurological [3, 26, 28–42], cardiovascular [34, 36, 37, 43–46] and renal [47–49]. Furthermore, although development of dependence to marijuana is rare, chronic use of SCBs can lead to tolerance, dependence, and withdrawal [50–52]. Most alarming, however, are reports that SCB abuse in some individuals can result in death [53]. Taken collectively, clinical cases reported in recent scientific literature clearly indicate that SCBs found in K2/Spice products are not simply safe or alternative forms of “synthetic marijuana”.
Table 1.
SCB Toxicity in Humans: Comparison with Marijuana
| Adverse Effects and Toxicities |
Observed with K2/ Spice Products (SCBs) |
Observed with Marijuana (THC) |
Citations |
|---|---|---|---|
| Gastrointestinal | |||
| • Nausea | Common | Rare | [1, 3, 25, 26] |
| • Vomiting | Common | Rare | |
| • Hyperemesis Syndrome | Common | Rare | [1, 3, 25, 26] [27] |
| Neurological | |||
| • Euphoria | Common | Common | [26, 29, 30] |
| • Appetite Stimulation | Common | Common | |
| • Nystagmus | Reported | Reported | [26, 31] |
| • Slurred Speech | Reported | Reported | [32] |
| • Ataxia/Lethargy | Reported | Reported | [33] |
| • Psychosis in Susceptible Individuals |
Extreme | Mild | [34] [3, 35] |
| • Hypothermia | Reported | None Reported | |
| • Hallucinations | Common | Rare | [188] |
| • Delusions | Common | Rare | [3, 36] |
| • Confusion | Common | Rare | [3] |
| • Anxiety | Common | Rare | [33] |
| • Panic Attacks | Common | Rare | [37, 38] |
| • Agitation | Common | Rare | [34] |
| • Irritability | Common | Rare | [3, 34] |
| • Confusion | Common | Rare | [39] |
| • Memory Disturbances | Reported | Common | [33] |
| • Self-Mutilation | Reported | None Reported | [26] |
| • Seizures | Reported | None Reported | [40] |
| • Catatonia | Reported | Very Rare | [41] |
| • Acute Cerebral Ischemia |
Reported | None Reported | [42] [28] |
| Cardiovascular | |||
| • Tachycardia | Reported (can lead to Tachyarrhythmia) |
Reported (devoid of Tachyarrhythmia) |
[34, 37, 43] |
| • Hypertension | Reported | None Reported | |
| • Hypotension | None Reported | Reported | [44] |
| • Chest Pain | Reported | None Reported | [45] |
| • Cardiotoxicity (i.e. Myocardial toxicity) |
Reported | None Reported | [36] [46] |
| Renal | |||
| • Acute Tubular Necrosis | Reported | None Reported | [47, 48] |
| • Acute Interstitial Nephritis |
Reported | None Reported | [48] |
| • Acute Kidney Failure | Reported | Non Reported | [49] |
| Effects of Chronic Use | |||
| • Tolerance | Common | Common | [50, 51] |
| • Marked Withdrawal | Reported | Mild | [52] |
| • Dependence | Reported | Rare | [51, 52] |
| Deaths (between 2011–2014) | Over 20 deaths reported | None Reported | [53] |
Distinct SCB toxicity in humans - issues for special concern
Pro-convulsant effects
Despite current interest in medical cannabis as a treatment for epilepsy and other seizure disorders (recently reviewed in [54], for example), the clinical literature is rife with reports of seizures and convulsions elicited by SCBs in humans ([44, 55–58]), and in one case, their exposed pets [59]. As may be expected from case reports, forensic determination of the specific SCBs responsible for these effects occurs only rarely, and the co-use of other drugs often confounds the causal attribution of these convulsant effects to SCBs. Nevertheless, controlled laboratory studies in animals have recently been performed and support the notion that high efficacy SCBs exhibit unexpected pro-convulsant effects. For example, the SCB AKB48 and its fluorinated analogue 5F-AKB48 [23] as well as the aminoalkylindoles JWH-015 and JWH-073 [60] all induced impaired visual, acoustic and tactile sensorimotor responses, convulsions, myoclonia and hyperreflexia in mice. Similarly, the aminoalkylindole SCBs JWH-018, JWH-073, JWH-210, AM-2201 and JWH-167 all increased central nervous system excitability in a functional observation battery in mice, with JWH-018 being “especially active” in this regard [61]. Further characterization of the pro-convulsant effects of the SCBs is clearly warranted.
Pro-psychotic effects
SCBs are typically much more potent and efficacious than Δ9-THC at both CB1 and CB2 cannabinoid receptors, suggesting a capacity to induce far more intense in vivo effects than cannabis. This is sometimes – but not always – the case. With regards to toxicity of cannabinoid agonists, epidemiological studies suggest that cannabis use, particularly in adolescence, increases risk for psychotic episodes later in life [62, 63], and preclinical studies have also demonstrated pro-psychotic effects of Δ9-THC in rodents treated during the adolescent period [64]. Alarmingly, reports of acute and lasting psychosis elicited by use of SCBs are rapidly accumulating in the clinical literature [4, 34, 35, 38, 50, 65–72], but the mechanism of psychosis remains poorly understood and no controlled studies have yet characterized the pro-psychotic effects of SCBs in humans. Even acute use of SCBs can elicit psychosis-like symptoms of paranoia, disorganized behavior, visual and auditory hallucinations, and suicidal thoughts which persist much longer than more typical cannabinoid effects of motor depression and anxiety [65]. Interestingly, SCBs induce these effects in users with previous histories of psychosis and schizophrenia, as well as in users with no previous morbidity. Pro-psychotic effects of the low-efficacy cannabinoid agonists Δ9-THC, dronabinol, and nabilone have been studied under controlled laboratory conditions in humans (reviewed by [73]); however, evidence for a causal relationship between SCB use and re-emergence of previous psychotic symptoms or induction of new-onset psychosis is hampered by the fact that the literature surrounding this topic consists entirely of case reports. Indeed, differences in patient assessment methods, the general lack of forensic confirmation of which exact SCB compound was used, the possible confound of co-use of SCBs with prescribed therapeutics or other drugs of abuse, and the potential for preexisting mental illness should give one pause in attributing a causative link between SCB exposure and psychosis.
SCB abuse liability, tolerance, dependence and withdrawal
Abuse liability
The “gold standard” for preclinical abuse liability testing is the intravenous self-administration assay [74], where an animal subject within a modified cage or experimental chamber can self-inject a drug through a surgically-implanted venous catheter after a specific behavior has been emitted (typically a lever press or a nose poke; for more details of the procedure, see [75]). Thus, these studies directly assess the reinforcing effects of drugs. Importantly, not all drugs which are abused by humans maintain cointingent responding in laboratory species. For example, serotonergic hallucinogens are not reliably reinforcing in self-administration studies in laboratory animals [74], and the psychotropic effects of cannabinoids may be similar to those of the psychedelics. Thus, despite constant recreational use and abuse of cannabinoids throughout human history, the reinforcing effects of cannabinoids have not been widely investigated in laboratory animals. Some of the earliest studies on the reinforcing effects of cannabinoids failed to establish intravenous self-administration of Δ9-THC in rhesus monkeys [76] or in rats [77]. In later years, attempts were made to compensate for the relatively slow-onset, long-lasting behavioral effects of existing cannabinoids by establishing self-administration procedures with widely spaced drug deliveries, but these efforts also failed to establish reliable responding maintained by either Δ9-THC or CP-55,940 [78], leading to the widespread perception that cannabinoids, like serotonergic hallucinogens, were simply ineffective in self-administration assays. However, it was eventually noted that these previous studies utilized intravenous unit doses which were higher than those calculated from human studies, perhaps indicating that the unit doses available for self-administration would be aversive to laboratory animals. Similarly, the lipophilic nature of Δ9-THC and its poor solubility in water suggested that the Δ9-THC solutions used were, at best, in suspension and therefore not likely to be biologically active. Indeed, the use of lower doses of Δ9-THC which clearly dissolve in solution, allowing the drug to rapidly penetrate the brain after intravenous administration, seems to readily maintain self-administration in squirrel monkeys [79, 80]. Additionally, self-administration of endogenous cannabinoids anandamide [81] and 2-arachidonoylglycerol [82] by squirrel monkeys has also been demonstrated. To date, Δ9-THC has not been reported to maintain reliable self-administration behavior in rodents, although the higher efficacy cannabinoids WIN 55,212 and HU-210 have been reported to maintain intravenous self-administration behavior in mice and rats [83–85]. These data may suggest that other high efficacy cannabinoids, such as those present in K2/Spice products, might also display reinforcing effects in self-administration procedures, but thus far, no published reports bolster this supposition. Indeed, a recent paper failed to demonstrate reinforcing effects with JWH-030, JWH-175 or JWH-176 in rats [86]. Nevertheless, the majority of self-administration reports in squirrel monkeys and rats demonstrate that the reinforcing effects of intravenous cannabinoids are significantly attenuated by pretreatment with CB1 cannabinoid receptor antagonists, strongly suggesting that the abuse-related effects of these substances are indeed mediated by central cannabinoid systems.
Another way to indirectly assess abuse-related effects of cannabinoids in experimental animals is to study their capacity to elicit a conditioned place preference. After a few pairings of a drug with a novel context, an increase in time spent in the drug-paired context relative to control is deemed a “conditioned place preference” and may indicate that the drug has positive motivational properties, while a decrease in time spent in the drug-paired context is termed a “conditioned place aversion” and may indicate that the drug has aversive stimulus properties [87]. Presumably, the learned association between the context and the interoceptive stimulus properties of the drug dictates both the magnitude and directionality of the place conditioning effect. Studies investigating the capacity of cannabinoids to induce conditioned place preference present a complex and contradictory picture. For example, administration of high dose Δ9-THC or higher efficacy cannabinoids (including CP 55,940, WIN 55,212, and HU 210) to rodents typically produces either no effect in place conditioning assays, or induces place aversion (reviewed in [88] and [89]). Nevertheless, robust preferences for Δ9-THC-paired contexts have sometimes been reported in rats [90] and mice [91–93] if the animals have been “pre-exposed” to cannabinoids prior to beginning conditioning trials. These results probably indicate that methodological factors within place conditioning experiments are critical mediators of the magnitude and directionality (preference versus aversion) of the effects observed with cannabinoids. Interestingly, as previously demonstrated with Δ9-THC, prior exposure to Δ9-THC was required to “unmask” rewarding effects of JWH-018 [94] and pre-exposure to HU-210 prior to CPP conditioning sessions was necessary to obtain place preference with HU-210. [95]. At the time of this writing, very few SCBs have been tested in CPP experiments. With the exception of JWH-018 (described in [94]), only CP 55,940, WIN 55,212, and HU 210 have been tested for rewarding or aversive effects using place conditioning methods, and the results of such studies have been highly variable ([88, 89]). Given the general failure of cannabinoids to elicit reinforcing effects in laboratory animals, it may be the case that place conditioning could be more sensitive to abuse-related effects of these compounds. Further efforts to characterize newly emerging novel SCBs should consider profiling these drugs in place conditioning assays.
