Abstract
Objectives
Although the Hox transcript antisense intergenic RNA (HOTAIR), a vital long non-coding RNA, is known to participate in the development and progression of a wide range of carcinomas, there are still no published reports regarding its expression in adenocarcinoma of esophagogastric junction (AEJ). The aims of this study were to investigate the expression of HOTAIR, and to analyze the association of its expression with PI3K/Akt pathway activation in clinical AEJ patients.
Methods
Nine normal epithelial tissues and 41 samples of AEJ were studied comparably. The expression of HOTAIR was detected by real-time PCR according to the different tumor grades in these AEJ tissues. Western blot was performed to reveal the Ser473-phosphorylated Akt and total Akt levels. Results: HOTAIR was found to be up-regulated in higher grades of AEJ tissues compared to low grades and/or noncancerous tissues. pAkt expression was also found to be up-regulated in tissues of higher tumor stages. We found that the overexpression of HOTAIR finely correlated with elevated Ser473-phosphorylated Akt levels. Conclusion: Upregulated HOTAIR was associated with abnormal activated PI3K/Akt pathway, which might serve as a promising therapeutic strategy for AEJ treatment.
Keywords: AEJ, HOTAIR, PI3K/Akt pathway
1. Introduction
Adenocarcinoma of the esophagogastric junction (AEJ) is firstly defined as the carcinoma that across the esophagogastric junction line, no matter where the tumor epicenter locates, including both distal esophageal and proximal gastric carcinomas [1]. The incidence of AEJ has risen faster than any other malignancies in western countries, especially over the last two decades [2,3]. There remain many controversies in its definition, classification, diagnosis and treatment. Increasing evidences suggest that AEJ is different from gastric and esophageal adenocarcinoma in molecular features, pathological evolution, and clinical behavior [4]. The prognosis of AEJ patients remains still poor (five-year survival rate, 10%-15%), which is poorer than those with other gastric cancers [5], because most of these patients are diagnosed at an advanced stage. The diagnosis and prognosis promise of many molecular targeted agents that have been characterized in other tumors are still unclear in AEJ [6–8].
Long non-coding RNAs (lncRNAs) are non-protein coding RNA transcripts that range from 200 bases to about 100 kb bases in length. They are mostly transcribed by RNA POLII from different regions across the genome. Recently, lnRNAs are known as the key regulators of gene expression via transcriptional and/or post-transcriptional regulation [9,10]. In addition, lncRNA could also regulate the activity of epigenetic machinery during cell differentiation [11]. For instance, some lncRNAs were proved to be able to recruit chromatin-modifying proteins, for example, polycomb repressive complex 2 (PRC2), to specific sites of genome and affect gene expression through regulating chromatin status [12]. Homeobox transcript antisense intergenic RNA (HOTAIR) is among these lncRNAs that could act epigenetically to repress gene expression [13].
HOTAIR, a trans-acting lncRNA, is located on chromosome 12q13.13, and is aberrantly up-regulated in various human cancers, such as breast cancer, gastric cancer, and non-small cell lung cancer [14,15]. HOTAIR has been found to be involved in cooperation with chromatin modifying enzymes, like PRC2, to promote epigenetic activation or silencing gene expression [16]. One study has showed that the oncogenic role of HOTAIR in human laryngeal squamous cell cancer is to promote phosphatase and tensin homolog (PTEN) methylation [17]. PTEN is a tumor suppressor by inhibiting PI3K/Akt pathway [18]. Activation of PI3K/Akt signaling enhances cancer cell proliferation, survival, cell cycle progression, and angiogenesis [19]. Methylation of PTEN results in inhibition of its expression at both mRNA and protein levels. Hence, methylation of PTEN is one of the mechanisms why HOTAIR acts as an oncogenic lncRNA leading to tumorigenesis. However, there are still no reports addressing the association of HOTAIR expression with PI3K/Akt signaling pathway in human cancers, even not to mention in AEJ. We thus sought to investigate the expression of HOTAIR in clinical AEJ tissues, and further analyze the relationship between its expression levels and the PI3K/Akt signaling pathway activation.
