Abstract
Objectives
Increasing evidence showed that microRNAs (miRNAs) were implicated in the chemical resistance of human cancers. We intended to investigate the role of miR-218 in cisplatin sensitivity of esophageal cancer cells.
Methods
Quantitative real-time polymerase chain reaction (qRT-PCR) was carried out to analyze miR-218 expression in human esophageal cancer cell line Eca9706 and a cisplatin-resistant subline (ECa9706-CisR cells). The effects of miR-218 transfection on ECa9706 and ECa9706-CisR cell viability, including cell viability and apoptosis rate were confirmed using MTT assay, or flow cytometry, respectively. qRT-PCR was used to validate survivin as a direct target gene of miR-218 in our system.
Results
We found that miR-218 was significantly decreased in ECa9706-CisR cells compared with parent Eca9706 cells. Overexpression of miR-218 by mimics transfection would enhance cisplatin sensitivity evaluated by cell viability inhibition and apoptosis promotion. We validated here survivin as a direct target of miR-218 in ECa9706 cells, which might contribute to the chemoresistance of esophageal cancer cells to cisplatin.
Conclusions
In summary, our data suggest that miR-218 might represent as a promising sensitizer of cisplatin therapy in clinical esophageal cancer patients.
Keywords: Esophageal cancer, miR-218, cisplatin sensitivity, survivin
1. Introduction
Esophageal cancer (EC) is now one of the most aggressive and lethal type of malignant digestive tract tumor [1]. Currently, esophagectomy resection remains as the common and regular treatment strategy for EC patients at the early stage of this disease [2]. However, after resections, approximately half of patients develop systemic or local recurrences gradually along with the progression of EC, which would give bad outcomes [3-4]. Therefore, to improve prognosis, chemotherapy combined with surgery are widely used in the clinic. The results come out that effect of chemotherapy is not satisfying and the chemosensitivity differs between patients [5]. Low sensitivity or even chemoresistance give poor prognosis and also increase economic burden to patients. Cisplatin is a widely used chemotherapeutic agent. Resistance to cisplatin is frequently reported in EC patients [6-8]. Thus, efforts to understand the mechanisms of cisplatin resistance and elevate its sensitivity are of great importance in EC treatment.
MicroRNAs (miRNAs), typically 18-25 nucleotides in length, constitute a class of endogenous, small non-coding RNAs that are highly conserved across species [9]. Recent reports revealed that some miRNAs in cancers are associated with the response of chemotherapy [10-13]. For miR-218, a growing body of evidence supports that miR-218 acts as a potent tumor suppressor and participates in tumor progression, development and metastasis and also confers sensitivity to certain chemotherapeutic drugs in several types of human cancers [14]. For instance, in cervical cancer, miR-218 could impair cell growth, induce cell apoptosis and increase chemosensitivity to cisplatin in vitro [15]. It acts similarly in gastric cancer cells [16]. In EC cells, it was recently proved that miR-218 could inhibit cell proliferation and metastasis by targeting BMI1 [17]. Actually, it was also demonstrated by another group and they considered that miR-218 could both suppress tumor growth and enhance the chemosensitivity of EC cells to cisplatin, via regulating the expression of phosphorylated PI3K, AKT and mTOR [18]. However, the involvement of miR-218 in cisplatin sensitivity still lacks sufficient clues. In addition, the mechanisms that govern its activity in chemosensitivity remain to be more discussed.
2. Materials and methods
2.1. Cell Lines
Human esophageal squamous cell carcinoma cell line ECa9706 was purchased from Shanghai Institutes for Biological Sciences. ECa9706 cells were maintained in 1640 medium supplemented with 10% FBS at 37°C in the 5% CO2. According to Wang’s methods [19], a cisplatin-resistant subline named ECa9706-CisR cell line was obtained from parental ECa9706 cells.
2.2. Reagents and Transfections
Cisplatin was purchased from Sigma. MiR-218 mimic duplexes were synthesized by Gene Pharma. RNAimax was purchased from Life Science. Transfections were performed using RNAimax as per the manufacturer’s instructions. 48 hours post-transfection cells were harvested for further experiments.
2.3. Cell Viability MTT Assay
MTT assay using tetrazolium 3-(4,5)-dimethylthiahiazo(-z-y1)-3,5-di-phenytetrazoliumromide dye was employed to determine cell viability. Briefly, approximately 5×103 cells per well were seeded into a 96-well plate overnight. At certain time, 20ml of MTT solution was added into each well, and the plate was subsequently incubated for 4h in the incubator. Then, the liquid was removed from the plate, and 150ml of DMSO was added to each well. All plates were read at 490nm.