Tolerance
A recent survey [96] finds prevalent use of SCBs among cannabis smokers (e.g., 32.3%), including a subset of individuals reporting daily use of SCBs. This agrees with previous reports that most college students who abuse SCBs also regularly use marijuana [30, 97], raising the possibility of cross-tolerance between Δ9-THC and the SCBs. Repeated administration of cannabinoid agonists has been shown to result in tolerance to several central and peripheral effects in laboratory animals [98–100], and to cellular effects observed in vitro (reviewed by [10]). In human marijuana users, tolerance to numerous cannabinoid effects has also been reported following smoked [101–103] and oral [104–106] administration of Δ9-THC. Thus, a history of Δ9-THC administration might also render individuals less sensitive to some effects of the higher efficacy SCBs through the phenomenon of cross-tolerance. This is especially important because drug users often increase the amount of drugs they consume in an attempt to surmount tolerance to desired psychoactive effects, and any factors which lead SCB users to escalate dose will necessarily increase the risk for adverse effects. Thus, if it is the case that non-cannabinoid receptors prove to be involved in some of the adverse effects of SCBs, tolerant individuals might be particularly susceptible to these effects as they escalate their drug doses.
The role of intrinsic efficacy in tolerance and cross-tolerance among the cannabinoids is underdeveloped, and the data in this domain are often contradictory. For example, some studies show similar tolerance to hypothermic effects of Δ9-THC and the high efficacy cannabinoids CP-55,940 and WIN 55,212-2 following a Δ9-THC pretreatment regimen [107], while others show these same high efficacy cannabinoids to partially surmount Δ9-THC-induced tolerance to locomotor suppression, hypothermia and antinociception [108]. More recently, the high efficacy SCBs JWH-018 and JWH- 073 were unable to induce hypothermia in mice previously made tolerant to hypothermic effects of low efficacy Δ9-THC, suggesting that cross-tolerance developed to the hypothermic effects of the high efficacy SCBs, despite the relatively large disparity in intrinsic activity [109]. In other words, unlike what is typically observed with other drugs (such as the opioids), tolerance to an effect induced by low efficacy Δ9-THC was not surmounted by administration of higher efficacy SCBs. Furthermore, cross-tolerance was still present 14 days after Δ9-THC cessation, suggesting that this cross-tolerance may be as persistent as the tolerance induced by repeated administration of the high efficacy SCBs themselves [109]. Indeed, chronic treatment with high efficacy SCBs results in rapid and persistent tolerance to some, but not all, in vivo effects, accompanied by region-specific down-regulation and desensitization of central CB1 cannabinoid receptors [109–111] (Figure 3).
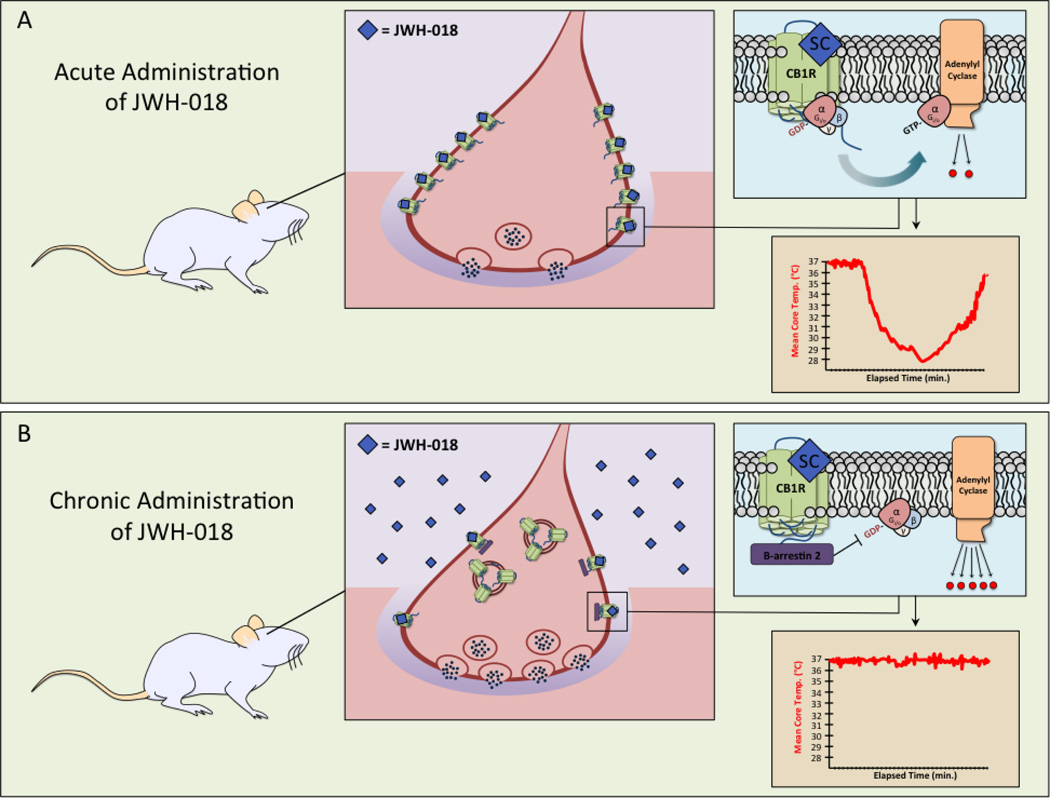
Figure 3. Chronic administration of JWH-018 results in cellular desensitization and down-regulation of CB1 cannabinoid receptors, as well as tolerance to SCB-induced behavioral responses in animals.
In panel A, the SCB JWH-018 (blue diamonds) is administered to a naïve rodent. JWH-018 binds CB1 cannabinoid receptors on pre-synaptic neurons and mediates full agonist Gi/o protein coupling, adenylyl cyclase inhibition and modulation of other effectors including ion channels (not depicted). A single behavioral end-point demonstrates that acute administration of JWH-018 also results in a marked, time-dependent decrease in core body temperature in a SCB-naïve rodent. In panel B, JWH-018 is administered chronically. Complex molecular signaling reveals significant desensitization of CB1 cannabinoid receptors by phosphorylation and recruitment of β-arrestin 2 (purple rectangles), marked down-regulation and internalization of the receptor, and finally dis-inhibition of quantal release of neurotransmitter by the pre-synaptic neuron. Attenuation of CB1 cannabinoid receptor signaling via desensitization, down-regulation and receptor internalization contributes to the blunting of JWH-018-induced hypothermia. The complexity of these cellular mechanisms and how they translate to reduced behavioral responses (such as hypothermia) is indicative of tolerance in the rodent following chronic administration of JWH-018 [187].
In the case report literature, there are a few published instances of what might seem to be a paradoxical hyperthermic response to SCB use in humans. One report describes the clinical course of 11 patients exposed to the SCB MAB-CHMINACA [58]. For the most part, the symptoms reported are consistent with known SCB effects, including altered consciousness, severe agitation, seizures and death, however, a 20-year-old male patient developed a malignant hyperthermia and died on his seventh day in the hospital. Similarly, another report (which did not forensically determine the specific compound ingested) described hyperthermia in a patient who reportedly had smoked a commercial SCB preparation known as “Mr Big Shot” [112] and was successfully treated after 60 minutes of evaporative cooling and ice pack exposure. At present the mechanism for SCB-induced hyperthermia – if indeed SCBs were the cause of these two reported instances – remains unknown.
Dependence and Withdrawal
Drug dependence cannot be directly observed in vivo, but it is assumed to be present when either sudden abstinence from chronic drug use or administration of an antagonist elicits a withdrawal syndrome. Most studies indicate that simple cessation of chronic Δ9-THC administration does not cause spontaneous signs of withdrawal in laboratory animals [113], but robust withdrawal signs are reported after discontinuing SCBs in human users [114]. Unfortunately, clinical reviews of withdrawal following SCB discontinuation in humans (see, for example, [52, 96, 114–116] do not report specific SCB compounds used, most likely because the users themselves do not know the identity of the specific drugs. However, in mice, CB1 cannabinoid receptor antagonist-precipitated withdrawal is reliably characterized by readily quantifiable signs, including wet dog shakes, head shakes, front paw tremor, and motor hyperactivity [113, 117]. Importantly, withdrawal contributes to relapse to drug use for a wide range of abused substances. Thus, if withdrawal from high efficacy SCBs is exacerbated compared to Δ9-THC, this implies that SCB users attempting to cease SCB use may be powerfully motivated to relapse to escape aversive withdrawal effects. However, it is not currently known whether abuse of high-efficacy SCBs would result in a more extreme abstinence syndrome than typically observed following discontinuation of cannabis use, but reports of SCB withdrawal are accumulating in the literature [52, 114–116]. Given the adverse effects associated with acute and long-term abuse of SCBs [118–120] it is increasingly apparent that research into potential therapeutics is warranted. Currently, there is no accepted medical treatment for cannabinoid dependence. The most well-researched pharmacotherapeutic for the treatment of cannabinoid dependence is the CB1 cannabinoid receptor antagonist / inverse agonist rimonabant [121], however, the inverse agonist properties of this drug result not only in blockade of the pharmacological effects elicited by cannabinoid agonists, but also in disruption of constitutive CB1 cannabinoid receptor activity. This fundamental difference between inverse agonists (which decrease signaling below levels observed in normal physiology) and what are conceptualized as neutral antagonists (which block agonist-elicited stimulation in signaling, but preserve constitutive activity) has pharmacological relevance within the cannabinboid system, as rimonabant has been shown to increase cAMP levels in a forskolin-stimulated cAMP assay [122, 123], while the neutral CB1R antagonist AM4113 has no effect on forskolin-stimulated cAMP production [123]. Systems-level behavioral differences between inverse agonists and neutral antagonists have also been demonstrated, where AM4113 did not induce conditioned gaping in rats or emesis in ferrets, both of which occur with inverse agonists, such as rimonabant and AM251 [124, 125]. In humans, adverse events reported after rimonabant exposure, including suicidal ideation, nausea, seizure [126], anxiety and depression [127], eventually led to withdrawal of the drug from the European market and importantly, these adverse events have been ascribed to the inverse agonist properties of rimonabant at CB1 cannabinoid receptors in the CNS [117, 128]. This suggests that cannabinoid withdrawal precipitated by rimonabant, and the direct adverse effects of rimonabant in subjects not previously exposed to exogenous cannabinoids, may not be solely attributed to blockade of CB1 cannabinoid receptors, but may also be exacerbated by rimonabant’s negative efficacy at those binding sites. Development of a truly neutral CB1 cannabinoid receptor antagonist as a possible pharmacotherapeutic for cannabis dependence is currently an active area of research, and may be useful in treatment of cannabinoid dependence.