2. Materials and methods
2.1. Clinical samples
Freshly resected tissue was immediately frozen in liquid nitrogen for subsequent total RNA extraction. All grades of adenocarcinoma of esophagogastric junction tissues with clinical data were collected from the First Affiliated Hospital of AnHui Medical University. All patients have given their informed consent prior to their inclusion. The fresh tissues were snap-frozen in liquid nitrogen and stored at –80°C until analysis.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
2.2. RNA extraction and real-time PCR analysis
Total RNA was extracted by TRIzol (Life Technology). QRT-PCR assay was performed to measure the expression levels of HOTAIR and GAPDH. Real-time PCR was performed using SYBR Premix Ex Taq mix (takara) according to the Manufacturer’s protocols. GAPDH were used for normalization of HOTAIR expression. The results were analyzed by the comparative Ct method.
2.3. Western Blot analysis
The phosphorylated Akt (pAkt) and non-phosphorylated Akt (tAkt) level was assayed by western blot analysis in human tissue lysates (40µg of protein in RIPA lysis buffer). Proteins were separated by 9% SDS-PAGE and transferred to a PVDF membrane. After blocking by 5% milk for 1h, the membranes were incubated with pAkt antibody (Cell Signaling) or tAkt antibody (Cell Signaling) at a dilution of 1:1000 in 5% BSA, followed by a secondary antibody against rabbit IgG. Signals were visualized with the ECL chemiluminescence system Quantification of the intensity of pAkt and tAkt in was performed by ImageJ software.
2.4. Statistical analysis
Statistical analyses were performed by SAS v8. The significance of differences between normal tissues and tumors or between different grades was estimated by one-way ANOVA. Correlation between expression levels of HOTAIR and pAkt was analyzed using Spearman’s linear correlation. Two-sided P-values were calculated, and a P < 0.05 was chosen for statistical significance.
3. Results
3.1. HOTAIR is upregulated in AEJ patients
Since we were interested in examining if HOTAIR was altered at RNA levels in tumors vs normal tissues, we used real-time PCR to identify HOTAIR expression patterns within AEJ tumors of WHO grade I, II, III and IV. A total of 41 AEJ patients were enrolled in this study. GAPDH was used for normalization of HOTAIR expression. We observed that over 90% of the AEJ tissues exhibited higher HOTAIR expression than in the non-cancerous tissues. High (more than 2 fold) HOTAIR expression levels were identified in 71% (29/41) of all tested AEJ samples. As shown in Figure 1, HOTAIR was upregulated in higher grades of AEJ tissues. Our findings clearly demonstrated that HOTAIR was abnormally increased in AEJ tumor tissues compared to normal controls, in agreement with its overexpression and oncogenic potential in gastric cancer.
Figure 1.
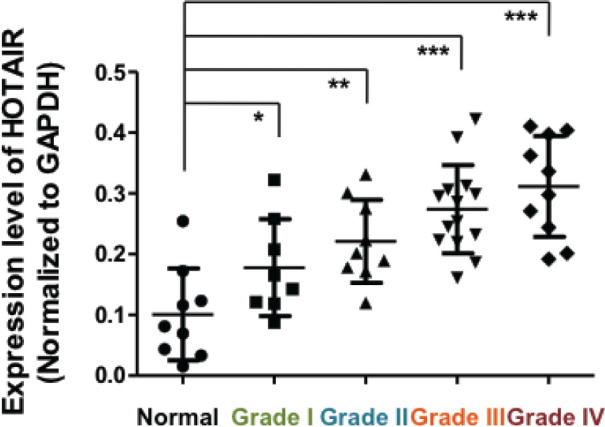
HOTAIR expression is upregulated in adenocarcinoma of esophagogastric junction (WHO grade I to IV) tissues. Compared with non-tumor tissue (Normal, n=9), AEJ tissues of WHO grade I (n=8), grade II (n=9), grade III (n=14) and grade IV (n=10), tissues from AEJ (n=41) displayed higher HOTAIR expression as determined by real-time PCR. GAPDH was used as internal control. Different grades were showed in different colors. *: P<0.05, **: P<0.01, ***: P<0.001 (One-Way ANOVA).
