Abstract
At present time, both CT and MRI are valuable techniques in the study of the thoracic aorta. Nowadays, CT represents the most widely employed technique for the study of the thoracic aorta. The new generation CTs show sensitivities up to 100% and specificities of 98-99%. Sixteen and wider row detectors provide isotropic pixels, mandatory for the ineludible longitudinal reconstruction. The main limits are related to the X-ray dose expoure and the use of iodinated contrast media. MRI has great potential in the study of the thoracic aorta. Nevertheless, if compared to CT, acquisition times remain longer and movement artifact susceptibility higher. The main MRI disadvantages are claustrophobia, presence of ferromagnetic implants, pacemakers, longer acquisition times with respect to CT, inability to use contrast media in cases of renal insufficiency, lower spatial resolution and less availability than CT. CT is preferred in the acute aortic disease. Nevertheless, since it requires iodinated contrast media and X-ray exposure, it may be adequately replaced by MRI in the follow up of aortic diseases. The main limitation of MRI, however, is related to the scarce visibility of stents and calcifications.
Keywords: Thoracic Aorta, CT; Thoracic Aorta, MRI; Aortic diseases
1. Introduction
At the present time, both CT and MR imaging are valuable techniques in the study of the thoracic aorta. Nevertheless, differences in spatial resolution, movement artifact susceptibility, acquisition time, contraindications, and risks make a critical re-evaluation of the state of the technology useful to optimize their employment in relation to different clinical situations and apparatus performances.
2. Computed Tomography (CT)
2.1. Computed Tomography (CT)
Computed Tomography (CT) is currently the most widely employed technique for the study of the thoracic aorta. Fast scanning achieved with the new wide area detector, low artifact sensibility due to fast velocity tube rotation and 24-hour availability in the emergency rooms are the main advantages of CT usage in the medical practice [1-2].
The new generation CTs show sensitivities up to 100% and specificities of 98-99%, allowing the possibility to evaluate the entire aorta including lumen and wall, the possible thrombotic apposition and the peri-aortic area. Identification of anatomic variants is also possible (Figure 1), as well as distinction among acute syndromes. The acquisitions require quite short times and nowadays they are almost universally available [3,4].
Figure 1.
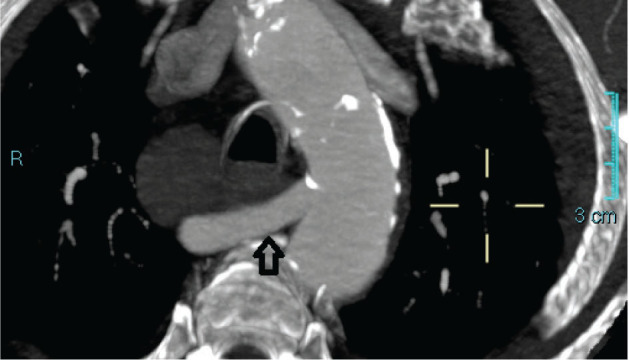
CT showing the anomaly of the right subclavian artery (arrow) with an evident retroesophageal course (also called lusory artery) acquisitions require quite short times and nowadays they are almost universally available [3,4].
Sixteen and wider row detectors provide isotropic pixels, mandatory for the ineludible longitudinal reconstruction. Nevertheless, in the thoracic area the ECG-gated technique is strongly suggested to avoid the evidence of false positive flap due to high pulsatility of the root and ascending aorta. Thanks to the ECG-gated technique, it is possible to perform the kinetic evaluation of the aortic leaflets and is useful to assess the valve function. Unfortunately, the ECG-gated technique increases the acquisition time as well as the breath-hold required. This is why today the employment of a 64 or wider ECG-gated row detector system is suggested.
The main limits of CT are related to the X-ray dose exposure and the use of iodinated contrast media. The first aspect is amplified by the gated technique. When using the ECG-gated retrospective technique the acquisition is performed during the entire cardiac cycle. In post-processing, the best diastolic phase is chosen to reconstruct the thorax and thoracic aorta [5-6]. The remaining irradiated cardiac cycle is deleted, making a large part of the irradiation time useless. In only a few cases is the entire cardiac cycle reconstructed to show evidence of systo-diastolic movements of the aortic leaflets. ECG-gated technique performed at the thoracic aortic level is responsible for a more than 1000 mGy X-ray exposure compared to a less than 500 mGy exposure in the non- gated acquisition. Of course, these data are variable and depend on patients’ body mass, detector-efficiency, techniques of automatic tube current modulation and iterative reconstruction (Figure 2). Nevertheless, the retrospective ECG-gated technique significantly increases the dose exposure. The prospective ECG-gated technique is an interesting alternative [7]. Using this modality, the X-ray exposure is limited to the end-diastolic cardiac phase with a strongly effective dose exposure reduction. This acquisition is obtained using axial acquisition and step a shot moving table technique until the entire coverage of the studied organ is obtained. The main drawback is related to the misalignment of the subsequent steps responsible for the more or less evident lateral step. This aspect is minimized by using 16 cm wide area detectors to obtain the entire thoracic aorta with only one step. This is enough to acquire the thoracic region and for that eventually responsible of misalignment in a single level. This modality has the advantage of combining the ECG-gated technique and low level X-ray exposure (Figure 3). Using a 320 row detector to acquire 16 cm in a single tube rotation, we obtained a mean value of 550±45mGy exposure in a cohort of 50 patients (mean BMI 26,1) [8-10]
Figure 2.