Factors potentially contributing to greater SCB toxicity relative to Δ9-THC
In vitro pharmacodynamics of SCBs
The mechanisms responsible for the enhanced toxicity of SCBs relative to Δ9-THC (see Table 1) are currently unknown. Many factors may contribute, including potential off-target action of SCBs at non-cannabinoid receptors [61], a complete lack of quality control in abuse-ready smoking products [129], and the absence of potentially toxicity-mitigating non-psychoactive phytocannabinoids in SCB products, as compared to those co-occurring endogenously in cannabis sativa [130]. However, the following section will focus on reported differences in CB1 cannabinoid receptor affinity, potency and efficacy between SCBs and Δ9-THC, for which the most quantitative data are available.
SCBs exhibit higher CB1 cannabinoid receptor affinity than Δ9-THC
Δ9-THC produces psychotropic actions by activating CB1 cannabinoid receptors in the CNS [131]. SCBs also bind and activate CB1 cannabinoid receptors (see following discussion), and thus the abuse liability of both Δ9-THC and SCBs likely results from their agonist actions at these receptors. Therefore, this review will limit discussion of recent reports that have examined the affinity of SCBs for CB1 cannabinoid receptors. Almost all SCBs studied to date have higher affinity for CB1 cannabinoid receptors than Δ9-THC. Depending on the radioligand or specific assay conditions employed, the affinity (or Ki) of Δ9-THC has been reported to range from 3.87 [5] to 41 nM [132]. In contrast, SCBs contained in seized K2/Spice products often exhibit sub-nanomolar CB1 cannabinoid receptor affinity (e.g, 5F-PB-22 [133], AK-B48 [133], AB-FUBINACA [134], ADB-FUBINACA, [134], JWH-122 [24], JWH-210 [21]). Newer SCBs derived from the indazole carboxamide scaffold, including AB-CHIMINACA [135] and AKB48 [23, 133], also have sub-nanomolar affinity for CB1 cannabinoid receptors. In addition to such “ultra-high affinity” compounds, many other SCBs also bind with high affinity to CB1 cannabinoid receptors, exhibiting Ki values between 1 and 20 nM (e.g., JWH-018 [136], AB-PINACA [135], JWH-250, [137], STS-135 [133]). Importantly, since the majority of SCBs bind to CB1 cannabinoid receptors with higher affinity relative to Δ9-THC, it might be anticipated that these compounds may also more potently modulate signaling pathways, potentially contributing to increased toxicity observed for SCBs.
SCBs exhibit high potency and greater efficacy for modulation of CB1 cannabinoid receptor-mediated signaling than Δ9-THC
In addition to exhibiting higher affinity for CB1 cannabinoid receptors than Δ9-THC, most SCBs also modulate intracellular signaling pathways via cannabinoids receptors with high potency and full efficacy when compared to the partial agonist Δ9-THC (Figure 2). CB1 and CB2 cannabinoid receptors are G-protein coupled receptors that, upon agonist binding, activate Gi/o-proteins [138, 139] that then proceed to inhibit activity of the downstream intracellular effector adenylyl cyclase (AC), resulting in reduction in cAMP levels [140]. Therefore, most studies quantify the intrinsic activity of SCBs by measuring the potency (e.g., EC50/IC50) and efficacy (e.g., Emax/Imax) of these compounds to activate G-proteins and/or inhibit AC-activity in brain homogenates (G-protein activation) or whole cells expressing native or transfected CB1 cannabinoid receptors (AC-activity). Activation of CB1 cannabinoid receptors by Δ9-THC results in potent activation of G-proteins (ED50 values 81 [141] to 167 [136] nM), but only as a partial agonist. SCBs examined in this assay instead act full CB1 cannabinoid receptor agonists when compared to Δ9-THC (e.g, JWH-018 [136], 5F-PB-22 [133], MAM-2201 [142], JWH-250 [137], STS-135 [133], XLR-11 [143]). Δ9-THC also potently inhibits AC-activity via CB1 cannabinoid receptors (e.g., IC50 values ranging from 5.0 [140] to 44 nM [23]), although with reduced efficacy indicative of a partial agonist. In marked contrast, almost all SCBs examined in this assay also inhibit AC-activity with high potency in the nM range, but similar to modulation of G-protein activity, act as full agonists (e.g, 5F-PB-22 [133], AB-PINACA [142], EAM-2201 [142], MAM-2201 [142], JWH-250 [137], PB-22, AK-B48 [133], STS-135 [133] and XLR-11 [143]). Similar full agonist activity for SCBs at CB1 cannabinoid receptors has been demonstrated by use of additional methods including a fluorometric assay to measure membrane potential [144] and in primary hippocampal neurons by quantifying calcium transients [142]. As would be expected if SCBs are modulating G-protein activation and AC-inhibition via CB1 cannabinoid receptors, in most instances, the rank order of affinity of the investigated SCBs for CB1 cannabinoid receptors parallels the potency of these compounds to modulate CB1 cannabinoid receptor-mediated signaling pathways [135] and those CB1-mediated functional effects induced by SCBs can be reversed by CB1 cannabinoid receptor antagonists [23, 135, 144]. Importantly, the higher efficacy of SCBs likely results in not only greater acute effects that may contribute to toxicity, but also in enhanced chronic effects occurring at both cellular and whole animal levels that perhaps lead to tolerance and dependence.
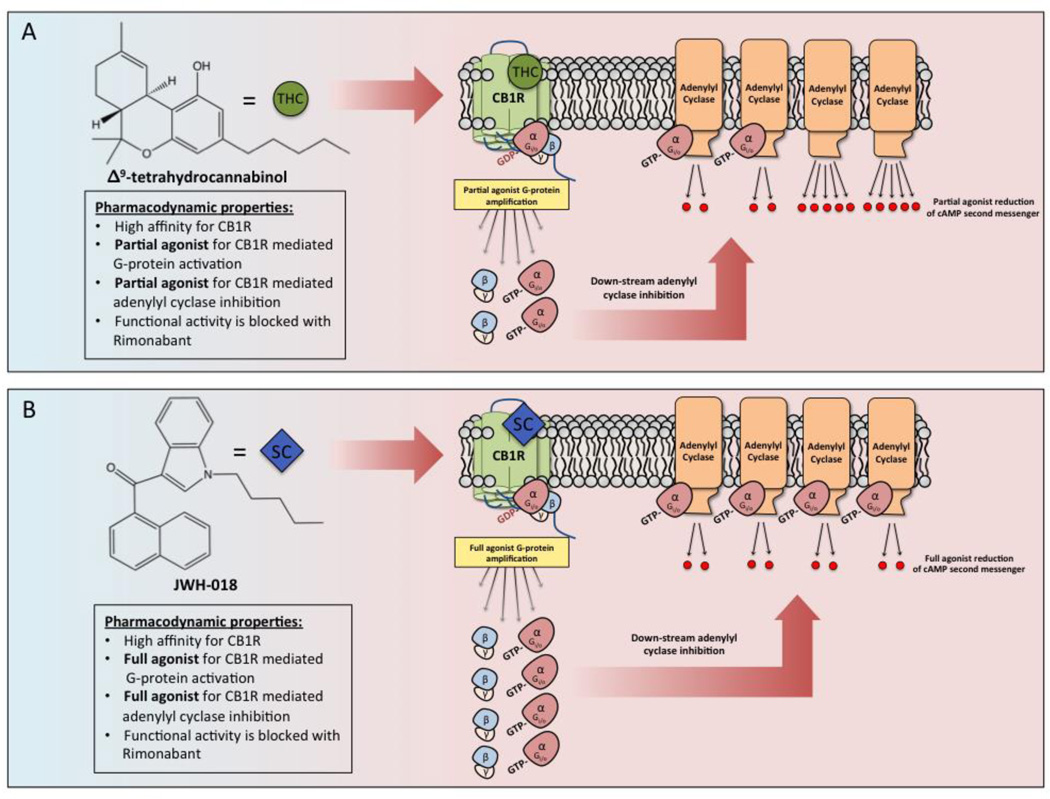
Figure 2. The SCB JWH-018 is a full agonist at CB1 cannabinoid receptors when compared to Δ9-THC at the cellular level.
In panel A, Δ9-THC (green circles) binds and stabilizes the active confirmation of CB1 cannabinoid receptors with high affinity. Δ9-THC induces amplification that results in highly potent, and moderately efficacious coupling to Gi/o proteins, that then proceed to inhibit activity of the downstream intracellular effector, adenylyl cyclase [138–140]. Δ9-THC in marijuana is classified as a partial CB1 cannabinoid receptor agonist due to its sub-maximal recruitment of Gi/o proteins and inhibition of adenylyl cyclase. Comparatively, in panel B, the SCB, JWH-018 (blue diamonds) also binds to CB1 cannabinoid receptors with a very high affinity and couples Gi/o proteins to inhibit adenylyl cyclase. The major distinction between Δ9-THC and JWH-018 is the efficacy of JWH-018-induced Gi/o coupling and adenylyl cyclase inhibition. As depicted, binding of JWH-018 to CB1 cannabinoid receptors results in marked increases in Gi/o protein coupling and inhibition of adenylyl cyclase when compared to the partial agonist Δ9-THC. As such, JWH-018 present in K2/Spice products can be classified as a full CB1 cannabinoid receptor agonist [136].
SCB in vivo Pharmacodynamics
The higher in vitro efficacy of SCBs as compared to Δ9-THC is intriguingly recapitulated at the systems level for some endpoints, but not for all endpoints. Administration of cannabinoid agonists from multiple structural classes elicits a characteristic cluster of effects in laboratory animals. This cluster of the four classical endpoints of hypothermia, analgesia, catalepsy, and locomotor suppression has been termed the cannabinoid tetrad [18, 145]. Consistent with the higher in vitro efficacy of SCBs as compared to Δ9-THC, multiple laboratories reliably report that hypothermic effects obtained with SCBs are greater in magnitude than those observed after administration of maximally effective doses of Δ9-THC in mice [109, 146, 147]. In contrast, multiple research groups consistently demonstrate that Δ9-THC elicits a similar degree of locomotor suppression as higher efficacy SCBs [109, 148] [135]. No consistent efficacy-dependent results are obtained with the cannabinoid tetrad endpoints of analgesia and catalepsy, presumably due to numerous methodological variables associated with data collection across laboratories.