2.4. qRT-PCR
Total RNA was extracted by using TRIzol. Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) was performed from 2μ;g of total RNA transcribed with random primers. Amplification of cDNA was performed according to the manufacturer’s protocol using Takara SYBR Premix Ex Taq II kit. U6 was used as an internal control for microRNAs, and GAPDH was used as the internal control for genes. The relative expression level was calculated using the comparative 2-∆∆Ct method.
2.5. Apoptosis Analysis
For apoptosis analysis, ECa9706 and ECa9706-CisR cells were collected and Annexin V-FITC assay kit was used according to the manufacturer’s protocol after treated with 5mM cisplatin for 24h.
2.6. Statistical Analysis
Data in the present study were represented as means ± sd of at least three independent experiments. Student’s t-test was used for comparison between two groups. A P value < 0.05 was considered significantly different.
3. Results
3.1. Expression of miR-218 is lower in cisplatin-resistant EC cell lines
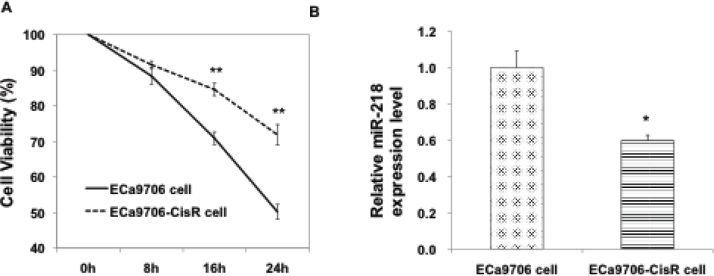
To determine whether miR-218 is implicated in cisplatin resistance in EC cells, ECa9706 cell line and its cisplatin-resistant subline named ECa9706-CisR was firstly established through a continuous exposure to increasing doses of cisplatin (from 1.5 to 12μ;M) over 12 months, according to Wang’s method [19]. The resistance to cisplatin of ECa9706-CisR cells was proven in Figure 1A. The expression level of miR-218 was then evaluated in this ECa9706-CisR cells and its parental line. qRT-PCR analysis showed significantly lower expression of miR-218 in cisplatin-resistant ECa9706-CisR cells (Figure 1B). These results suggest an association between miR-218 downregulation and cisplatin resistance in EC cells.
Figure 1.
Expression of miR-218 is lower in cisplatin-resistant esophageal cancer cell lines. (A)Cell viability inhibition effect of 5mM cisplatin on ECa9706 cells and ECa9706-CisR cells.**: P<0.01. (B)Relative miR-218 expression level in ECa9706 cells and ECa9706-CisR cells. *: P<0.05.
3.2. miR-218 transfection enhances cisplatin sensitivity
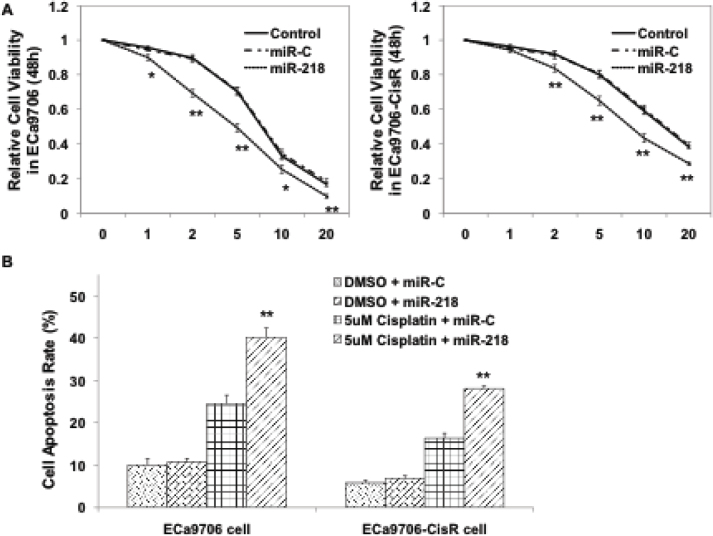
To explore whether miR-218 could increase cisplatin sensitivity of ECa9706 and established ECa9706-CisR cells, we transfected mimics of miR-218 or control duplexes into the indicated cells. As expected, following transfection of miR-218 mimics, the sensitivity of both ECa9706 and ECa9706-CisR cells to cisplatin was increased compared with negative control cells or miR-C cells, as demonstrated by MTT assay (Figure 2A). In addition, as shown in Figure 2B, flow cytometry demonstrated that miR-218 overexpression elevated the apoptosis rate in both cell lines upon treatment with 5μ;M of cisplatin. These results indicated that miR-218 expression is closely related to the sensitivity of EC cells to cisplatin.