3.2. Activated Akt in advanced AEJ tissues
As a crucial signaling pathway, the PI3K/Akt pathway, or Akt pathway, serves as an important signal transduction pathway that promotes cell growth and survival in response to extracellular signals. Full activation of Akt requires phosphorylation of Ser473 (S473), which can be catalyzed by multiple protein kinases. Since HOTAIR was proved to regulate PTEN, a tumor suppressor that negatively impacts Akt pathway. Indeed, loss of PTEN function leads to over activation of Akt and is common in cancer cells. Because we showed an upregulation of HOTAIR in AEJ tissues, we began to suspect that PI3K/Akt pathway might also be activated in this disease. We then performed western blot assays to confirm our hypothesis. Figure 2A showed the representative results of pAkt and tAkt in normal and AEJ tissues, which suggested an upregulation of pAkt level in AEJ adenocarcinomas. As shown in Figure 2B, the ratio of pAkt/tAkt expression was plotted, and the S473-phosphorylated Akt exhibited hyper-activated in AEJ tissues of higher tumor grades. tAkt was used asinternal controls.
Figure 2.
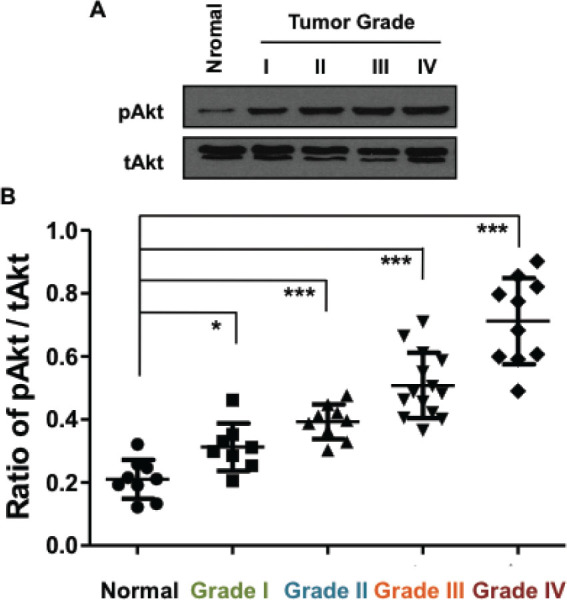
Level of pAkt is upregulated in adenocarcinoma of esophagogastric junction (WHO grade I to IV) tissues. (A) Western blot results of pAkt and tAkt in normal and tumor tissues with different grades, representatively. (B) Compared with non-tumor tissue (Normal, n=9), AEJ tissues of WHO grade I (n=8), grade II (n=9), grade III (n=14) and grade IV (n=10), tissues from AEJ (n=41) displayed higher ratio of pAkt/tAkt expression as determined by western blot. Different grades were showed in different colors. *: P<0.05, **: P<0.01, ***: P<0.001 (One-Way ANOVA).
3.3. Positive correlation of HOTAIR levels with pAkt
In order to assess whether higher HOTAIR levels correlates with higher pAkt levels, Spearman’s correlation analysis was performed in 41 AEJ patients. As shown in Figure 3, we found that after logarithmic transformation, HOTAIR expression closely associated with the pAkt/tAkt levels in these AEJ tissues, suggesting a putative linear relationship between these two molecules. This finding supports the hypothesis that an altered HOTAIR may contribute to tumor progression of AEJ. Although direct evidence was still lacking, our results at least provide clues.
Figure 3.
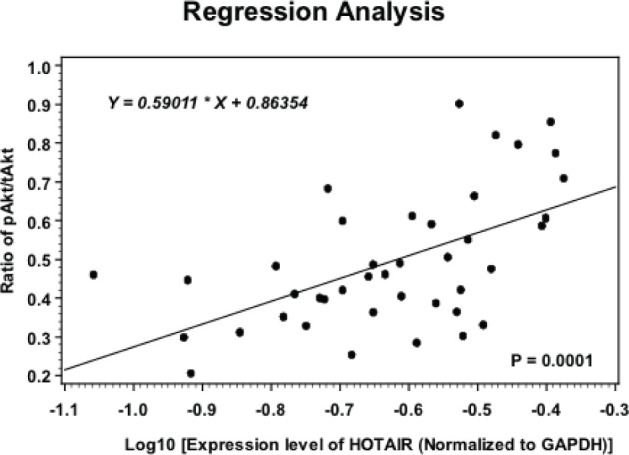
Spearman’s correlation analysis between HOTAIR expression and the pAkt levels. The positive correlation between HOTAIR expression and pAkt in 41 AEJ samples was determined using Spearman’s correlation analysis (P=0.0001).