Perhaps the most vexing finding with regards to the relationship between intrinsic efficacy and behavioral effects comes from the realm of drug discrimination. In humans, cannabinoids exert numerous effects on perception and other unobservable psychological endpoints, thus, drug discrimination is useful as an animal model of these subjective effects. The drug discrimination assay can be thought of as an in vivo drug detection procedure whereby animals are trained to recognize the stimulus effects of a given dose of a particular training drug. Once trained, animals may be administered different doses of the same training drug, or different doses of a novel compound suspected to have similar subjective effects to the training drug. Indeed, SCBs reliably induce Δ9-THC-like effects in animals trained to discriminate Δ9-THC. For example, full substitution for Δ9-THC was observed with JWH-018 and JWH-073 in mice [149], which is consistent with the notion that a full agonist would substitute for a partial agonist in this assay. However, instead of a partial-substitution profile expected from the study of other drugs as discriminative stimuli, Δ9-THC reliably produces full substitution for the training stimulus across rodent and non-human primate species trained to discriminate a variety of full CB1 cannabinoid receptor agonists. For example, Δ9-THC fully substituted for the high efficacy SCB JWH-018 in rhesus monkeys [150] and in rats [151]. This same pattern of results was also obtained in squirrel monkeys trained to discriminate the full CB1 cannabinoid receptor agonist AM4054 [152]. It is clear that the role of intrinsic cannabinoid efficacy in systems-level effects is poorly understood, and likely to be a fertile topic for research for some time.
SCB Pharmacokinetics
SCBs are metabolized to many active phase I and II metabolites, Δ9-THC is not
Mounting evidence indicates that SCBs can cause severe, adverse responses in users (Table 1), but little is known about the potential influence of metabolism on the toxicological effects of these novel compounds. It is recognized that oxidative metabolism (Phase I) generally terminates the activity of the parent compounds and functionalizes them for future conjugation reactions (Phase II) such as glucuronidation and sulfation [153], considered the final step for terminating biological activity. Δ9-THC metabolism has been well studied and is extensively metabolized by cytochrome P450 enzymes CYP2C9 and CYP3A4, but only to a single major active metabolite (11-OH-Δ9-THC) with equivalent CB1 cannabinoid receptor affinity [154] and slightly higher potency in antinociceptive assays [155] as compared to the parent drug. 11-OH-Δ9-THC is subsequently oxidized to an inactive intermediate 11-nor-9-carboxy-Δ9-THC that is conjugated to form the O-ester glucuronide, the major metabolite detected in urine [156]. In marked contrast, it has been reported that numerous hydroxylated metabolites of the SCBs JWH-073, JWH-018 and AM-2201 bind to CB1 and CB2 cannabinoid receptors with affinity similar to that of the parent compound, and possess biological activity in both in vitro and in vivo assays [157–159]. Furthermore, several of the hydroxylated compounds detected following administration of the SCBs JWH-018, AM-2201, JWH-122, JWH-210, PB-22, MAM-2201, EAM-2201 and 5F-PB-22 [158, 160] are the major phase I metabolites formed, and importantly retain higher in vitro affinity and activity than Δ9-THC. Although a major glucuronide conjugate of JWH-018 exhibits reduced affinity for CB1 cannabinoid receptors, this metabolite still binds to CB1 cannabinoid receptors in the high nM range and acts as a competitive CB1 cannabinoid receptor antagonist [161]. Therefore, in susceptible individuals (see following genetic polymorphism section), metabolism to active CB1 cannabinoid receptor metabolites may contribute to increased half-life, efficacy and toxicity of SCBs compared to Δ9-THC, while metabolism to competitive CB1 cannabinoid receptor antagonists could lead to increased SCB consumption in an attempt to overcome blunted psychoactive effects. In any case, since many hydroxylated and conjugated derivatives of SCBs (but not Δ9-THC) retain biological activity, defining the metabolic processing of SCBs is required to fully understand the pharmacokinetic and pharmacodynamic properties of known and future novel classes of these abused compounds.
Genetic polymorphisms of P450 and UGT metabolic enzymes may contribute to idiosyncratic SCB toxicity
Based on the reported complex metabolism of SCBs to potentially pharmacologically relevant metabolites (see previous section), identifying specific phase I and II enzymes involved in SCB biotransformation is critical to provide essential direction for future pharmacokinetic and pharmacogenetic studies of current and future classes of these virtually unknown compounds. Without this information, understanding of adverse drug reactions potentially related to polymorphisms of drug metabolizing enzymes and drug-drug interactions cannot be achieved. For example, CYP1A2 and CYP2C9 are the major cytochrome P450 enzymes responsible for metabolism of the SCB JWH-018 [158], while CYP3A4 is most important for oxidation of AKB-48 [162]. SCBs of the napthoylindole class inhibit [163], while cigarette smoking induce [164], CYP1A activity. Several clinically relevant polymorphisms that affect activity of both CYP1A2 and CYP2C9 also have been reported [165]. Furthermore, two human brain UGT isoforms (UGT1A3 and UGT2B7) show relatively high activity toward two metabolites of the SCB JWH-018 commonly found in human urine (JWH-018-ω-OH and JWH-018-ω-COOH) [166], implying that these UGT enzymes may control neuronal concentrations of glucuronidated conjugates available for interaction with CB1 cannabinoid receptors after SCB exposure. In summary, genetic polymorphisms of drug metabolizing enzymes in susceptible individuals may contribute to unique idiosyncratic toxicity often observed following abuse of SCBs.
SCB toxicity - CB1 versus non-CB1 cannabinoid receptor targets
To develop efficacious treatments for SCB toxicity, it is important to first determine potential targets responsible for mediating the adverse effects produced by these drugs. Since SCBs were originally synthesized to exhibit high affinity and activity at CB1 and/or CB2 cannabinoid receptors, it might be expected that many of the adverse effects produced by these drug occur via action at cannabinoid receptors. Indeed, as discussed previously in this review, SCBs exhibit several distinct in vitro and in vivo pharmacodynamic properties when acting at CB1 cannabinoid receptors that likely contribute to the different and greater toxic effects of these drugs relative to Δ9-THC. In support of this suggestion, SCBs examined to date (AM-2201, JWH-018, JWH-073, JWH-081, JWH-210, JWH-167 and JWH-391) lack appreciable affinity for a number of non-CB1 cannabinoid receptor targets including norepinephrine, histamine, opioid, sigma, GABAA or benzodiazepine receptor subtypes [61]. The selective CB1 cannabinoid antagonist rimonabant reverses the effects of these SCBs in a functional observational battery in mice (e.g., muscle tone, equilibrium, sensorimotor activity, alertness, ease of handling and autonomic effects). Furthermore, sensorimotor dysfunction in mice produced by JWH-018, JWH-250 and JWH-073 [60, 167], and impaired motor activity and seizures resulting from JWH-018 and JWH-018-Br [168] are all normalized by co-administration with CB1 cannabinoid receptor antagonists rimonabant and AM-251. Finally, antinociception and hypothermic effects produced by the SCBs CP-55,950, WIN-55,212-2, JWH-073, A-834,735D and CP-47,497 are absent in CB1 cannabinoid receptor knockout mice [169]. These in vitro and in vivo studies collectively indicate that many SCB adverse effects observed in humans are likely mediated via CB1 cannabinoid receptors.
SCBs also exhibit high affinity and activity at CB2 cannabinoid receptors, the second major cannabinoid receptor subtype [159, 170, 171]. Although CB2 cannabinoid receptors are expressed in relatively low levels in the CNS [172] and are not directly associated with psychoactive effects produced by SCBs [173], prolonged activation of CB2 cannabinoid receptors has been shown to upregulate 5-HT2A serotonin receptors in mouse prefrontal cortex [174, 175]. 5-HT2A serotonin receptors are the primary site of action for hallucinogenic drugs [176] and 5-HT2A serotonin receptor dysfunction has been associated with mental disorders including anxiety [177] and psychosis [178]. Common adverse effects of SCBs, but not Δ9-THC, are anxiety and psychosis [179] (Table 1). Based on the reported interaction between CB2 cannabinoid and 5-HT2A serotonin receptors, it is possible that chronic activation of CB2 cannabinoid receptors by SCBs results in an enhancement of 5-HT2A serotonin receptor function that contributes to anxiety and psychosis often observed following exposure to SCBs.
SCBs are structurally diverse (Figure 1) and produce many toxic effects that are not observed with Δ9-THC (Table 1). Therefore, it might be anticipated that these uncharacterized compounds might also exhibit appreciable affinity and activity for cellular targets other than CB1 or CB2 cannabinoid receptors that contribute to the distinct toxicity associated with SCB abuse. In support of this suggestion, some (AM-2201, JWH-018, JWH-073, JWH-167 and JWH-391), but not other (JWH-081 and JWH-210) SCBs act as low potency (EC50 > 3 mM), but high efficacy (> 59%) inhibitors of hERG channels [61]. hERG channel inhibition contributes to prolonged QT intervals and ventricular tachycardia [180], and thus may underlie cardiovascular toxicity reported following SCB use [34] (Table 1). Although potential links to toxicity are currently unknown, SCBs have also been shown to inhibit currents through 5HT3 receptors in the high nanomolar (nM) range (100–600 nM) [181], and at high micromolar (µM) concentrations inhibit monoamine oxidase activity [182], antagonize 5HT2B serotonin receptors [61], and activate strychnine-sensitive α1 glycine receptors by direct and allosteric mechanisms [183]. In summary, although more research is required, the distinct toxicity profile observed with abuse of SCBs relative to Δ9-THC likely results from actions at both CB1 and non-CB1 cannabinoid receptor targets.
“Antidote” for acute SCB toxicity – selective CB1 cannabinoid receptor antagonists?
Since SCBs lack appreciable affinity for non-CB1 cannainoid receptor targets [61] and almost all acute SCB responses in animals are blocked by co-administration with CB1 cannabinoid receptor antagonists (e.g., rimonabant or AM-251) [60, 61, 167–169], it is unfortunate that clinical studies to examine the potential use of CB1 cannabinoid receptor antagonists for treatment of acute SCB overdose in emergency departments have not been conducted. The CB1 cannabinoid receptor antagonist/inverse agonist rimonabant was withdrawn from clinical trials for use in obesity by the European Medicines Agency in October of 2008 due to adverse psychiatric consequences [184]. Following this decision, all major pharmaceutical companies developing CB1 cannabinoid receptor antagonist/inverse agonists quickly discontinued ongoing clinical research of drugs in this class. As might be expected, these safety concerns have likely limited investigation of CB1 cannabinoid receptor antagonists for treatment of acute or chronic SCB toxicity in humans. However, since the most serious adverse effects of rimonabant were primarily observed following chronic therapy, and in a relatively small subset of patients [185], the important and potential life saving use of CB1 cannabinoid receptor antagonists for treatment of acute SCB overdose should perhaps be examined.