Figure 2.
miR-218 enhances cisplatin sensitivity in both Eca9706 and ECa9706-CisR cells. (A)Cells were transfected with either control miRNA or miR-218 mimics. Then cells were treated with increasing doses of cisplatin (0, 1, 2, 5, 10 and 20mM). 48h later, MTT assay was performed. (B)Overexpression of miR-218 increased apoptosis rate when treated with 5mM cisplatin in both Eca9706 and ECa9706-CisR cells. *: P<0.05, **: P<0.01.
3.3. miR-218 targets survivin to increase cisplatin sensitivity
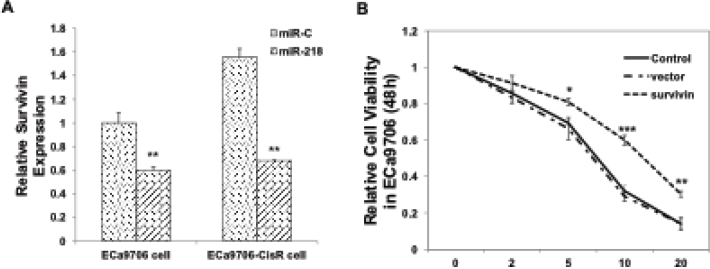
To investigate whether miR-218-induced cisplatin sensitivity was mediated by its downstream targets, we first examined in silico predicted targets using miRDB (http://mirdb. org/miRDB/). Besides, a study from breast cancer cells had pointed out that survivin, encoded by BIRC5, was significantly reduced after miR-218 transfection and this targeting regulated resistance to chemotherapeutics in breast cancer cells [20]. Thus, we also speculated that in our EC cells, the miR-218/survivin pathway contributed to the increase of cisplatin sensitivity. To confirm this hypothesis, we carried out qRT-PCR experiments, and found that miR-218 significantly downregulated survivin expression in both ECa9706 and ECa9706-CisR cells (Figure 3A). Notably, we found that in the miR-C transfected cells, survivin expression was a bit increased in ECa9706-CisR than the parent ECa9706 cells. To further test whether the targeting of survivin might contribute a lot for miR-218-induced cisplatin resistance, we overexpressed survivin in ECa9706 cells and monitored the chemoresistance effect of this overexpression. As shown in Figure 3B, we proved that survivin could enhance cisplatin resistance in this cell line.
Figure 3.
miR-218 downregulates survivin to increase cisplatin sensitivity in both Eca9706 and Eca9706-CisR cells. (A)Either control microRNA or miR-218 mimics were transfected into both ECa9706 and ECa9706-CisR cells. 48h later, cells were harvested for qRT-PCR test. **: P<0.01. (B)Survivin was overexpressed in Eca9706 cells by plasmid transfection. Then cells were treated with increasing doses of cisplatin (0, 2, 5, 10 and 20mM). 24h later, MTT assay was performed. *: P<0.05, **: P<0.01, ***: P<0.001.
4. Discussion
Our current study showed that miR-218 was significantly decreased in cisplatin-resistant EC cell line, which in turn, was associated with cisplatin resistance in these cells. Specifically, we demonstrated that miR-218 transfection by its mimics could increase chemosensitivity of both ECa9706 cells and ECa9706-CisR cells to cisplatin in vitro, probably via targeting survivin expression. Our findings might provide an important layer of genetic regulation in EC progression, especially in its chemoresistance to cisplatin, and ultimately would benefit developing valuable therapeutic tools for EC therapy.