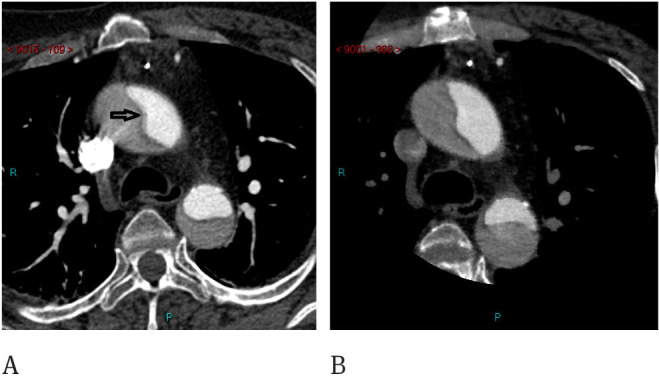
CT findings of type A Aortic Dissection. A) In the ungated acquisition, pulsatility artifacts are evident (arrow). B Same patient studied with cardiac gating, no pulsatility artifacts are evident.
Figure 3.
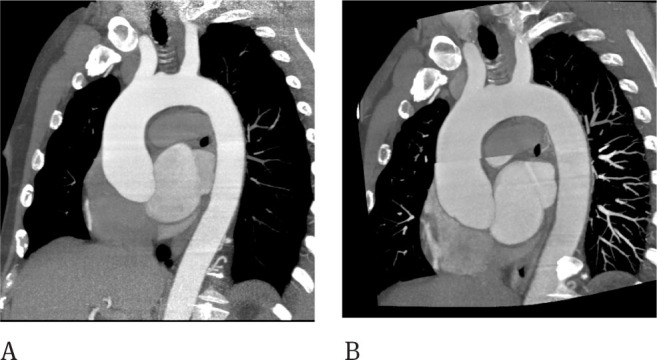
CT of the thoracic aorta showing moderate dilatation of the ascending aorta. The same patient was studied with the retrospective gated technique with a 320 row-detector, the effective dose was 919 mGy (A). Step and shoot technique used at follow up was able to spare dose (DLP 425 mGy) but misalignment artifacts are evident at the ascending aorta (B).
2.2. Contrast Media Administration
Injection of iodinated contrast-media remains another potential risk related to CT acquisition. In reality, the dimensional evaluation of the aorta can be obtained without contrast-medium injection on the axial slice. This may be useful in the monitoring of the aortic dilatation but is poor on the longitudinal reformatted images and inadequate in the endoluminal evaluation. In most of cases, the technique allows the acquisition of images without contrast media. This is particularly useful in cases of suspected intramural hematoma, with contrast medium injection useful for the complete evaluation of lumen and wall. Of course, the use of contrast media remains ineludible in the study of the intimal flap.
Double syringe injector, fast imaging and high flow rate are adequate to reduce contrast medium quantity. Double injection of contrast media followed by saline injection is the first step in reducing contrast medium. The pushing action of the saline injection avoids temporary persistence of the contrast-medium into the arm veins, and allows sparing of about 12 ml of iodine solution. Second, in addition toreducing the acquisition time, fast imaging also reduces the injection time needed to maintain intra-aortic high iodine concentration, resulting in a decrease of contrast medium volume down to 70 ml. Third, high rate injection is useful to reach a high density level using lower concentrated solution, leading to contrast medium volume reduction. The obtained total iodine dose reduction increases patients’ renal tolerability [11-12].
3. Magentic Resonance Imaging (MRI)
3.1. Magnetic resonance imaging (MRI)
Magnetic resonance imaging has great potential in the study of the thoracic aorta. However, compared to CT, acquisition times remain longer and movement artifact susceptibility higher [13-17].
Different techniques are presently used in the thoracic aorta studies. Both ECG gated and ungated sequences are employed. The employment of contrast media still represents a controversial issue.
The most common acquisition modality is the breath hold 3D Fast SPGR ungated sequences obtained after paramagnetic contrast medium injection. This is a quite fast acquisition obtained using a spoiled gradient echo sequence with multiple k-space line simultaneous acquisition. Shorter Repetition time and Echo time are employed to nullify stationary tissues and enhance the intravascular contrast medium injection. These acquisitions are generally able to reduce the acquisition time to about 10-14 seconds with a sub millimeter voxel resolution. This modality requires 0.1 mmol/kg gadolinium injection followed by 20 ml of saline solution both at 1.0 ml/sec. The correct timing is a crucial point for the quality of the aortic images. The fluoro technique is generally preferred to the automatic bolus test. It is obtained by performing a preliminary very fast 2D acquisition at the level of the aorta to image the progress of the contrast medium into the vascular system and detect its arrival at the level of the aortic root. This is considered the optimal timing to start the 3D acquisition. A mostly 1.0 ml/sec flow velocity is considered adequate. Nevertheless, the correct calculation should be a flow velocity equal to maintain maximal arterial concentration during 2/3 of the scan time acquisition. Calculation includes the total gadolinium dose and acquisition time. The standard dose is 0.1 mmol/kg, but when contrast injection is required to resolve fine details, as in case of severe stenosis, a dose of 0.15-02mmol/kg can increase the detectability of pathological details. Similar considerations can be made for large aortic aneurysms, aortic dissection and when compensating for errors in bolus timing (Figure 4).
Figure 4.