Concluding Remarks
SCBs were originally synthesized to aid in the development of therapeutically useful cannabinoid receptor ligands [18–21]. Unfortunately, since the early 2000’s, SCBs have been synthesized by clandestine labs and marketed to vulnerable populations as safe and legal alternatives to marijuana, despite the numerous serious adverse effects posed to human health (Table 1). Although production and usage of K2/Spice products has significantly increased over the years, very few mechanistic studies have established direct mechanisms responsible for the increased toxicity of these high affinity, high efficacy “marijuana substitutes” at CB1 and/or non-CB1-cannabinoid receptor targets (Outstanding Questions 1 and 2). In addition to lack of mechanistic insight, very little continues to be known concerning potential contributions of phase I and II metabolism of SCBs to toxicity observed following K2/Spice use (Outstanding Question 3). As reviewed here, the consequences of acute and chronic K2/Spice abuse have been examined in studies ranging from basic science reports to clinical cases [5, 21, 24, 44, 55–58, 133, 134]. Although much useful information has been gained, unfortunately, many important questions remain; such as, why adolescents appear to be more susceptible to the pro-psychotic actions of SCBs (reviewed in, for example, [186] and in [64]) (Outstanding Question 4). Additionally, it is curious why current treatment of SCB toxicity is purely supportive, lacking clinically available efficacious antidotes for serious life-threatening situations (Outstanding Questions 5 and 6)? Upon review of the structural diversity, distinct pharmacodynamic, pharmacokinetic and clinical effects produced by these compounds, it can readily be concluded that SCBs are neither similar, nor safe, substitutes for marijuana. As shown in Figure 4 (Key Figure) by comparing several characteristics of SCBs reviewed here with marijuana, distinctions between the pharmacological and clinical actions are quite dramatic. Although scheduling of SCBs has been a priority by the DEA since 2010, the inability for legislation to stay ahead of the production of novel SCBs continues to fail in preventing the ongoing abuse of these very dangerous and occasionally deadly drugs (Outstanding Question 7).
Outstanding Questions Box.
Why is there an apparent lack of research directed toward determination of non-CB1 receptor-mediated targets possibly responsible for human toxicity produced by SCB, but not Δ9-THC abuse?
Does high affinity and efficacy of SCBs at CB1 receptors, when compared to Δ9-THC, contribute to greater toxicity associated with use of K2/Spice products?
Do genetic polymorphisms of drug metabolizing enzymes, potentially producing pharmacologically active phase I and phase II metabolites, contribute to SCB toxicity in susceptible individuals?
Does brain development during adolescence make K2/Spice users in this group more susceptible the potential psychotic and/or pro-convulsant effects of SCBs?
Why is no research being conducted to examine the potential use of the CB1 receptor antagonist/inverse agonist rimonabant for cases of acute K2/Spice overdose in emergency departments?
Why is there an apparent lack of research toward development of other safe and widely available antidotes for treatment of SCB overdose?
What legislative steps can be taken to stay ahead of production of novel SCBs by clandestine laboratories?
Key Figure 4. SCBs present in K2/Spice products are not safe alternatives to marijuana.
SCBs in K2/Spice products are structurally diverse psychoactive compounds, exhibiting distinct pharmacodynamic, pharmacokinetic and clinical effects when compared to Δ9-THC in marijuana. The studies reviewed here clearly demonstrate that SCBs are neither similar, nor suitable, substitutes for marijuana and that use of these compounds can result in tolerance and dependence, as well as numerous other documented adverse, toxic and potentially fatal effects.
Trends Box.
Synthetic cannabinoids (SCBs) are a large collection of man-made chemicals, reported in the scientific literature over decades of research to have affinity for CB1 and CB2 cannabinoid receptors.
Products known as K2 or Spice contain a mixture of SCBs that have been illicitly synthesized and sprayed onto inert plant material, in order to mimic the appearance and psychotropic effects of Δ9-THC in marijuana.
K2/Spice products are falsely marketed to adolescent and other vulnerable populations as “safe” and/or “legal” alternatives to marijuana, and are widely known to avoid detection in standard drug screens due to their lack of structural similarity to Δ9-THC.
SCBs present in K2/Spice products produce a variety of dangerous acute and chronic adverse effects, including psychosis, seizures, tolerance, dependence and death, with a greater severity and frequency than observed following marijuana use.
Very little is known concerning the mechanisms underlying the distinct toxic effects of SCBs compared to Δ9-THC, but it is likely that they result from actions at both CB1 and non-CB1 cannabinoid receptor targets.
Acknowledgments
This work was supported in part by NIDA/NIH grant number DA039143.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors have no significant conflicts of interest to declare.
REFERENCES
- 1.Gurney SM, et al. Pharmacology, Toxicology, and Adverse Effects of Synthetic Cannabinoid Drugs. Forensic Science Review. 2014;26(1):53–78. [PubMed] [Google Scholar]
- 2.DEA Office of Diversion Control. Department of Justice. 2016 http://www.deadiversion.usdoj.gov/
- 3.Zawilska JB, Wojcieszak J. Spice/K2 drugs--more than innocent substitutes for marijuana. Int J Neuropsychopharmacol. 2014;17(3):509–525. doi: 10.1017/S1461145713001247. [DOI] [PubMed] [Google Scholar]
- 4.Spaderna M, et al. Spicing things up: synthetic cannabinoids. Psychopharmacology (Berl) 2013;228(4):525–540. doi: 10.1007/s00213-013-3188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hess C, et al. Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice. Forensic Toxicol. 2016;34:329–343. doi: 10.1007/s11419-016-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mechoulam R, Gaoni Y. Hashish. IV. The isolation and structure of cannabinolic cannabidiolic and cannabigerolic acids. Tetrahedron. 1965;21(5):1223–1229. doi: 10.1016/0040-4020(65)80064-3. [DOI] [PubMed] [Google Scholar]
- 7.Mechoulam R, Gaoni Y. Recent advances in the chemistry of hashish. Fortschr Chem Org Naturst. 1967;25:175–213. doi: 10.1007/978-3-7091-8164-5_6. [DOI] [PubMed] [Google Scholar]
- 8.Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 9.Matsuda LA, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 10.Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol. 2005;(168):1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- 11.Pertwee RG, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol Rev. 2010;62(4):588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin BR, et al. Discovery and characterization of endogenous cannabinoids. Life Sci. 1999;65(6–7):573–595. doi: 10.1016/s0024-3205(99)00281-7. [DOI] [PubMed] [Google Scholar]
- 13.Hanus LO, et al. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016 doi: 10.1039/c6np00074f. [DOI] [PubMed] [Google Scholar]
- 14.Di Marzo V, Petrocellis LD. Plant, synthetic, and endogenous cannabinoids in medicine. Annu Rev Med. 2006;57:553–574. doi: 10.1146/annurev.med.57.011205.135648. [DOI] [PubMed] [Google Scholar]
- 15.Mills B, et al. Synthetic Cannabinoids. Am J Med Sci. 2015;350(1):59–62. doi: 10.1097/MAJ.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 16.Bow EW, Rimoldi JM. The Structure-Function Relationships of Classical Cannabinoids: CB1/CB2 Modulation. Perspect Medicin Chem. 2016;8:17–39. doi: 10.4137/PMC.S32171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mechoulam R, Gaoni Y. A Total Synthesis of Dl-Delta-1-Tetrahydrocannabinol, the Active Constituent of Hashish. J Am Chem Soc. 1965;87:3273–3275. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- 18.Little PJ, et al. Pharmacology and stereoselectivity of structurally novel cannabinoids in mice. J Pharmacol Exp Ther. 1988;247(3):1046–1051. [PubMed] [Google Scholar]
- 19.Compton DR, et al. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992;263(3):1118–1126. [PubMed] [Google Scholar]
- 20.Huffman JW. Cannabimimetic indoles, pyrroles and indenes. Curr Med Chem. 1999;6(8):705–720. [PubMed] [Google Scholar]
- 21.Huffman JW, et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg Med Chem. 2005;13(1):89–112. doi: 10.1016/j.bmc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- 22.Huffman JW, et al. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett. 1994;4:563–566. [Google Scholar]
- 23.Canazza I, et al. Effect of the novel synthetic cannabinoids AKB48 and 5F-AKB48 on"tetrad", sensorimotor, neurological and neurochemical responses in mice. In vitro and in vivo pharmacological studies. Psychopharmacology (Berl) 2016 doi: 10.1007/s00213-016-4402-y. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 24.Huffman JW, et al. 3-Indolyl-1-naphthylmethanes: new cannabimimetic indoles provide evidence for aromatic stacking interactions with the CB(1) cannabinoid receptor. Bioorg Med Chem. 2003;11(4):539–549. doi: 10.1016/s0968-0896(02)00451-0. [DOI] [PubMed] [Google Scholar]
- 25.Besli GE, et al. Synthetic Cannabinoid Abuse in Adolescents: A Case Series. The Journal of Emergency Medicine. 2015;49(5):644–650. doi: 10.1016/j.jemermed.2015.06.053. [DOI] [PubMed] [Google Scholar]
- 26.Abrams DI, Guzman M. Cannabis in cancer care. Clin Pharmacol Ther. 2015;97(6):575–586. doi: 10.1002/cpt.108. [DOI] [PubMed] [Google Scholar]
- 27.Ukaigwe A, et al. A Gut Gone to Pot: A Case of Cannabinoid Hyperemesis Syndrome due to K2, a Synthetic Cannabinoid. Case Rep Emerg Med. 2014;2014:167098. doi: 10.1155/2014/167098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takematsu M, et al. A case of acute cerebral ischemia following inhalation of a synthetic cannabinoid. Clinical toxicology (Philadelphia, Pa.) 2014;52(9):973–975. doi: 10.3109/15563650.2014.958614. [DOI] [PubMed] [Google Scholar]
- 29.Vardakou I, et al. Spice drugs as a new trend: mode of action, identification and legislation. Toxicol Lett. 2010;197(3):157–162. doi: 10.1016/j.toxlet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Vandrey R, et al. A survey study to characterize use of Spice products (synthetic cannabinoids) Drug Alcohol Depend. 2012;120(1–3):238–241. doi: 10.1016/j.drugalcdep.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheikh IA, et al. Spice/K2 synthetic marijuana-induced toxic hepatitis treated with N-acetylcysteine. American Journal of Case Reports. 2014;15:584–588. doi: 10.12659/AJCR.891399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeakel JK, Logan BK. Blood synthetic cannabinoid concentrations in cases of suspected impaired driving. J Anal Toxicol. 2013;37(8):547–551. doi: 10.1093/jat/bkt065. [DOI] [PubMed] [Google Scholar]