The roles of miRNAs in the chemoresistance of EC cells have recently attracted attention. For example, miR-141 was found to be the most highly expressed miRNA in the cisplatin-resistant EC cell lines, and it confers resistance to cisplatin-induced apoptosis by targeting YAP121. A report from another group also showed that, miR-96 serves as an oncogene role in EC cells through downregulating RECK, which also promotes chemo- or radioresistance in these cells [22]. As regards miR-218, its downregulation has been reported in several types of human cancers. In EC cells, it has been proved that miR-218 could inhibit cell proliferation and metastasis [17]. Additionally, it was also reported by another group that miR-218 could both suppress tumor growth and enhance the chemosensitivity of EC cells to cisplatin, through downregulating the levels of phosphorylated PI3K, AKT and mTOR [17]. Consistent with this, in the present study, we observed that miR-218 was downregulated in cisplatin-resistant EC cells, and its forced expression sensitized cells to cisplatin treatment. We further validated here survivin was targeted by miR-218. However, a complete understanding of survivin-mediated chemoresistance remains still unclear. Future work will be required to further explore the detailed mechanisms by which survivin affects chemoresistance.
In conclusion, this study confirmed that miR-218 could enhance cisplatin sensitivity of EC cells via targeting survivin. Based on these results, we suggested that the miR-218/survivin axis is a potential therapeutic target for EC treatment. And anti-survivin drugs might be developed with a focus on enhanced sensitivity of EC patients to chemotherapy.
Conflict of interest statement:Authors state no conflict of interest
References
- Hu W, Liang Y, Zhang S, Hu Y, Liu J. The significance of subcarinal dissection in esophageal cancer surgery. Asia Pac J Clin Oncol. 2014;10(2):183–189. doi: 10.1111/ajco.12095. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Park JK, Moon SW. Usefulness of positron emission tomography-computed tomography in pre-operative evaluation of intra-thoracic esophageal cancer. Thoracic cancer. 2015;6(6):687–694. doi: 10.1111/1759-7714.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ge J, Zhang XH, Liu JY, Yang CM, Zhao SL. Endoscopic submucosal dissection versus endoscopic mucosal resection for the treatment of early esophageal carcinoma: a meta-analysis. Asian Pac J Cancer Prev. 2014;15(4):1803–1806. doi: 10.7314/apjcp.2014.15.4.1803. [DOI] [PubMed] [Google Scholar]
- Miyata H, Yamasaki M, Kurokawa Y, Takiguchi S, Nakajima K, Fujiwara Y, Konishi K, Mori M, Doki Y. Survival factors in patients with recurrence after curative resection of esophageal squamous cell carcinomas. Ann Surg Oncol. 2011 Nov;18(12):3353–3361. doi: 10.1245/s10434-011-1747-7. [DOI] [PubMed] [Google Scholar]
- Miyata H, Yoshioka A, Yamasaki M, Nushijima Y, Takiguchi S, Fujiwara Y, Nishida T, Mano M, Mori M, Doki Y. Tumor budding in tumor invasive front predicts prognosis and survival of patients with esophageal squamous cell carcinomas receiving neoadjuvant chemotherapy. Cancer. 2009 Jul 15;115(14):3324–3334. doi: 10.1002/cncr.24390. [DOI] [PubMed] [Google Scholar]
- Zhu L, Du H, Shi M, Chen Z, Hang J. ATG7 deficiency promote apoptotic death induced by Cisplatin in human esophageal squamous cell carcinoma cells. Bull Cancer. 2013 Jul-Aug;100(7-8):15–21. doi: 10.1684/bdc.2013.1749. [DOI] [PubMed] [Google Scholar]
- Guo W, Zhao YP, Jiang YG, Wang RW, Hong L, Fan DM. Upregulation of ZNRD1 enhances cisplatin resistance in human esophageal cancer cells by regulation of ERCC1 and Bcl-2. Tumour Biol. 2008;29(3):188–194. doi: 10.1159/000146864. [DOI] [PubMed] [Google Scholar]
- Takashima A, Shirao K, Hirashima Y, Takahari D, Okita N, Akatsuka S, Nakajima TE, Matsubara J, Yasui H, Asakawa T, Kato K, Hamguchi T, Muro K, Yamada Y, Shimada Y. Chemosensitivity of patients with recurrent esophageal cancer receiving perioperative chemotherapy. Dis Esophagus. 2008;21(7):607–611. doi: 10.1111/j.1442-2050.2008.00821.x. [DOI] [PubMed] [Google Scholar]
