Abstract
Non-small cell lung cancer (NSCLC) is the leading cause of cancer related deaths worldwide. But no one type of serum biomarker was found to be highly sensitive and specific for detection of lung cancer at present. So, the aim of this study was to evaluate a diagnostic value of serum carcinoembryonic antigen (CEA), neuron specific enolase (NSE) and matrix metallo-proteinase (MMP-9) for non-small cell lung cancer. Thirty-six cases with pathology confirmed non-small cell lung cancer and thirty-two of subjects with benign lung disease were reviewed in our hospital and included in this retrospective study. The serum level of CEA, NSE and MMP-9 were tested and compared between the non-small cell lung cancer patients and benign lung disease. The diagnosis sensitivity, specificity and area under the receiver-operating characteristic (ROC) curve (AUC) for serum CEA, NSE and MMP-9 were calculated with STATA10.0 software. The serum CEA, NSE and MMP-9 were 32.0±16.7 ng/mL, 51.6±68.3 ng/mL, 30.6 ±15.7 μg/L for the NSCLC patients and 15.1±10.9 ng/mL, 4.9±3.1 ng/mL, 9.3±5.9 μg/L for the benign lung disease patients with statistical difference (Pall<0.05); The diagnosis sensitivity, specificity and AUC were 80.0%, 72.2%, 0.84 for the serum CEA; 71.0%, 83.3% and 0.80 for NSE and 87.1%, 80.56%, 0.89 for MMP-9, respectively. The serum CEA, NSE and MMP-9 were generally elevated in patients with non-small cell lung cancer and could be used as potential bio-markers for non-small cell lung cancer diagnosis.
Keywords: Non-small cell lung cancer, biomarker, diagnosis, sensitivity, specificity
1. Introduction
According to the global cancer statistical reports, lung cancer especially the non-small cell lung cancer (NSCLC), is the 1st most commonly diagnosed malignant solid tumor worldwide [1]. In the Unites States, it was estimated that 221,200 new lung cancer cases and 158,040 deaths were found for both male and female during 2015 [2]. In China, there is no accurate epidemiology data for lung cancer morbility and mortality for the whole country. But local epidemiology studies indicated that lung cancer incidence is on the rise in China [3]. Generally speaking, the prognosis of lung cancer is poor for lacking tools of early diagnosis. Serum biomarker was known as effective for lung cancer diagnosis being minimally invasive. But no one type of biomarker was found to be highly sensitive and specific for detection of lung cancer at present [4]. So, combination detection multiple biomarker was general used for lung cancer diagnosis [5].
2. Material and methods
2.1. Patients
Patients included in this retrospective study were selected from the electronic medical records of our hospital from February 2012 to January 2015. The inclusion criteria were: (1) Patients were diagnosed with non-small cell lung cancer with pathology or cytology confirmation; (2) The serum CEA, NSE and MMP-9 can be drawn from the electronic medical records; (3) No other malignant tumors could be found in the included patients except for non-small cell lung cancer; (4) The benign lung disease was diagnosed by clinical investigations for patients in the control group. According to the inclusion criteria, 36 patients with pathology or cytology confirmed non-small cell lung cancer and 32 subjects with diagnosis of benign lung disease were included in this study. For the non-small cell lung cancer group, the mean age was 58.2±16.8 with 21 cases of adenocarcinoma and 15 cases of squamous cell carcinoma. For the benign lung disease group, the mean age of patients was 50.4±19.2 with 20 subjects of pulmonary infection, 6 cases of pulmonary tuberculosis and 6 cases of obstructive pulmonary disease.
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent: Informed consent has been obtained from all individuals included in this study.
2.2. Data extraction
The data of each included patient were extracted by two reviewers independently and checked by the third reviewer. The extraction information of each participating patient included (1) Name of patient; (2) gender; (3) age; (4) diagnosis with pathology type; (5) serum level of CEA, NSE and MMP-9.