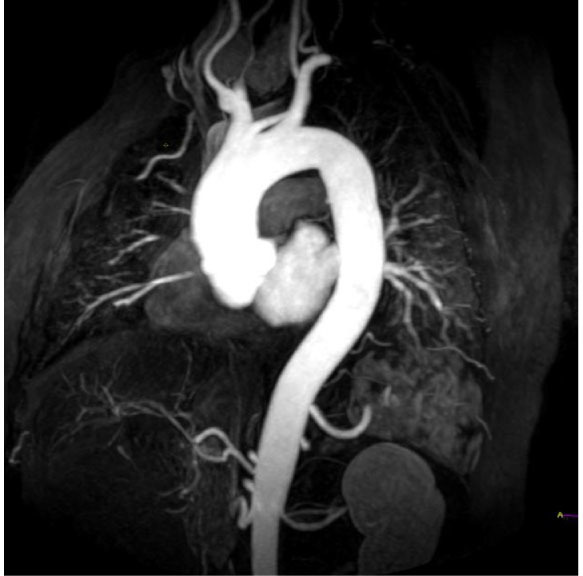
Aortic root ectasia is evident on 3D FSPGR MRI sequences obtained after gadolinium injection.
Time resolved techniques may also be applied in the evaluation of the thoracic aorta. This modality also requires contrast medium injection and is acquired without cardiac gating.
The best advantages are related to the contrast medium dose reduction and short time acquisition avoiding the need for accurate timing after injection. These sequences are able to acquire the aortic volume in 1-4 seconds and multiple acquisitions are performed upon the contrast media arrival with the possibility to choose the best timing after acquisition. Only 4-5 ml of paramagnetic contrast media are required. This sequence is also called Projection Radial Time Resolved Imaging of contrast Kinetic (PR-TRIKS) (18). The acquisition oversamples the central region of K space combining radial sampling and conventional Cartesian encoding. This modality combines the possibility to acquire high temporal and spatial resolution (Figure 5).
Figure 5.
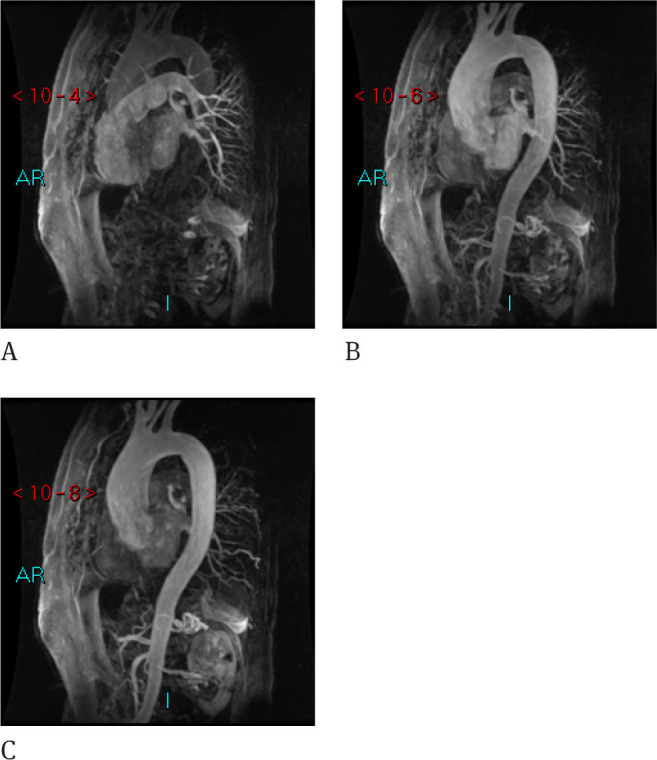
PR-TRIKS sequences are able to evaluate the progression of the contrast media at the pulmonary arteries (A), at the proximal aorta (B) and at the distal aorta (C).
All these contrast graphic acquisitions need post processing, usually using MIP reconstruction. Only in a few cases (aortic dissection) is the volume rendering reconstruction preferred.
3.2. Non contrast graphic ECG gated acquisitions
2D ECG gated steady state acquisitions are also useful in the evaluation of the aortic thoracic disease. Topographic MRI evaluation is feasible using both axial and oblique sagittal view according to the direction of the aorta (Figure 6). With this technique, the use of thin contiguous slices provides a complete evaluation of the aortic diameters and wall, giving useful information on the presence of aortic dilatation and presence of endoluminal thrombotic material and flap dissection. Dimensional comparison provides high agreement in the dimensional evaluation between 2D ECG gated steady state acquisition, ungated Contrast Enhanced 3D SPGR sequences and ungated Contrast Enhanced PR-TRICS sequences at the level of the middle ascending aorta, at the arc and the descending tract. Higher intra- and interobserver variability was found in the dimensional evaluation of the aortic root on gated acquisitions due to the absence of pulsatility artifacts with respect to ungated acquisitions in a series of 40 patients [19]. 2D sequences present limits in the evaluation of aortic branches and in very tortuous aortic vessels. An interesting solution is the use of ECG gated 3D fat saturated Steady State acquisition, first employed in the evaluation of the coronary arteries also called v-cats. Gated 3D steady State sequences are also useful in the evaluation of the aortic valve. They present an advantage over CT and can be applied routinely in patients with aortic root enlargement, adding information on the valvular dysfunction. By the use of acquisition through the outflow ventricular tract and parallel to the aortic annulus, it is possible to detect the presence of turbulent flow and leaflet defects. Moreover, using Gradient Encoding sequences it is possible to evaluate the velocity through the valve and calculate the pressure gradient or regurgitant volume [20].