- Obermayer B, Levine E. Exploring the miRNA regulatory network using evolutionary correlations. PLoS Comput Biol. 2014 Oct 9;10(10):e1003860. doi: 10.1371/journal.pcbi.1003860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamano R, Miyata H, Yamasaki M, Kurokawa Y, Hara J, Moon JH, Nakajima K, Takiguchi S, Fujiwara Y, Mori M, Doki Y. Overexpression of miR-200c induces chemoresistance in esophageal cancers mediated through activation of the Akt signaling pathway. Clin Cancer Res. 2011 May 1;17(9):3029–3038. doi: 10.1158/1078-0432.CCR-10-2532. [DOI] [PubMed] [Google Scholar]
- Imanaka Y, Tsuchiya S, Sato F, Shimada Y, Shimizu K, Tsujimoto G. MicroRNA-141 confers resistance to cisplatin-induced apoptosis by targeting YAP1 in human esophageal squamous cell carcinoma. J Hum Genet. 2011 Apr;56(4):270–276. doi: 10.1038/jhg.2011.1. [DOI] [PubMed] [Google Scholar]
- Hummel R, Watson DI, Smith C, Kist J, Michael MZ, Haier J, Hussey DJ. Mir148a improves response to chemotherapy in sensitive and resistant oesophageal adenocarcinoma and squamous cell carcinoma cells. J Gastrointest Surg. 2011 Mar;15(3):429–438. doi: 10.1007/s11605-011-1418-9. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li M, Han Y, Hong L, Gong T, Sun L, Zheng X. Down-regulation of miR-27a might reverse multidrug resistance of esophageal squamous cell carcinoma. Dig Dis Sci. 2010 Sep;55(9):2545–2551. doi: 10.1007/s10620-009-1051-6. [DOI] [PubMed] [Google Scholar]
- Lu YF, Zhang L, Waye MM, Fu WM, Zhang JF. MiR-218 mediates tumorigenesis and metastasis: Perspectives and implications. Exp Cell Res. 2015 May 15;334(1):173–182. doi: 10.1016/j.yexcr.2015.03.027. [DOI] [PubMed] [Google Scholar]
- Li J, Ping Z, Ning H. MiR-218 impairs tumor growth and increases chemo-sensitivity to cisplatin in cervical cancer. Int J Mol Sci. 2012 Nov 28;13(12):16053–16064. doi: 10.3390/ijms131216053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XL, Shi HJ, Wang JP, Tang HS, Wu YB, Fang ZY, Cui SZ, Wang LT. MicroRNA-218 is upregulated in gastric cancer after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy and increases chemosensitivity to cisplatin. World J Gastroenterol. 2014 Aug 28;20(32):11347–11355. doi: 10.3748/wjg.v20.i32.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Chen T, Niu H, Li C, Xu C, Li Y, Huang R, Zhao J, Wu S. MicroRNA-218 inhibits the proliferation and metastasis of esophageal squamous cell carcinoma cells by targeting BMI1. Int J Mol Med. 2015 Jul;36(1):93–102. doi: 10.3892/ijmm.2015.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Hou L, Xiong YM, Huang JX, She YJ, Bi XB, Song XR. miR-218 suppresses tumor growth and enhances the chemosensitivity of esophageal squamous cell carcinoma to cisplatin. Oncol Rep. 2015 Feb;33(2):981–989. doi: 10.3892/or.2014.3657. [DOI] [PubMed] [Google Scholar]
- Zhang T, Rong N, Chen J, Zou C, Jing H, Zhu X, Zhang W. SIRT1 expression is associated with the chemotherapy response and prognosis of patients with advanced NSCLC. PLoS One. 2013 Nov 5;8(11):e79162. doi: 10.1371/journal.pone.0079162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Xu K, Yagüe E. miR-218 targets survivin and regulates resistance to chemotherapeutics in breast cancer. Breast Cancer Res Treat. 2015 Jun;151(2):269–280. doi: 10.1007/s10549-015-3372-9. [DOI] [PubMed] [Google Scholar]
- Imanaka Y, Tsuchiya S, Sato F, Shimada Y, Shimizu K, Tsujimoto G. MicroRNA-141 confers resistance to cisplatin-induced apoptosis by targeting YAP1 in human esophageal squamous cell carcinoma. J Hum Genet. 2011 Apr;56(4):270–276. doi: 10.1038/jhg.2011.1. [DOI] [PubMed] [Google Scholar]
- Xia H, Chen S, Chen K, Huang H, Ma H. MiR-96 promotes proliferation and chemo- or radioresistance by down-regulating RECK in esophageal cancer. Biomed Pharmacother. 2014 Oct;68(8):951–958. doi: 10.1016/j.biopha.2014.10.023. [DOI] [PubMed] [Google Scholar]