- 33.Chase PB, et al. Differential physiological and behavioral cues observed in individuals smoking botanical marijuana versus synthetic cannabinoid drugs. Clinical toxicology (Philadelphia, Pa.) 2016;54(1):14–19. doi: 10.3109/15563650.2015.1101769. [DOI] [PubMed] [Google Scholar]
- 34.Hermanns-Clausen M, et al. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108(3):534–544. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- 35.Peglow S, et al. Synthetic cannabinoid induced psychosis in a previously nonpsychotic patient. Am J Addict. 2012;21(3):287–288. doi: 10.1111/j.1521-0391.2012.00222.x. [DOI] [PubMed] [Google Scholar]
- 36.Young AC, et al. Cardiotoxicity associated with the synthetic cannabinoid, K9, with laboratory confirmation. Am J Emerg Med. 2012;30(7):1320 e5–1320 e7. doi: 10.1016/j.ajem.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Schneir AB, et al. "Spice" girls: synthetic cannabinoid intoxication. The Journal of Emergency Medicine. 2011;40(3):296–299. doi: 10.1016/j.jemermed.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Benford DM, Caplan JP. Psychiatric sequelae of Spice, K2, and synthetic cannabinoid receptor agonists. Psychosomatics. 2011;52(3):295. doi: 10.1016/j.psym.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Muller H, et al. Panic attack after spice abuse in a patient with ADHD. Pharmacopsychiatry. 2010;43(4):152–153. doi: 10.1055/s-0029-1243252. [DOI] [PubMed] [Google Scholar]
- 40.Meijer KA, et al. Smoking synthetic marijuana leads to self-mutilation requiring bilateral amputations. Orthopedics. 2014;37(4):391–394. doi: 10.3928/01477447-20140401-62. [DOI] [PubMed] [Google Scholar]
- 41.de Havenon A, et al. The secret "spice": an undetectable toxic cause of seizure. Neurohospitalist Medicine. 2011;1(4):182–186. doi: 10.1177/1941874411417977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haro G, et al. Could spice drugs induce psychosis with abnormal movements similar to catatonia? Psychiatry. 2014;77(2):206–208. doi: 10.1521/psyc.2014.77.2.206. [DOI] [PubMed] [Google Scholar]
- 43.Clark BC, et al. Myocardial Ischemia Secondary to Synthetic Cannabinoid (K2) Use in Pediatric Patients. J Pediatr. 2015;167(3):757–761. doi: 10.1016/j.jpeds.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Schneir AB, Baumbacher T. Convulsions associated with the use of a synthetic cannabinoid product. J Med Toxicol. 2012;8(1):62–64. doi: 10.1007/s13181-011-0182-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones RT. Cardiovascular system effects of marijuana. The Journal of Clinical Pharmacology. 2002;42(11 Suppl):58S–63S. doi: 10.1002/j.1552-4604.2002.tb06004.x. [DOI] [PubMed] [Google Scholar]
- 46.McIlroy G, et al. Acute myocardial infarction, associated with the use of a synthetic adamantyl-cannabinoid: a case report. BMC Pharmacology and Toxicology. 2016;17:2. doi: 10.1186/s40360-016-0045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhanushali GK, et al. AKI associated with synthetic cannabinoids: a case series. Clinical Journal of the American Society of Nephrology. 2013;8(4):523–526. doi: 10.2215/CJN.05690612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kazory A, Aiyer R. Synthetic marijuana and acute kidney injury: an unforeseen association. Clinical Kidney Journal. 2013;6(3):330–333. doi: 10.1093/ckj/sft047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Centers for Disease, C. and Prevention. Acute kidney injury associated with synthetic cannabinoid use--multiple states, 2013. Morbidity and Mortality Weekly Report. 2012;62(6):93–98. [PMC free article] [PubMed] [Google Scholar]
- 50.Every-Palmer S. Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. 2011;117(2–3):152–157. doi: 10.1016/j.drugalcdep.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 51.Wikler A. Aspects of tolerance to and dependence on cannabis. Ann N Y Acad Sci. 1976;282:126–147. doi: 10.1111/j.1749-6632.1976.tb49893.x. [DOI] [PubMed] [Google Scholar]
- 52.Zimmermann US, et al. Withdrawal phenomena and dependence syndrome after the consumption of "spice gold". Dtsch Arztebl Int. 2009;106(27):464–467. doi: 10.3238/arztebl.2009.0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Trecki J, et al. Synthetic Cannabinoid-Related Illnesses and Deaths. N Engl J Med. 2015;373(2):103–107. doi: 10.1056/NEJMp1505328. [DOI] [PubMed] [Google Scholar]
- 54.Reddy DS, Golub VM. The Pharmacological Basis of Cannabis Therapy for Epilepsy. J Pharmacol Exp Ther. 2016;357(1):45–55. doi: 10.1124/jpet.115.230151. [DOI] [PubMed] [Google Scholar]
- 55.Lapoint J, et al. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol. 2011;49(8):760–764. doi: 10.3109/15563650.2011.609822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McQuade D, et al. First European case of convulsions related to analytically confirmed use of the synthetic cannabinoid receptor agonist AM-2201. Eur J Clin Pharmacol. 2013;69(3):373–376. doi: 10.1007/s00228-012-1379-2. [DOI] [PubMed] [Google Scholar]
- 57.Schep LJ, et al. Delayed seizure-like activity following analytically confirmed use of previously unreported synthetic cannabinoid analogues. Hum Exp Toxicol. 2015;34(5):557–660. doi: 10.1177/0960327114550886. [DOI] [PubMed] [Google Scholar]
- 58.Katz KD, et al. Case Series of Synthetic Cannabinoid Intoxication from One Toxicology Center. West J Emerg Med. 2016;17(3):290–294. doi: 10.5811/westjem.2016.2.29519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gugelmann H, et al. 'Crazy Monkey' poisons man and dog: Human and canine seizures due to PB-22, a novel synthetic cannabinoid. Clin Toxicol. 2014;52(6):635–638. doi: 10.3109/15563650.2014.925562. [DOI] [PubMed] [Google Scholar]
- 60.Ossato A, et al. Effect of JWH-250, JWH-073 and their interaction on "tetrad", sensorimotor, neurological and neurochemical responses in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2016;67:31–50. doi: 10.1016/j.pnpbp.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Wiley JL, et al. Evaluation of first generation synthetic cannabinoids on binding at non-cannabinoid receptors and in a battery of in vivo assays in mice. Neuropharmacol. 2016;110(Pt A):143–153. doi: 10.1016/j.neuropharm.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.D'Souza DC, et al. Cannabis and psychosis/schizophrenia: human studies. Eur Arch Psychiatry Clin Neurosci. 2009;259(7):413–431. doi: 10.1007/s00406-009-0024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Evins AE, et al. The effect of marijuana use on the risk for schizophrenia. J Clin Psychiatry. 2012;73(11):1463–1468. doi: 10.4088/JCP.12012co1c. [DOI] [PubMed] [Google Scholar]
- 64.Rubino T, Parolaro D. Cannabis abuse in adolescence and the risk of psychosis: a brief review of the preclinical evidence. Prog Neuropsychopharmacol Biol Psychiatry. 2014;52:41–44. doi: 10.1016/j.pnpbp.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 65.Glue P, et al. Hospitalisation associated with use of the synthetic cannabinoid K2. N Z Med J. 2013;126(1377):18–23. [PubMed] [Google Scholar]
- 66.Durand D, et al. Psychosis and Severe Rhabdomyolysis Associated with Synthetic Cannabinoid Use. Clin Schizophr Relat Psychoses. 2013:1–13. doi: 10.3371/CSRP.DUDE.031513. [DOI] [PubMed] [Google Scholar]
- 67.Oluwabusi OO, et al. Synthetic cannabinoid-induced psychosis: two adolescent cases. J Child Adolesc Psychopharmacol. 2012;22(5):393–395. doi: 10.1089/cap.2012.0004. [DOI] [PubMed] [Google Scholar]
- 68.Tung CK, et al. Acute mental disturbance caused by synthetic cannabinoid: a potential emerging substance of abuse in Hong Kong. East Asian Arch Psychiatry. 2012;22(1):31–33. [PubMed] [Google Scholar]
- 69.Brakoulias V. Products containing synthetic cannabinoids and psychosis. Aust N Z J Psychiatry. 2012;46(3):281–282. doi: 10.1177/0004867411433974. [DOI] [PubMed] [Google Scholar]
- 70.Hurst D, et al. Psychosis associated with synthetic cannabinoid agonists: a case series. Am J Psychiatry. 2011;168(10):1119. doi: 10.1176/appi.ajp.2011.11010176. [DOI] [PubMed] [Google Scholar]
- 71.Every-Palmer S. Warning: legal synthetic cannabinoid-receptor agonists such as JWH-018 may precipitate psychosis in vulnerable individuals. Addiction. 2010;105(10):1859–1860. doi: 10.1111/j.1360-0443.2010.03119.x. [DOI] [PubMed] [Google Scholar]
- 72.Muller H, et al. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res. 2010;118(1–3):309–310. doi: 10.1016/j.schres.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 73.Sherif M, et al. Human Laboratory Studies on Cannabinoids and Psychosis. Biol Psychiatry. 2016;79(7):526–538. doi: 10.1016/j.biopsych.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 74.Goodwin AK. An intravenous self-administration procedure for assessing the reinforcing effects of hallucinogens in nonhuman primates. J Pharmacol Toxicol Methods. 2016;82:31–36. doi: 10.1016/j.vascn.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 75.Katz JL, Goldberg SR. Preclinical assessment of abuse liability of drugs. Agents Actions. 1988;23(1–2):18–26. doi: 10.1007/BF01967174. [DOI] [PubMed] [Google Scholar]
- 76.Harris RT, et al. Evaluation of reinforcing capability of delta-9-tetrahydrocannabinol in rhesus monkeys. Psychopharmacologia. 1974;37(1):23–29. doi: 10.1007/BF00426679. [DOI] [PubMed] [Google Scholar]
- 77.Van Ree JM, et al. Intravenous self-administration of drugs in rats. TJournal of Pharmacology and Experimental Therapeutics. 1978;204(3):547–557. [PubMed] [Google Scholar]
- 78.Mansbach RS, et al. Failure of Delta(9)-tetrahydrocannabinol and CP 55,940 to maintain intravenous self-administration under a fixed-interval schedule in rhesus monkeys. Behav Pharmacol. 1994;5(2):219–225. doi: 10.1097/00008877-199404000-00014. [DOI] [PubMed] [Google Scholar]
- 79.Tanda G, et al. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat Neurosci. 2000;3(11):1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- 80.Justinova Z, et al. Self-administration of delta9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology (Berl) 2003;169(2):135–140. doi: 10.1007/s00213-003-1484-0. [DOI] [PubMed] [Google Scholar]
- 81.Justinova Z, et al. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci. 2005;25(23):5645–5650. doi: 10.1523/JNEUROSCI.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Justinova Z, et al. The endogenous cannabinoid 2-arachidonoylglycerol is intravenously self-administered by squirrel monkeys. J Neurosci. 2011;31(19):7043–7048. doi: 10.1523/JNEUROSCI.6058-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martellotta MC, et al. Self-administration of the cannabinoid receptor agonist WIN 55,212-2 in drug-naive mice. Neuroscience. 1998;85(2):327–330. doi: 10.1016/s0306-4522(98)00052-9. [DOI] [PubMed] [Google Scholar]