Figure 6.
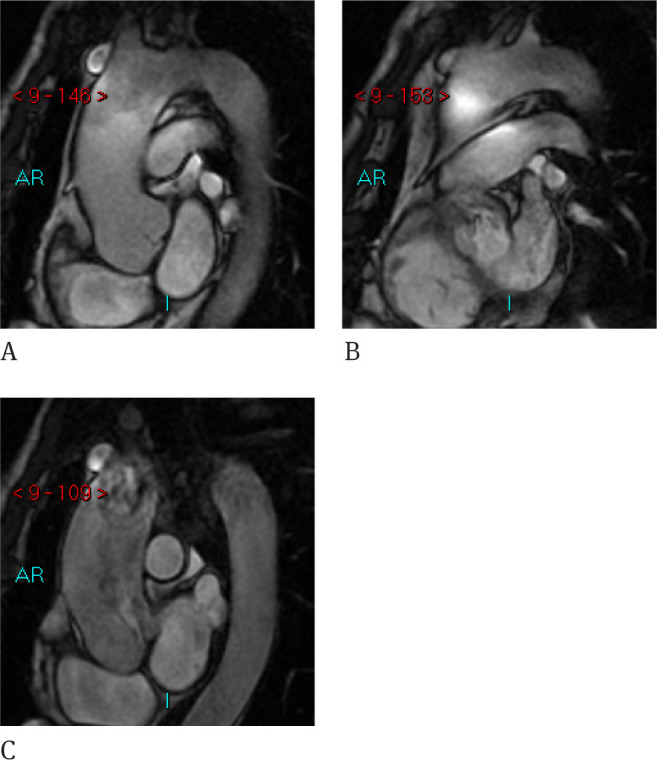
D Gated Steady-state CINE RM provide a natural contrast between the lumen and wall and no pulsatility artifacts are evident. Dimensional evaluation is also possible but by means the analysis in different slices for the ascending aorta, the arch and descending aorta.
3.3. MRI disavantages
The main MRI disadvantages are claustrophobia, presence of ferromagnetic implants, pacemakers, longer acquisition times with respect to CT, inability to use contrast media in cases of renal insufficiency, lower spatial resolution and less accesibility than CT. Nevertheless, these limitations can now be overcome: claustrophobic patients can be evaluated using open high field magnets; most of the metallic implants are not ferromagnetic; MRI safe pacemakers [21] are being currently produced; the acquisition time can be reduced (i.e. PR-TRICS sequences); the use of steady state sequences help overcome the limitation on patients with renal failure; spatial resolution is increasing with fast imaging and, lastly, MRI unit availability is also increasing.
4. Aortic measurements
A crucial point in the evaluation of both CT and MRI aortic acquisition is the modality in which the measurements are taken. Many radiologists are accustomed to taking measurements on the axial planes. Diameter measurements taken from the axial planes are inherently incorrect unless the aorta being measured is perfectly aligned in the cross-section on the image (22). For this reason, we suggest to taking measurements on the longitudinal reformatted imaging. Standard measurements should be obtained at the aortic valve, at the maximal diameter of the aortic root, at the sino-tubular junction, at the middle ascending tract, at the arc, between the anonymous trunk and common left carotid artery and in the descending tract posterior to the left atrium. The signed levels are useful to repeat measurements at the same level in subsequent controls. Nevertheless, additional measurements are mandatory if enlargement is evident in any other level. Dimensional evaluation should be obtained on two longitudinal planes and the final diameters should be obtained by algebraic mean. Moreover, in the analysis of the root, the optimal size measurement should be obtained on images parallel to the root on the so called short axis view. Both CT and MRI images obtained on the short axis of the root are particularly useful in the evaluation of the root diameter when the latter is not circular but oval as normally occurs in the bicuspid aortic root. Of course, both CT and MRI are able to detect the presence of an aortic bicuspid valve.
5. Normal diameters
Normal diameters are generally considered ≤ 3.9 cm at the root level, ≤ 3.8 cm at the ascending tract, ≤3.0 at aortic arch and ≤ 2.5 cm at the descending area [23]. Of course, these parameters do not consider sex differences and body mass index (BMI). Indexed values are strongly suggested and are considered as normal BMI indexed values ≤ 1.4 at the level of the annulus, ≤ 2 at the Valsalva sinus, ≤1.7 in the sinotubular junction and ≤ 1.9 in the ascending aorta (Table 1).
Table 1.
In the table are reported the normal values of the thoracic aorta
| Site | Normal values (mm) |
|---|---|
| Aortic Root | ≤ 39 |
| Sinotubular Junction | ≤ 30 |
| Ascending Aorta | ≤ 37 |
| Aortic arch | ≤ 30 |
| Descending Aorta | ≤ 25 |
6. Aneurysmatic disease
Aneurysms are often an incidental imaging report with a prevalent involvement of the ascending aorta. The most common cause is related to a degenerative process of the media. Hypertension is also a common finding in these patients. Nevertheless, aortic dilatation is also associated with inherited conditions, Such as Marfan’s, Ehlers-Danlos and Turner’s syndromes [24].
The correct measurement of dilation is of extreme importance if you consider that an aneurysm greater than 6 cm has a rupture risk higher than 30% in 5 years and between 4 and 5.9 cm higher than 15% [25].