2.3. Statistical analysis
Statistical analysis was done by the STATA10.0 software package. The data were expressed as mean±S.D. The significance of CEA, NSE and MMP-9 between non-small cell lung cancer patients and benign lung disease patients was analyzed using Student’s t –test. Receiver operator characteristic (ROC) curves were calculated and the area under the curve (AUC) was generated to compare the diagnostic accuracy of CEA, NSE and MMP-9 for predicting non-small cell lung cancer. The correlation between CEA and NSE or CEA and MMP-9 was calculated by Pearson correlation test. Differences with P<0.05 were considered as statistically significant
3. Results
3.1. Serum level of CEA, NSE and MMP-9
The serum CEA, NSE and MMP-9 were 32.0±16.7 ng/mL, 51.6±68.3 ng/mL, 30.6 ±15.7 μg/L for the NSCLC group and 15.1±10.9 ng/mL, 4.9±3.1 ng/mL, 9.3±5.9 μg/L for the benign lung disease group with statistical difference (Pall<0.05). The serum level of CEA, NSE and MMP-9 of non-small cell lung cancer patients were significantly higher than that of benign lung disease patients. Table 1.
Table 1.
The serum level of CEA, NSE and MMP-9in non-small cell lung cancer
| NSE(ng/mL) | CEA (ng/mL) | MMP-9 (μg/L) | ||||
|---|---|---|---|---|---|---|
| Group | Mean | SD | Mean | SD | Mean | SD |
| NSCLC (n=36) | 32.0 | 16.7 | 51.6 | 68.3 | 30.6 | 15.7 |
| BLD (n=32) | 15.1 | 10.9 | 4.9 | 3.1 | 9.3 | 5.9 |
NSCLC: non-small cell lung cancer; BLD: benign lung disease; SD: Standard deviation
3.2. Diagnostic sensitivity and specificity
We calculated the diagnosis sensitivity, specificity of the serum CEA, NSE and MMP-9. The diagnosis sensitivity, specificity were 80.0% and 72.2% for the serum CEA at the cut off value of 5.2 ng/mL; 71.0% and 83.3% for NSE at the cut off value of and 19.1 ng/mL; 87.1% and 80.56% at the cut off value of 15.3 μg/L for MMP-9.
3.3. The areas under the ROC curves
The ROC curves were calculated in order to evaluate the diagnosis efficacy for serum CEA, NSE and MMP-9 as a biomarker in diagnosis of non-small cell lung cancer. The areas under the ROC curve were 0.84 (95%CI: 0.74–0.93, P<0.05), 0.80 (95%CI: 0.69–0.91, P<0.05) and 0.89 (95%CI: 0.81–0.97, P<0.05) for CEA, NSE and MMP-9, respectively as a biomarker for non-small cell lung cancer diagnosis (Figure 1).
Figure 1.
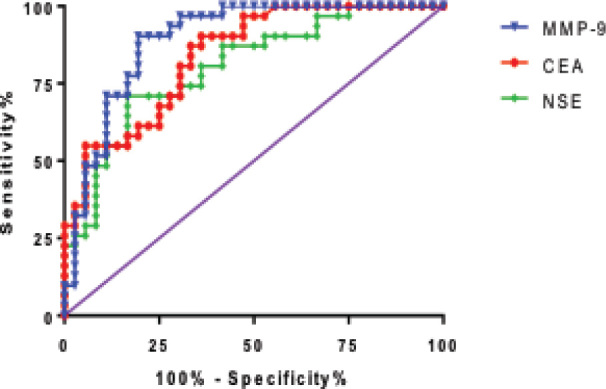
The diagnosis ROC curve for serum CEA, NSE and MMP-9
3.4. The correlation between serum CEA, NSE and MMP-9
The correlation between CEA, NSE and MMP-9 was calculated by Pearson correlation analysis. But no significant correlation was found between NSE and CEA (rPearson=0.28, P=0.10) or MMP-9 and CEA (rPearson=0.13, P=0.50), Figure 2.
Figure 2.