4. Discussion
Here, we employed regular approaches to provide the first limited analysis of lncRNA HOTAIR expression in a small scale of 50 samples from 41 primary AEJ patients and nine non tumoral controls. We found that both HOTAIR and pAkt were significantly upregulated in tumor tissues compared with that of the non-cancerous tissues. Notably, we observed that these two molecules both exhibited a higher expression in advanced AEJ tumors. Furthermore, we found a fine correlation between HOTAIR and pAkt levels in the AEJ tumors, which is consistent with Chen’s recent report in breast cancer [20], though they pointed out that HOTAIR was a downstream target of pAkt. Our data support the notion that HOTAIR is an oncogenic lncRNA in human malignancies. Since Akt is an important node in the cellular PI3K/Akt pathway and might serve as a targetable molecule for AEJ treatment, our study here provided another possibility and mechanism to address the role of HOTAIR in cancer cells.
Currently, the understanding of biomarkers and therapeutic targets for AEJ have largely been unclear and lagged behind those of gastric or esophageal cancers. The reasons that lead to the current difficulty in AEJ research include: 1) unavailability in obtaining model systems, such as cultured cell lines; 2) difficulty in giving clear conclusions from the previous reports, since the majority of previous gastroesophageal tumor sample studies havie already been mixed by tumor sites (esophagus, gastroesophageal junction and stomach). The study of molecular biological biomarkers has become a hot topic worldwide.
Different studies have provided evidence for addressing the roles of HOTAIR in the development and progression in cancers. It is known that HOTAIR interacts with PRC2 and regulates chromosome occupancy by EZH2 (a subunit of PRC2), which leads to histone H3 lysine 27 (H3K27) trimethylation of the homeobox D locus, enforcing a silent chromatin state at Hox and additional genes, which contributes to cancer development and metastasis [21]. Another group demonstrated that HOTAIR could promote methylation of PTEN [17]. Its methylation leads to its downregulation at both mRNA and protein levels. Loss of PTEN would activate the PI3K/Akt pathway. Hence, HOTAIR might indirectly impact this signaling pathway through the mediator, PTEN. More efforts should be paid for deep understanding of HOTAIR/Akt signaling transduction afterwards.
In summary, we concluded from our study that lncRNA HOTAIR expression was increased in AEJ adenocarcinomas. Overexpressed HOTAIR was more significantly observed in higher grades tumors. Importantly, upregulation of HOTAIR was associated with activated PI3K/Akt pathway. The exact mechanism involved in this regulation requires more studies in future.
Conflict of interest statement
Authors state no conflict of interest.
References
- [1].Monig SP, Schroder W, Beckurts KT, Holscher AH.. Classification, diagnosis and surgical treatment of carcinomas of the gastroesophageal junction. Hepatogastroenterology. 2001 Sep-Oct;48(41):1231–1237. [PubMed] [Google Scholar]
- [2].Pohl H, Welch HG.. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005 Jan 19;97(2):142–146. doi: 10.1093/jnci/dji024. [DOI] [PubMed] [Google Scholar]
- [3].Moehler M, Lyros O, Gockel I, Galle PR, Lang H.. Multidisciplinary management of gastric and gastroesophageal cancers. World J Gastroenterol. 2008 Jun 28;14(24):3773–3780. doi: 10.3748/wjg.14.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yu X, Xiao H, Zhao B, Zhang X, Wang G.. DNA repair gene ERCC1 C118T polymorphism predicts sensitivity of recurrent esophageal cancer to radiochemotherapy in a Chinese population. Thoracic cancer. 2015;6(6):741–748. doi: 10.1111/1759-7714.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hu W, Liang Y, Zhang S, Hu Y, Liu J.. The significance of subcarinal dissection in esophageal cancer surgery. Asia Pac J Clin Oncol. 2014;10(2):183–189. doi: 10.1111/ajco.12095. [DOI] [PubMed] [Google Scholar]