A correct measurement is of utmost importance for surgical decisions. Most of surgeons generally consider 5.5 cm in diameter the surgical threshold for an ascending aortic aneurysm. The decision for surgery is made for aneurysms greater than 4.5 cm in diameter in Marfan’s, Erlers-Danlos and Turner Syndromes, as well as in Bicuspid aortic valve disease. Similarly, 4.5 cm diameter aneurysms are generally considered appropriate for surgical approach in patients undergoing surgical aortic valvular repair. Due to the higher risk incidence in the descending thoracic aorta, surgical intervention is considered for aneurysms greater than 5.5 cm in diameter. Annual follow-up is generally required in these patients to evaluate whether they reach the previously mentioned limits or to evaluate an annual increase exceeding 0.5 cm, the latter being considered as an appropriate parameter for surgical decision, whether or not the standard diameters are reached [26].
7. Morphology
In addition to diameter, morphology is also essential in making the decision to proceed with surgery. Saccular aneurysms have a theoretically higher risk than fusiform ones. This may be explained by the pressure exercised by the turbulent flow in the saccular aneurysm, which is different from the pressure distribution in the fusiform one. Differentiations are present in the case of thrombosis. This represents a protection with respect to the risk of rupture, but increases the risks related to the thrombo-embolic disease.
8. Wall elasticity
In borderline cases, it may be wise to perform an evaluation of the aortic wall elasticity that is considered an ancillary risk factor. Loss of elasticity is considered a low prognostic factor in the risk of rupture and may add parameters, especially in borderline diameters. The analysis is generally obtained using ultrasound imaging that provides data more cheaply and in real time. Nevertheless, Cine MRI may provide similar results with high grade reproducibility [27].
9. Acute Aortic Syndrome
In patients with Acute Aortic Syndrome, CT is generally preferred. The choice is due to faster imaging, which makes this technique more reliable in cases of uncooperative or unstable patients, or in presence of electronic devices. Moreover, CT is generally active 24hours in an emergency room. Due to the need of assistance and the higher susceptibility to movements artifacts, MRI plays a limited role in the management of these patients [28-29].
10. Intramural hematoma
CT is particularly useful in the diagnosis of acute hematoma, providing imaging on the unenhanced graphic acquisition of a thickened hyperdense wall. After contrast medium injection, the false lumen is not present and the thickened wall is better delineated 29.
11. Aortic Dissection
CT is the method of choice in the acute phase [30,34].
11.1. Aortic Dissection: Type A
CT provides a clear delineation of the intimal flap, its location and exertion involving the ascending aorta. Presence of thrombotic material into the false lumen is clearly detected after contrast medium injection. Moreover, it is possible to observe the entry door and the distal extension of the flap including the involvement of the aortic branches. Dimensional analysis may be obtained and pericardial blood effusion is clearly detected (Figure 7).
Figure 7.
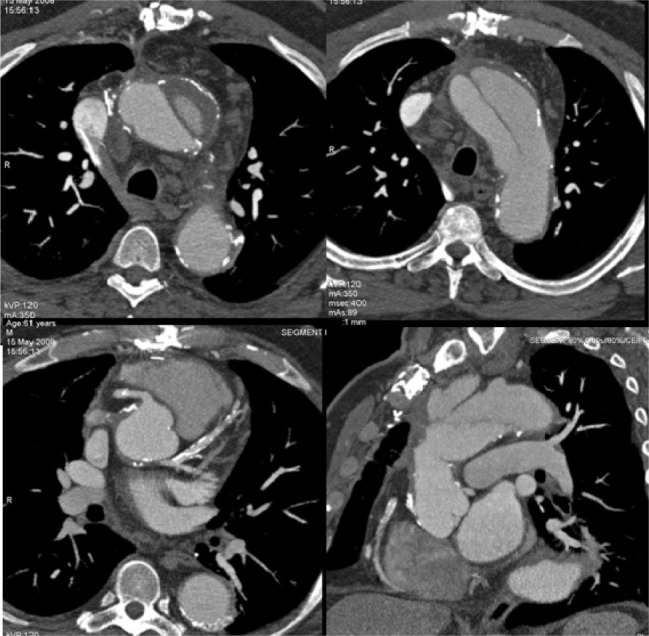
Type A Aortic dissection. CT is able to evaluate the flap, true and false lumen, the thrombotic material into the false lumen and the relationship of the flap with the thoracic aortic branches.
11.2. Aortic Dissection: Type B
CT is usually employed as first choice. This technique is able to demonstrate the presence of the dissection that does not involve the ascending area. Involvement of the abdominal aorta and aortic branches can be clearly detected. Dimensional analysis is generally performed and the ratio between the patent aortic false lumen and the true lumen is considered to evaluate the rupture risk of the aorta [35].
11.3. Follow-up
Both A and B types of aortic dissection need follow-up. In the case of type A, the follow-up is performed to evaluate the post-surgical condition. Early stage imaging may provide information on mediastinal surgical bleeding and patency of the residual dissection, if present. Subsequently, 1- and 6- month follow-up studies are required followed by annual controls, in order to evaluate size of the dissected aorta, persistence of false lumen patency or thrombotic evolution, evolution of the dissection and presence of aortic vessel involvement, anastomosis and prosthesis status [36].
Similar considerations can be made in the case of follow-up for the dissection of type B, but in most cases, they are limited by the natural evolution of the dissection due to non-surgical standard therapy. Nevertheless, the possibility to repair the type B dissection with endoprosthesis is today the most reliable treatment in a considerable group of patients. The evidence of the stent position is achievable only with CT. In reality, metallic stents are hardly visible on MRI. However, both techniques are adequate in the evaluation of the repair after the procedure and follow-up is required before discharge at 1 and 6 months, and yearly thereafter [37-42].