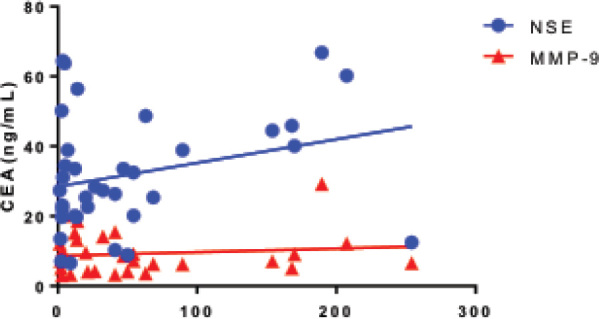
The correlation between serum CEA, NSE and MMP-9 in patients with NSCLC
4. Discussion
As is well known, lung cancer is one of the most diagnosed malignant solid carcinomas for males and the second for females and remains the leading cause of cancer related deaths worldwide [6, 7]. The general prognosis for lung cancer is poor due to the lack of effective methods of early detection [8]. Patients with the early stage of non-small cell lung cancer can be treated with surgery plus combined treatment modality with relatively well prognosis [9]. But for advanced stages, the prognosis is relatively poor with chemotherapy or radiation therapy. So, the early diagnosis is most important for improving the prognosis and increasing the life expectancy for patients with non-small cell lung cancer.
Serum biomarker as diagnostic tool with less invasive and rapid detection was widely used for malignant tumor diagnosis [10]. But for non-small cell lung cancer, there was no one type of serum biomarker with highly diagnostic sensitivity and specificity. So, the combined detection of multiple serum biomarkers was generally used for lung cancer diagnosis in a clinic.
Carcinoembryonic antigen (CEA) is usually generated by gastrointestinal tissue during fetal development, but stops before birth. So, it is low in the serum of healthy subjects compared to patients with malignant tumors such as lung cancer [11] and colorectal cancer [12]. Therefore, it could be used as a type of serum biomarker for lung cancer detection. It was reported that the diagnostic sensitivity and specificity was 40.9% and 99.2% for serum CEA in detection of lung cancer [13]. The diagnostic sensitivity was relatively low but the specificity was very high. In our present study, the diagnostic sensitivity and specificity was 80.0% and 72.2% respectively with the AUC of 0.84. Neuron specific enolase (NSE) is also known as phosphoenolpyruvic invertase, and it is isoenzyme of neuron-specific enolase. It is reported that the NSE was significantly elevated in the serum in patients with small cell lung cancer [14]. But is also increased in the serum of patients with non-small cell lung cancer [15]. In our study, we found that the serum level of NSE was 51.6±68.3 ng/mL in non-small cell lung cancer patiens and was significantly higher than that of benign lung disease patients with the diagnostic sensitivity of 71.0% and 83.3%. Matrix metallo-proteinase (MMP-9), a key member of multifunctional family of zinc dependent end peptidases has been found to be unregulated during inflammation and in some cancers. Barany et al. [16] reported that the serum level of MMP-9 was significantly elevated in non-small cell lung cancer patients, which could be considered as a potential biomarker. We also found that the serum level of MMP-9 in non-small cell lung cancer patients was much higher than that of benign lung disease patients with statistical difference.
In conclusion, serum level of CEA, NSE and MMP-9 was significantly elevated in non-small cell lung cancer patients compared with benign lung disease subjects. The diagnostic efficacy for CEA, NSE and MMP-9 as a serum biomarker was relatively high. But with the small number of cases included in this study and retrospective study design, the conclusion should be confirmed by other well designed prospective multiple center diagnostic trials.