- 84.Fattore L, et al. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology (Berl) 2001;156(4):410–416. doi: 10.1007/s002130100734. [DOI] [PubMed] [Google Scholar]
- 85.Navarro M, et al. Functional interaction between opioid and cannabinoid receptors in drug self-administration. J Neurosci. 2001;21(14):5344–5350. doi: 10.1523/JNEUROSCI.21-14-05344.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tampus R, et al. Assessment of the Abuse Liability of Synthetic Cannabinoid Agonists JWH-030, JWH-175, and JWH-176. Biomol Ther. 2015;23(6):590–596. doi: 10.4062/biomolther.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology (Berl) 2000;153(1):31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- 88.Justinova Z, et al. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol Biochem Behav. 2005;81(2):285–299. doi: 10.1016/j.pbb.2005.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murray JE, Bevins RA. Cannabinoid conditioned reward and aversion: behavioral and neural processes. ACS Chem Neurosci. 2010;1(4):265–278. doi: 10.1021/cn100005p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lepore M, et al. Conditioned place preference induced by delta 9-tetrahydrocannabinol: comparison with cocaine, morphine, and food reward. Life Sci. 1995;56(23–24):2073–2080. doi: 10.1016/0024-3205(95)00191-8. [DOI] [PubMed] [Google Scholar]
- 91.Valjent E, Maldonado R. A behavioural model to reveal place preference to delta 9-tetrahydrocannabinol in mice. Psychopharmacology (Berl) 2000;147(4):436–438. doi: 10.1007/s002130050013. [DOI] [PubMed] [Google Scholar]
- 92.Valjent E, et al. Behavioural and biochemical evidence for interactions between Delta 9-tetrahydrocannabinol and nicotine. Br J Pharmacol. 2002;135(2):564–578. doi: 10.1038/sj.bjp.0704479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castane A, et al. Cannabinoid withdrawal syndrome is reduced in double mu and delta opioid receptor knockout mice. Eur J Neurosci. 2003;17(1):155–159. doi: 10.1046/j.1460-9568.2003.02409.x. [DOI] [PubMed] [Google Scholar]
- 94.Hyatt WS, Fantegrossi WE. Delta9-THC exposure attenuates aversive effects and reveals appetitive effects of K2/'Spice' constituent JWH-018 in mice. Behav Pharmacol. 2014;25(3):253–257. doi: 10.1097/FBP.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu T, et al. Cannabinoid-Elicited Conditioned Place Preference in a Modified Behavioral Paradigm. Biol Pharm Bull. 2016;39(5):747–753. doi: 10.1248/bpb.b15-00834. [DOI] [PubMed] [Google Scholar]
- 96.Cooper ZD. Adverse Effects of Synthetic Cannabinoids: Management of Acute Toxicity and Withdrawal. Curr Psychiatr Rep. 2016;18(5):52. doi: 10.1007/s11920-016-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hu X, et al. College students and use of K2: an emerging drug of abuse in young persons. Subst Abuse Treat Prev Policy. 2011;6:16. doi: 10.1186/1747-597X-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dewey WL. Cannabinoid pharmacology. Pharmacol Rev. 1986;38(2):151–178. [PubMed] [Google Scholar]
- 99.Abood ME, Martin BR. Neurobiology of marijuana abuse. Trends Pharmacol Sci. 1992;13(5):201–206. doi: 10.1016/0165-6147(92)90064-d. [DOI] [PubMed] [Google Scholar]
- 100.Maldonado R, Rodriguez de Fonseca F. Cannabinoid addiction: behavioral models and neural correlates. J Neurosci. 2002;22(9):3326–3331. doi: 10.1523/JNEUROSCI.22-09-03326.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jones RT, et al. Clinical relevance of cannabis tolerance and dependence. J Clin Pharmacol. 1981;21(8–9 Suppl):143S–152S. doi: 10.1002/j.1552-4604.1981.tb02589.x. [DOI] [PubMed] [Google Scholar]
- 102.Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38(1):1–20. [PubMed] [Google Scholar]
- 103.Ramaekers JG, et al. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology (Berl) 2011;214(2):391–401. doi: 10.1007/s00213-010-2042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Benowitz NL, Jones RT. Cardiovascular effects of prolonged delta-9-tetrahydrocannabinol ingestion. Clin Pharmacol Ther. 1975;18(3):287–297. doi: 10.1002/cpt1975183287. [DOI] [PubMed] [Google Scholar]
- 105.Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther. 1980;215(1):35–44. [PubMed] [Google Scholar]
- 106.Gorelick DA, et al. Tolerance to effects of high-dose oral delta9-tetrahydrocannabinol and plasma cannabinoid concentrations in male daily cannabis smokers. J Anal Toxicol. 2013;37(1):11–16. doi: 10.1093/jat/bks081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pertwee RG, et al. Cross-tolerance between delta-9-tetrahydrocannabinol and the cannabimimetic agents, CP 55,940, WIN 55,212-2 and anandamide. Br J Pharmacol. 1993;110(4):1483–1490. doi: 10.1111/j.1476-5381.1993.tb13989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fan F, et al. Development of cross-tolerance between delta 9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther. 1994;271(3):1383–1390. [PubMed] [Google Scholar]
- 109.Tai S, et al. Repeated administration of phytocannabinoid Delta(9)-THC or synthetic cannabinoids JWH-018 and JWH-073 induces tolerance to hypothermia but not locomotor suppression in mice, and reduces CB1 receptor expression and function in a brain region-specific manner. Pharmacol Res. 2015;102:22–32. doi: 10.1016/j.phrs.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Grim TW, et al. Pharmacological characterization of repeated administration of the first generation abused synthetic cannabinoid CP47,497. J Basic Clin Physiol Pharmacol. 2016;27(3):217–228. doi: 10.1515/jbcpp-2015-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hruba L, et al. Apparent inverse relationship between cannabinoid agonist efficacy and tolerance/cross-tolerance produced by Delta(9)-tetrahydrocannabinol treatment in rhesus monkeys. J Pharmacol Exp Ther. 2012;342(3):843–849. doi: 10.1124/jpet.112.196444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sweeney B, et al. Hyperthermia and severe rhabdomyolysis from synthetic cannabinoids. Am J Emerg Med. 2016;34(1):121 e1–121 e2. doi: 10.1016/j.ajem.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 113.Maldonado R. Study of cannabinoid dependence in animals. Pharmacol Ther. 2002;95(2):153–164. doi: 10.1016/s0163-7258(02)00254-1. [DOI] [PubMed] [Google Scholar]
- 114.Nacca N, et al. The Synthetic Cannabinoid Withdrawal Syndrome. J Addict Med. 2013 doi: 10.1097/ADM.0b013e31828e1881. [DOI] [PubMed] [Google Scholar]
- 115.Macfarlane V, Christie G. Synthetic cannabinoid withdrawal: a new demand on detoxification services. Drug Alcohol Rev. 2015;34(2):147–153. doi: 10.1111/dar.12225. [DOI] [PubMed] [Google Scholar]
- 116.Sampson CS, et al. Withdrawal seizures seen in the setting of synthetic cannabinoid abuse. Am J Emerg Med. 2015;33(11):1712 e3. doi: 10.1016/j.ajem.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 117.Tai S, et al. Cannabinoid withdrawal in mice: inverse agonist vs neutral antagonist. Psychopharmacology (Berl) 2015;232(15):2751–2761. doi: 10.1007/s00213-015-3907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fantegrossi WE, et al. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Delta(9)-THC: mechanism underlying greater toxicity? Life Sci. 2014;97(1):45–54. doi: 10.1016/j.lfs.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hohmann N, et al. Effects and risks associated with novel psychoactive substances: mislabeling and sale as bath salts, spice, and research chemicals. Dtsch Arztebl Int. 2014;111(9):139–147. doi: 10.3238/arztebl.2014.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luciano RL, Perazella MA. Nephrotoxic effects of designer drugs: synthetic is not better! Nat Rev Nephrol. 2014;10(6):314–324. doi: 10.1038/nrneph.2014.44. [DOI] [PubMed] [Google Scholar]
- 121.Hart CL. Increasing treatment options for cannabis dependence: a review of potential pharmacotherapies. Drug Alcohol Depend. 2005;80(2):147–159. doi: 10.1016/j.drugalcdep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 122.Mato S, et al. Cannabinoid receptor antagonism and inverse agonism in response to SR141716A on cAMP production in human and rat brain. Eur J Pharmacol. 2002;443(1–3):43–46. doi: 10.1016/s0014-2999(02)01575-3. [DOI] [PubMed] [Google Scholar]
- 123.Sink KS, et al. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33(4):946–955. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chambers AP, et al. A neutral CB1 receptor antagonist reduces weight gain in rat. Am J Physiol Regul Integr Comp Physiol. 2007;293(6):R2185–R2193. doi: 10.1152/ajpregu.00663.2007. [DOI] [PubMed] [Google Scholar]
- 125.Salamone JD, et al. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91(4):383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Braakman HM, et al. Rimonabant induces partial seizures in a patient with a history of generalized epilepsy. Epilepsia. 2009;50(9):2171–2172. doi: 10.1111/j.1528-1167.2009.02203.x. [DOI] [PubMed] [Google Scholar]
- 127.Alger BE. Getting high on the endocannabinoid system. Cerebrum. 2013;2013:14. [PMC free article] [PubMed] [Google Scholar]
- 128.Bergman J, et al. Some effects of CB1 antagonists with inverse agonist and neutral biochemical properties. Physiol Behav. 2008;93(4–5):666–670. doi: 10.1016/j.physbeh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Seely KA, et al. Forensic investigation of K2, Spice, and "bath salt" commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int. 2013;233(1–3):416–422. doi: 10.1016/j.forsciint.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 130.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol. 2008;153(2):199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fujiwara M, Egashira N. New perspectives in the studies on endocannabinoid and cannabis: abnormal behaviors associate with CB1 cannabinoid receptor and development of therapeutic application. J Pharmacol Sci. 2004;96(4):362–366. doi: 10.1254/jphs.fmj04003x2. [DOI] [PubMed] [Google Scholar]
- 132.Showalter VM, et al. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J Pharmacol Exp Ther. 1996;278(3):989–999. [PubMed] [Google Scholar]
- 133.De Luca MA, et al. Native CB1 receptor affinity, intrinsic activity and accumbens shell dopamine stimulant properties of third generation SPICE/K2 cannabinoids: BB-22, 5F-PB-22, 5F-AKB-48 and STS-135. Neuropharmacol. 2016;105:630–638. doi: 10.1016/j.neuropharm.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 134.Banister SD, et al. Pharmacology of Indole and Indazole Synthetic Cannabinoid Designer Drugs AB-FUBINACA, ADB-FUBINACA, AB-PINACA, ADB-PINACA, 5F-AB-PINACA, 5F-ADB-PINACA, ADBICA, and 5F-ADBICA. ACS Chem Neurosci. 2015;6(9):1546–1559. doi: 10.1021/acschemneuro.5b00112. [DOI] [PubMed] [Google Scholar]