- [6].Almhanna K.. Targeted therapy for gastric adenocarcinoma. Adv Pharmacol. 2012;65:437–470. doi: 10.1016/B978-0-12-397927-8.00014-2. [DOI] [PubMed] [Google Scholar]
- [7].Oshima T, Masuda M.. Molecular targeted agents for gastric and gastroesophageal junction cancer. Surg Today. 2012 Apr;42(4):313–327. doi: 10.1007/s00595-011-0065-9. [DOI] [PubMed] [Google Scholar]
- [8].Mukherjee K, Chakravarthy AB, Goff LW, El-Rifai W.. Esophageal adenocarcinoma: treatment modalities in the era of targeted therapy. Dig Dis Sci. 2010 Dec;55(12):3304–3314. doi: 10.1007/s10620-010-1187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Flynn RA, Chang HY.. Long noncoding RNAs in cell-fate programming and reprogramming. Cell Stem Cell. 2014 Jun 5;14(6):752–761. doi: 10.1016/j.stem.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Eades G, Zhang YS, Li QL, Xia JX, Yao Y, Zhou Q.. Long non-coding RNAs in stem cells and cancer. World J Clin Oncol. 2014 May 10;5(2):134–141. doi: 10.5306/wjco.v5.i2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rinn JL.. lncRNAs: linking RNA to chromatin. Cold Spring Harb Perspect Biol. 2014 Aug 1;6(8):pii: a018614. doi: 10.1101/cshperspect.a018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mercer TR, Mattick JS.. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013 Mar;20(3):300–307. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- [13].Bhan A, Mandal SS.. LncRNA HOTAIR: A master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015 Aug;1856(1):151–164. doi: 10.1016/j.bbcan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hajjari M, Salavaty A.. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med. 2015 Mar;12(1):1–9. doi: 10.7497/j.issn.2095-3941.2015.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cai B, Song XQ, Cai JP, Zhang S.. HOTAIR: a cancer-related long non-coding RNA. Neoplasma. 2014;61(4):379–391. doi: 10.4149/neo_2014_075. [DOI] [PubMed] [Google Scholar]
- [16].Marchese FP, Huarte M.. Long non-coding RNAs and chromatin modifiers: their place in the epigenetic code. Epigenetics. 2014 Jan;9(1):21–26. doi: 10.4161/epi.27472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li D, Feng J, Wu T, Wang Y, Sun Y, Ren J, Liu M.. Long intergenic noncoding RNA HOTAIR is overexpressed and regulates PTEN methylation in laryngeal squamous cell carcinoma. Am J Pathol. 2013 Jan;182(1):64–70. doi: 10.1016/j.ajpath.2012.08.042. [DOI] [PubMed] [Google Scholar]
- [18].Hales EC, Taub JW, Matherly LH.. New insights into Notch1 regulation of the PI3K-AKT mTOR1 signaling axis: targeted therapy of γ-secretase inhibitor resistant T-cell acute lymphoblastic leukemia. Cell Signal. 2014 Jan;26(1):149–161. doi: 10.1016/j.cellsig.2013.09.021. [DOI] [PubMed] [Google Scholar]
- [19].Petrulea MS, Plantinga TS, Smit JW, Georgescu CE, Netea-Maier RT.. PI3K/Akt/mTOR: A promising therapeutic target for non-medullary thyroid carcinoma. Cancer Treat Rev. 2015 Sep;41(8):707–713. doi: 10.1016/j.ctrv.2015.06.005. [DOI] [PubMed] [Google Scholar]
- [20].Chen J, Lin C, Yong W, Ye Y, Huang Z.. Calycosin and genistein induce apoptosis by inactivation of HOTAIR/p-Akt signaling pathway in human breast cancer MCF-7 cells. Cell Physiol Biochem. 2015;35(2):722–728. doi: 10.1159/000369732. [DOI] [PubMed] [Google Scholar]
- [21].Zhang J, Zhang P, Wang L, Piao HL, Ma L.. Long non-coding RNA HOTAIR in carcinogenesis and metastasis. Acta Biochim Biophys Sin (Shanghai) 2014 Jan;46(1):1–5. doi: 10.1093/abbs/gmt117. [DOI] [PMC free article] [PubMed] [Google Scholar]