Footnotes
Conflict of interest statement: Authors state no conflict of interest
References
- [1].Siegel R, Naishadham D, Jemal A.. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- [2].Siegel RL, Miller KD, Jemal A.. Cancer statistics. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- [3].Chen W, Zheng R, Zeng H, Zhang S.. Epidemiology of lung cancer in China. Thoracic cancer. 2015;6(2):209–215. doi: 10.1111/1759-7714.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yang RH, Tian RF, Ren QL. et al. Serum protein profiles of patients with lung cancer of different histological types. Asia Pac J Clin Oncol. 2015 doi: 10.1111/ajco.12441. [DOI] [PubMed] [Google Scholar]
- [5].Boyer M, Tsao MS, Janne P, Ramalingam S, Pitman LS, Alam M.. Preparing for tomorrow: molecular diagnostics and the changing nonsmall cell lung cancer landscape. Asia Pac J Clin Oncol. 2014;10(Suppl 2):2–10. doi: 10.1111/ajco.12189. [DOI] [PubMed] [Google Scholar]
- [6].Siegel R, Naishadham D, Jemal A.. Cancer statistics. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- [7].Siegel R, Desantis C, Jemal A.. Colorectal cancer statistics. CA Cancer J Clin. 2014;64(2):104–117. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- [8].Sawabata N.. Prognosis of lung cancer patients in Japan according to data from the Japanese Joint Committee of Lung Cancer Registry. Respir Investig. 2014;52(6):317–321. doi: 10.1016/j.resinv.2014.04.002. [DOI] [PubMed] [Google Scholar]
- [9].Gridelli C, Balducci L, Ciardiello F. et al. Treatment of Elderly Patients With Non-Small-Cell Lung Cancer: Results of an International Expert Panel Meeting of the Italian Association of Thoracic Oncology. Clin Lung Cancer. 2015;16(5):325–333. doi: 10.1016/j.cllc.2015.02.006. [DOI] [PubMed] [Google Scholar]
- [10].Ma PC, Blaszkowsky L, Bharti A. et al. Circulating tumor cells and serum tumor biomarkers in small cell lung cancer. Anticancer Res. 2003;23(1A):49–62. [PubMed] [Google Scholar]
- [11].Zhao W, Yu H, Han Z, Gao N, Xue J, Wang Y.. Clinical significance of joint detection of serum CEA, SCCA, and bFGF in the diagnosis of lung cancer. Int J Clin Exp Pathol. 2015;8(8):9506–9511. [PMC free article] [PubMed] [Google Scholar]
- [12].Stiksma J, Grootendorst DC, van der Linden PW.. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer. 2014;13(4):239–244. doi: 10.1016/j.clcc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- [13].Wang B, He YJ, Tian YX, Yang RN, Zhu YR, Qiu H.. Clinical utility of haptoglobin in combination with CEA, NSE and CYFRA21-1 for diagnosis of lung cancer. Asian Pac J Cancer Prev. 2014;15(22):9611–9614. doi: 10.7314/apjcp.2014.15.22.9611. [DOI] [PubMed] [Google Scholar]
- [14].Wang P, Piao Y, Zhang X, Li W, Hao X.. The concentration of CYFRA 21-1, NSE and CEA in cerebro-spinal fluid can be useful indicators for diagnosis of meningeal carcinomatosis of lung cancer. Cancer Biomark. 2013;13(2):123–130. doi: 10.3233/CBM-130338. [DOI] [PubMed] [Google Scholar]
- [15].Wang JL, Wu DW, Cheng ZZ, Han WZ, Xu SW, Sun NN.. Expression of high mobility group box–B1 (HMGB-1) and matrix metalloproteinase-9 (MMP-9) in non-small cell lung cancer (NSCLC) Asian Pac J Cancer Prev. 2014;15(12):4865–4869. doi: 10.7314/apjcp.2014.15.12.4865. [DOI] [PubMed] [Google Scholar]
- [16].El-Badrawy MK, Yousef AM, Shaalan D, Elsamanoudy AZ.. Matrix metalloproteinase-9 expression in lung cancer patients and its relation to serum mmp-9 activity, pathologic type, and prognosis. J Bronchology Interv Pulmonol. 2014;21(4):327–334. doi: 10.1097/LBR.0000000000000094. [DOI] [PubMed] [Google Scholar]