12. Report of Thoracic Aortic Imaging
The report should include:
-
–
Measurements of the aortic valve, root, sinotubular junction, middle ascending aorta, arc and mid descending aorta posterior to the left atrium
-
–
Site of pathological maximal diameter of the disease
-
–
Status of the wall including evidence of intramural hematoma or aortic dissection
-
–
Thrombotic material and evidence of atheroma or simple calcifications
-
–
Extension of the disease into the aortic branches
-
–
Peri-aortic, mediastinal hematoma, hematic pericar-dial fluid and contrast effusion (if present should be mentioned as sign of aortic rupture)
-
–
Image comparison with previous examination, if present
13. Conclusion
At present time, both CT and MRI are considered the best imaging modalities for the study of the thoracic aorta. CT is faster, less sensitive to movement artifacts and provides higher spatial resolution than MRI. For these reasons, CT is preferred in acute aortic disease. Nevertheless, since it requires iodinated contrast media and X-ray exposure, it may be adequately replaced by MRI in the follow up of aortic diseases. The main limitation of MRI, however, is related to the scarce visibility of stents and calcifications.
Conflict of interest statement
Authors state no conflict of interest.
References
- [1].Brenner DJ, Hall EJ.. Computed tomography—an increasing source of radiation exposure (Review) N Engl J Med. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- [2].Di Cesare E, Carbone I, Carriero A, Centonze M, De Cobelli F, De Rosa R, Di Renzi P, Esposito A, Faletti R, Fattori R, Francone M, Giovagnoni A, La Grutta L, Ligabue G, Lovato L, Marano R, Midiri M, Natale L, Romagnoli A, Russo V, Sardanelli F, Cademartiri F.. Clinical indications for cardiac computed tomography. From the working group of the Cardiac Radiology Section of the Italian Society of Medical Radiology (SIRM) Radiol Med. 2012;117:901–938. doi: 10.1007/s11547-012-0814-x. [DOI] [PubMed] [Google Scholar]
- [3].Di Cocco P, Orlando G, Di Cesare E, Mazzotta C, Rizza V, Pisani F, Famulari A, Gregorini R, Mazzola A, Lapecorella M.. Superiore vena cava Syndrome due to thrombotic occlusion in a thrombophilic renal transplant recipient: a case report http:// www.ncbi.nlm.nih.gov/pubmed/20534301 Transplant Proc. 2010;42:1358–1361. doi: 10.1016/j.transproceed.2010.03.076. [DOI] [PubMed] [Google Scholar]
- [4].Di Cesare E, Di Sibio A, Lanni G, Gennarelli A, Masciocchi C.. Magnetic resonance imaging of AMS (Aneurysm of the Membranous Septum), review of the literature and case report. J Radiol Case Rep. 2014;8(5):9–15. doi: 10.3941/jrcr.v8i5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Feuchtner GM, Jodocy D, Klauser A, Haberfellner B, Aglan I, Spoeck A. Radiation dose reduction by using 100-kV tube voltage in cardiac 64-slice computed tomography: a comparative study. Eur J Radiol. 2010;75:e51–6. doi: 10.1016/j.ejrad.2009.07.012. [DOI] [PubMed] [Google Scholar]
- [6].Ko SF, Hsieh MJ, Chen MC, Ng SH, Fang FM, Huang CC. Effects of heart rate on motion artifacts of the aorta on non-ECG-assisted 0.5-sec thoracic MDCT. AJR Am J Roentgenol. 2005;184:1225–1230. doi: 10.2214/ajr.184.4.01841225. [DOI] [PubMed] [Google Scholar]
- [7].Di Cesare E, Gennarelli A, Di Sibio A, Felli V, Splendiani A, Gravina GL, Barile A, Masciocchi C.. Assessment of dose exposure and image quality in coronary angiography performed by 640-slice CT: a comparison between adaptive iterative and filtered back-projection algorithm by propensity analysis. Radiol Med. 2014;119(8):642–649. doi: 10.1007/s11547-014-0382-3. [DOI] [PubMed] [Google Scholar]
- [8].Roos JE, Willmann JK, Weishaupt D, Lachat M, Marincek B, Hilfiker PR.. Thoracic aorta: motion artifact reduction with retrospective and prospective electrocardiography-assisted multi-detector row CT. Radiology. 2002;222:271–277. doi: 10.1148/radiol.2221010481. [DOI] [PubMed] [Google Scholar]
- [9].Di Cesare E, Gennarelli A, Di Sibio A, Felli V, Splendiani A, Gravina GL, Barile A, Masciocchi C.. Image quality and radiation dose of single heartbeat 640-slice coronary CT angiography: a comparison between patients with chronic Atrial Fibrillation and subjects in normal sinus rhythm by propensity analysis. 2014 doi: 10.1016/j.ejrad.2014.11.035. http://dx.doi.org/doi:10.1016/j.ejrad.2014.11.035 S0720-048X(14)00560-9. [DOI] [PubMed] [Google Scholar]