- 135.Wiley JL, et al. AB-CHMINACA, AB-PINACA, and FUBIMINA: Affinity and Potency of Novel Synthetic Cannabinoids in Producing Delta9-Tetrahydrocannabinol-Like Effects in Mice. J Pharmacol Exp Ther. 2015;354(3):328–339. doi: 10.1124/jpet.115.225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brents LK, et al. Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One. 2011;6(7):c21917. doi: 10.1371/journal.pone.0021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wiley JL, et al. 1-Pentyl-3-phenylacetylindoles and JWH-018 share in vivo cannabinoid profiles in mice. Drug Alcohol Depend. 2012;123(1–3):148–153. doi: 10.1016/j.drugalcdep.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ross R, et al. Agonist-inverse agonist characterization at CB1 and CB2 cannabinoid receptors of L759633, L759656, and AM630. Br J Pharmacol. 1999;126:665–672. doi: 10.1038/sj.bjp.0702351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shim JY, et al. Homology model of the CB1 cannabinoid receptor: sites critical for nonclassical cannabinoid agonist interaction. Biopolymers. 2003;71(2):169–189. doi: 10.1002/bip.10424. [DOI] [PubMed] [Google Scholar]
- 140.Bonhaus DW, et al. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther. 1998;287(3):884–888. [PubMed] [Google Scholar]
- 141.Breivogel CS, et al. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60(1):155–163. [PubMed] [Google Scholar]
- 142.Costain WJ, et al. Pharmacological characterization of emerging synthetic cannabinoids in HEK293T cells and hippocampal neurons. Eur J Pharmacol. 2016;786:234–245. doi: 10.1016/j.ejphar.2016.05.040. [DOI] [PubMed] [Google Scholar]
- 143.Wiley JL, et al. Cannabinoids in disguise: Delta9-tetrahydrocannabinollike effects of tetramethylcyclopropyl ketone indoles. Neuropharmacol. 2013;75:145–154. doi: 10.1016/j.neuropharm.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Banister SD, et al. Pharmacology of Valinate and tert-Leucinate Synthetic Cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and Their Analogues. ACS Chem Neurosci. 2016 doi: 10.1021/acschemneuro.6b00137. [DOI] [PubMed] [Google Scholar]
- 145.Compton DR, et al. Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther. 1992;260(1):201–209. [PubMed] [Google Scholar]
- 146.Paronis CA, et al. Delta(9)-Tetrahydrocannabinol acts as a partial agonist/antagonist in mice. Behav Pharmacol. 2012;23(8):802–805. doi: 10.1097/FBP.0b013e32835a7c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Singh H, et al. Tolerance and cross-tolerance to cannabinoids in mice: schedule-controlled responding and hypothermia. Psychopharmacology (Berl) 2011;215(4):665–675. doi: 10.1007/s00213-010-2162-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gatch MB, Forster MJ. Delta(9)-Tetrahydrocannabinol-like effects of novel synthetic cannabinoids in mice and rats. Psychopharmacology (Berl) 2016;233(10):1901–1910. doi: 10.1007/s00213-016-4237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Brents LK, et al. Differential Drug-Drug Interactions of the Synthetic Cannabinoids JWH-018 and JWH-073: Implications for Drug Abuse Liability and Pain Therapy. J Pharmacol Exp Ther. 2013;346(3):350–361. doi: 10.1124/jpet.113.206003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rodriguez JS, McMahon LR. JWH-018 in rhesus monkeys: differential antagonism of discriminative stimulus, rate-decreasing, and hypothermic effects. Eur J Pharmacol. 2014;740:151–159. doi: 10.1016/j.ejphar.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wiley JL, et al. Cross-substitution of Delta9-tetrahydrocannabinol and JWH-018 in drug discrimination in rats. Pharmacol Biochem Behav. 2014;124:123–128. doi: 10.1016/j.pbb.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kangas BD, et al. Cannabinoid discrimination and antagonism by CB(1) neutral and inverse agonist antagonists. J Pharmacol Exp Ther. 2013;344(3):561–567. doi: 10.1124/jpet.112.201962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Burchell B, Coughtrie MW. Genetic and environmental factors associated with variation of human xenobiotic glucuronidation and sulfation. Environ Health Perspect. 1997;105(Suppl 4):739–747. doi: 10.1289/ehp.97105s4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Compton DR, et al. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharmacol Exp Ther. 1993;265(1):218–226. [PubMed] [Google Scholar]
- 155.Ford RD, et al. delta 9-THC and 11-OH-delta 9-THC: behavioral effects and relationship to plasma and brain levels. Life Sci. 1977;20(12):1993–2003. doi: 10.1016/0024-3205(77)90178-3. [DOI] [PubMed] [Google Scholar]
- 156.Mazur A, et al. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos. 2009;37(7):1496–1504. doi: 10.1124/dmd.109.026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brents LK, et al. Mono-hydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol. 2012;83:952–961. doi: 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chimalakonda KC, et al. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos. 2012;40(11):2174–2184. doi: 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Rajasekaran M, et al. Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol Appl Pharmacol. 2013;269(2):100–108. doi: 10.1016/j.taap.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cannaert A, et al. Detection and activity profiling of synthetic cannabinoids and metabolites with a newly developed bio-assay. Anal Chem. 2016 doi: 10.1021/acs.analchem.6b02600. [DOI] [PubMed] [Google Scholar]
- 161.Seely KA, et al. A Major Glucuronidated Metabolite of JWH-018 Is a Neutral Antagonist at CB1 Receptors. Chem Res Toxicol. 2012;25(4):825–827. doi: 10.1021/tx3000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Holm NB, et al. CYP3A4 Mediates Oxidative Metabolism of the Synthetic Cannabinoid AKB-48. AAPS journal. 2015;17(5):1237–1245. doi: 10.1208/s12248-015-9788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ashino T, et al. Inhibitory effect of synthetic cannabinoids on CYP1A activity in mouse liver microsomes. J Toxicol Sci. 2014;39(6):815–820. doi: 10.2131/jts.39.815. [DOI] [PubMed] [Google Scholar]
- 164.Hughes AN, et al. Overcoming CYP1A1/1A2 mediated induction of metabolism by escalating erlotinib dose in current smokers. J Clin Oncol. 2009;27(8):1220–1226. doi: 10.1200/JCO.2008.19.3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhou SF, et al. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev. 2009;41(2):289–295. doi: 10.1080/03602530902843483. [DOI] [PubMed] [Google Scholar]
- 166.Chimalakonda KC, et al. Conjugation of Synthetic Cannabinoids JWH-018 and JWH-073, Metabolites by Human UDP-Glucuronosyltransferases. Drug Metab Dispos. 2011;39(10):1967–1976. doi: 10.1124/dmd.111.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ossato A, et al. JWH-018 impairs sensorimotor functions in mice. Neuroscience. 2015;300:174–188. doi: 10.1016/j.neuroscience.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 168.Vigolo A, et al. Novel halogenated derivates of JWH-018: Behavioral and binding studies in mice. Neuropharmacol. 2015;95:68–82. doi: 10.1016/j.neuropharm.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 169.Grim TW, et al. Stratification of Cannabinoid 1 Receptor (CB1R) Agonist Efficacy: Manipulation of CB1R Density through Use of Transgenic Mice Reveals Congruence between In Vivo and In Vitro Assays. J Pharmacol Exp Ther. 2016;359(2):329–339. doi: 10.1124/jpet.116.233163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Aung MM, et al. Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB(1) and CB(2) receptor binding. Drug Alcohol Depend. 2000;60(2):133–140. doi: 10.1016/s0376-8716(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 171.Chin CN, et al. The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, peripheral cannabinoid receptor. J Pharmacol Exp Ther. 1999;291(2):837–844. [PubMed] [Google Scholar]
- 172.Onaivi ES, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 173.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74(2):129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 174.Franklin JM, et al. Cannabinoid 2 receptor- and beta Arrestin 2-dependent upregulation of serotonin 2A receptors. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Franklin JM, Carrasco GA. G-Protein Receptor Kinase 5 Regulates the Cannabinoid Receptor 2-Induced Upregulation of Serotonin 2A Receptors. J Biol Chem. 2013 doi: 10.1074/jbc.M113.454843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fantegrossi WE, et al. The behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75(1):17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ghisleni G, et al. Diphenyl diselenide exerts anxiolytic-like effect in Wistar rats: putative roles of GABAA and 5HT receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1508–1515. doi: 10.1016/j.pnpbp.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 178.Morgan D, et al. Molecular and behavioral pharmacology of two novel orally-active 5HT2 modulators: Potential utility as antipsychotic medications. Neuropharmacol. 2013 doi: 10.1016/j.neuropharm.2013.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gunderson EW, et al. "Spice" and "K2" herbal highs: a case series and systematic review of the clinical effects and biopsychosocial implications of synthetic cannabinoid use in humans. Am J Addict. 2012;21(4):320–326. doi: 10.1111/j.1521-0391.2012.00240.x. [DOI] [PubMed] [Google Scholar]
- 180.Hancox JC, et al. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol Ther. 2008;119(2):118–132. doi: 10.1016/j.pharmthera.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 181.Barann M, et al. Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br J Pharmacol. 2002;137(5):589–596. doi: 10.1038/sj.bjp.0704829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Fisar Z. Inhibition of monoamine oxidase activity by cannabinoids. Naunyn Schmiedebergs Arch Pharmacol. 2010;381(6):563–572. doi: 10.1007/s00210-010-0517-6. [DOI] [PubMed] [Google Scholar]
- 183.Demir R, et al. Modulation of glycine receptor function by the synthetic cannabinoid HU210. Pharmacology. 2009;83(5):270–274. doi: 10.1159/000209291. [DOI] [PubMed] [Google Scholar]
- 184.Taylor D. Withdrawal of Rimonabant--walking the tightrope of 21st century pharmaceutical regulation? Curr Drug Saf. 2009;4(1):2–4. doi: 10.2174/157488609787354396. [DOI] [PubMed] [Google Scholar]
- 185.Christensen R, et al. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370(9600):1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 186.Renard J, et al. Long-term consequences of adolescent cannabinoid exposure in adult psychopathology. Front Neurosci. 2014;8:361. doi: 10.3389/fnins.2014.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Oleson EB, Cheer JF. A brain on cannabinoids: the role of dopamine release in reward seeking. Cold Spring Harb Perspect Med. 2012;2(8) doi: 10.1101/cshperspect.a012229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hermanns-Clausen M, et al. Acute intoxication by synthetic cannabinoids--four case reports. Drug Test Anal. 2013;5(9–10):790–794. doi: 10.1002/dta.1483. [DOI] [PubMed] [Google Scholar]