- [10].D’Orazio F, Splendiani A, Gallucci M. 320-Row Detector Dynamic 4D-CTA for the Assessment of Brain and Spinal Cord Vascular Shunting Malformations. A Technical Note. The neuroradiology journal. 2014;27(6):710–717. doi: 10.15274/NRJ-2014-10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ellis JH, Cohan RH.. Reducing the risk of contrast-induced nephropathy: a perspective on the controversies. AJR Am J Roentgenol. 2009;192:1544–1549. doi: 10.2214/AJR.09.2368. [DOI] [PubMed] [Google Scholar]
- [12].Rossi C, Reginelli A, D’Amora M, Di Grezia G, Mandato Y, D’Andrea A. Safety profile and protocol prevention of adverse reactions to uroangiographic contrast media in diagnostic imaging. J Biol Regul Homeost Agents. 2014 Jan-Mar;28(1):155–165. [PubMed] [Google Scholar]
- [13].Di Cesare E, Cademartiri F, Carbone I, Carriero A, Centonze M, De Cobelli F, De Rosa R, Di Renzi P, Esposito A, Faletti R, Fattori R, Francone M, Giovagnoni A, La Grutta L, Ligabue G, Lovato L, Marano R, Midiri M, Romagnoli A, Russo V, Sardanelli F. Natale. Clinical indications for the use of cardiac MRI. By the SIRM Study Group on Cardiac Imaging. Radiol Med. 2013;118:752–798. doi: 10.1007/s11547-012-0899-2. [DOI] [PubMed] [Google Scholar]
- [14].Francone M, Di Cesare E, Cademartiri F, Pontone G, Lovato L, Matta G, Secchi F, Maffei E, Pradella S, Carbone I, Marano R, Bacigalupo L, Chiodi E, Donato R, Sbarbati S, De Cobelli F, di Renzi P. and CMR Italian Registry Group.Italian registry of cardiac magnetic resonance. Eur J Radiol. 2014;83:e15–e22. doi: 10.1016/j.ejrad.2013.10.006. [DOI] [PubMed] [Google Scholar]
- [15].Di Cesare E, Battisti S, Di Sibio A. et al. Early assessment of sub-clinical cardiac involvement in systemic sclerosis (SSc) using delayed enhancement cardiac magnetic resonance (CE-MRI) Eur J Radiol. 2013;82(6):e268–e273. doi: 10.1016/j.ejrad.2013.02.014. [DOI] [PubMed] [Google Scholar]
- [16].Catalucci A, Anselmi M, Splendiani A, Smith JD, Limbucci N, Giangaspero F, Gallucci M.. Pediatric inflammatory diseases. Part I: multiple sclerosis. Neuroradiol J. 2012;25(6):684–694. doi: 10.1177/197140091202500608. [DOI] [PubMed] [Google Scholar]
- [17].Di Cesare E.. MRI of the cardiomyopathies. 2001;38(3):179–184. doi: 10.1016/s0720-048x(01)00311-4. [DOI] [PubMed] [Google Scholar]
- [18].Lohan DG, Krishnam M, Tomasian A, Saleh R, Finn JP.. Time-resolved MR angiography of the thorax (Review) Magn Reson Imaging Clin N Am. 2008;16(viii):235–248. doi: 10.1016/j.mric.2008.02.015. [DOI] [PubMed] [Google Scholar]
- [19].Krishnam MS, Tomasian A, Malik S, Desphande V, Laub G, Ruehm SG.. Image quality and diagnostic accuracy of unenhanced SSFP MR angiography compared with conventional contrast-enhanced MR angiography for the assessment of thoracic aortic diseases. Eur Radiol. 2010;20:1311–1320. doi: 10.1007/s00330-009-1672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Di Cesare E, Maurizi Enrici R, Paparoni S, Castaldo F, Alagia MG, Splendiani A, Bottone A.. Lupattelli L.Low-field magnetic resonance imaging in the evaluation of mechanical and biological heart valve function. European Journal of Radiology. 1995;20:224–228. doi: 10.1016/0720-048x(95)00656-b. [DOI] [PubMed] [Google Scholar]
- [21].Wollmann CG, Thudt K, Kaiser B, Salomonowitz E, Mayr H, Globits S.. Safe performance of magnetic resonance of the heart in patients with magnetic resonance conditional pacemaker systems: the safety issue of the ESTIMATE study. See comment in PubMed Commons below. J Cardiovasc Magn Reson. 2014;16:30. doi: 10.1186/1532-429X-16-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hiratzka LF, Bakris GL, Beckman JA, Bersin RM, Carr VF, Casey DE Jr. American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines; American Association for Thoracic Surgery; American College of Radiology; American Stroke Association; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions Society of Interventional Radiology; Society of Thoracic Surgeons; Society for Vascular Medicine. 2010 ACCF/AHA/ AATS/ACR/ASA/SCA/SCAI/SIR/ STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines American Association for Thoracic Surgery American College of Radiology American Stroke Association Society of Cardiovascular Anesthesiologists Society for Cardiovascular Angiography and Interventions Society of Interventional Radiology Society of Thoracic Surgeons and Society for Vascular Medicine. Circulation. 2010;121:e266–369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- [23].Wolak A, Gransar H, Thomson LE, Friedman JD, Hachamovitch R, Gutstein A. Aortic size assessment by noncontrast cardiac computed tomography: normal limits by age gender, and body surface area. JACC Cardiovasc Imaging. 2008;1:200–209. doi: 10.1016/j.jcmg.2007.11.005. [DOI] [PubMed] [Google Scholar]
- [24].Roman MJ, Rosen SE, Kramer-Fox R, Devereux RB.. Prognostic significance of the pattern of aortic root dilation in the Marfan syndrome. J Am Coll Cardiol. 1993;22:1470–1476. doi: 10.1016/0735-1097(93)90559-j. [DOI] [PubMed] [Google Scholar]
- [25].Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–1880. doi: 10.1016/s0003-4975(02)04147-4. [DOI] [PubMed] [Google Scholar]
- [26].Masuda Y, Takanashi K, Takasu J, Morooka N, Inagaki Y.. Expansion rate of thoracic aortic aneurysms and influencing factors. Chest. 1992;102:461–466. doi: 10.1378/chest.102.2.461. [DOI] [PubMed] [Google Scholar]
- [27].Di Cesare E.. Lo studio radiologico nell’invecchiamento dell’aorta e delle arterie coronarie. Radiol Med. 2003;106:57–59. (suppl 1) [PubMed] [Google Scholar]
- [28].Di Cesare E, Masciocchi C.. Radiologia d’urgenza Urgenze Toraciche: Cuore e pericardio. Radiol Med. 2002;103:35–44. (suppl 1) [Google Scholar]
- [29].Paonessa A, Limbucci N, Tozzi E, Splendiani A, Gallucci M.. Radiological strategy in acute stroke in children. Eur J Radiol. 2010;74(1):77–85. doi: 10.1016/j.ejrad.2009.01.016. [DOI] [PubMed] [Google Scholar]
- [30].Evangelista A, Mukherjee D, Mehta RH, O’Gara PT, Fattori R, Cooper JV. International Registry of Aortic Dissection (IRAD) Investigators Acute intramural hematoma of the aorta: a mystery in evolution. Circulation. 2005;111:1063–1070. doi: 10.1161/01.CIR.0000156444.26393.80. [DOI] [PubMed] [Google Scholar]
- [31].Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- [32].Tsai TT, Evangelista A, Nienaber CA, Trimarchi S, Sechtem U, Fattori R. International Registry of Acute Aortic Dissection (IRAD). Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD) Circulation. 2006;114(I):350–356. doi: 10.1161/CIRCULATIONAHA.105.000497. 1 Suppl. [DOI] [PubMed] [Google Scholar]
- [33].Splendiani A, Catalucci A, Limbucci N, Turner M, Krings T, Gallucci M.. Pediatric Inflammatory Diseases. Part III: Small Vessels Vasculitis. Neuroradiol J. 2012;25(6):715–724. doi: 10.1177/197140091202500611. [DOI] [PubMed] [Google Scholar]
- [34].Di Cesare E. MRI assessment of the right ventricular dysplasia. 2003;13:1387–1393. doi: 10.1007/s00330-002-1771-x. [DOI] [PubMed] [Google Scholar]
- [35].Kazerooni EA, Bree RL, Williams DM.. Penetrating atherosclerotic ulcers of the descending thoracic aorta: evaluation with CTA and distinction from aortic dissection. Radiology. 1992;183:759–765. doi: 10.1148/radiology.183.3.1584933. [DOI] [PubMed] [Google Scholar]
- [36].Sueyoshi E, Sakamoto I, Hayashi K, Yamaguchi T, Imada T.. Growth rate of aortic diameter in patients with type B aortic dissection during the chronic phase. Circulation. 2004;110II:256–261. doi: 10.1161/01.CIR.0000138386.48852.b6. (11Suppl 1) [DOI] [PubMed] [Google Scholar]
- [37].Di Cesare E, Giordano AV, Cerone G, De Remigis F, D’Eusanio G, Masciocchi C.. Comparative Evaluation of TEE conventional MRI and Contrast Enhanced 3D breat-holt MRA in the post-operative follow-up of dissecting aneurysms. Int J Cardiac Imaging. 2000;16:135–147. doi: 10.1023/a:1006404824873. [DOI] [PubMed] [Google Scholar]
- [38].Patrignani A, Di Cesare E, Cicogna S.. Echocardiography and cardiovascular magnetic resonance diagnostic role in Takotsubo cardiomyopathy. Intern J Cardiovasc Imaging. 2009;25:109–112. doi: 10.1007/s10554-008-9386-1. [DOI] [PubMed] [Google Scholar]
- [39].Sueyoshi E, Onitsuka H, Nagayama H, Sakamoto I, Uetani M.. Endovascular repair of aortic dissection and intramural hematoma: indications and serial changes. Springerplus. 2014;13(3):670. doi: 10.1186/2193-1801-3-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Dialetto G, Reginelli A, Cerrato M, Rossi G, Covino FE, Manduca S. Endovascular stent-graft treatment of thoracic aortic syndromes: a 7-year experience. Eur J Radiol. 2007 Oct;64(1):65–72. doi: 10.1016/j.ejrad.2007.06.019. Epub 2007 Aug 13. [DOI] [PubMed] [Google Scholar]
- [41].Reginelli A, Rossi C, Capasso R, Urraro F, Cagini L, Di Crescenzo V. Evaluation with multislice CT of the hilar pulmonary nodules for probable infiltration of vascular-bronchial structures. Recenti Prog Med. 2013 Jul-Aug;104(7-8):403–405. doi: 10.1701/1315.14584. [DOI] [PubMed] [Google Scholar]
- [42].Romano M., Mainenti P.P., Imbriaco M., Tamburrini O, Salvatore M.. Multidetector row CT angiography of the abdominal aorta and lower extremities in patients with peripheral arterial occlusive disease: Diagnostic accuracy and interobserver agreement. European Journal of Radiology. 2004;50(3):303–308. doi: 10.1016/S0720-048X(03)00118-9. [DOI] [PubMed] [Google Scholar]


