Abstract
Meningioma accounts for more than 30% of all intracranial tumours. It affects mainly the elderly above the age of 60, at a female:male ratio of 3:2. The prognosis is variable: it is usually favourable with no progression in tumour grade and no recurrence in WHO grade 1 tumours. However, a minority of tumours represent atypical (grade 2) or anaplastic (grade 3) meningiomas; this heterogeneity is also reflected in histopathological appearances. Irrespective of the grade, the size of the tumour and the localisation may have severe, sometimes lethal consequences. Following neurosurgical interventions to remove the tumour, recurrence and progression in WHO grade may occur. Our knowledge on predisposing histomorphological and molecular factors of recurrence is rather limited. These can be classified as I) demographic II) environmental, III) genetic and epigenetic IV) imaging, V) neuropathological, and VI) neurosurgical. In view of the complex background of tumour recurrence, the recognition of often subtle signs of increased risk of recurrence requires close collaboration of experts from several medical specialties. This multidisciplinary approach results in better therapy and fewer complications related to tumour recurrence.
Key words: Genetics, Immunohistochemistry, Meningioma, Molecular biology, Neuropathology, Pathology, Prognostic factors, Recurrence
1. Introduction
Meningioma is one of the most frequent intracranial tumours. The prevalence peaks between the age of 60 and 70 years, at a female:male ratio of 3:2. Nevertheless, the tumour may also occur in children. The most frequent localisations are parasagittal, lateral convexity, the wings of sphenoid bone, anterior fossa close to the olfactory nerve, the sellar region, and the posterior fossa in the vicinity of foramen magnum, respectively [1]. Most meningiomas are histologically benign. The WHO classification distinguishes three histological grades (1-2-3) and 15 subtypes [2]. Although meningiomas are usually histologically benign, space occupying lesions within the close skull can never be considered absolutely risk free: the increase of the intracranial pressure may lead to life-threatening complications. Because recurrence and progression are well known and not infrequent characteristics of meningiomas, a thorough analysis and better understanding of contributory factors are of crucial importance. In the current literature review, we assess the factors predictive of tumour recurrence with focus on clinical consequences, histological alterations, and risk factors related to the tumour treatment itself. Independent risk factors, such as sex and age, have also been analysed. Special emphasis has been devoted to predictive signs detectable by imaging techniques. The predictive values of immunohistochemical and other molecular markers in relation histological grade is increasingly recognised and therefore reviewed. Finally, the extent of surgical resection reflected by the Simpson grade appears to be very closely associated with the risk of recurrence. In summary, in this review we assess predictive factors derived from several neuro-specialties because their combined assessment can help to reach the best clinical decision on patient care (Figure 1).
Figure 1.
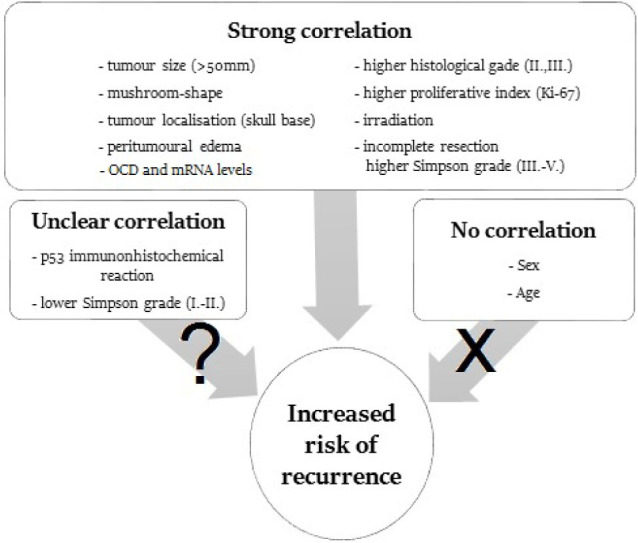
Possible factors in meningioma recurrence
2. Predisposing factors of meningioma recurrence
2.1. Demographic factors
The role of age and sex has been studied in relation to risk of recurrence. However, there has been no significant correlation shown between these two factors [3-6].
2.2. Environmental factors
An important environmental and/or iatrogenic factor is the effect of ionising radiation on the head and neck region. The increased incidence of meningiomas among survivals of the Hiroshima nuclear disaster at the end of the Second World War also supports this notion. However, most cases of radiation-induced meningiomas are iatrogenic and related to the diagnostic irradiation in dental practice. Therapeutic irradiation (radiotherapy) appears highly relevant, and there is growing body of evidence to support this [7-8]. Interestingly, traumatic head injury also increases the risk of meningioma 1 or 2 decades after the trauma in a dose-dependent manner and, apparently, unrelated to the severity of injury [9]. The female predominance of meningioma raises the possibility of hormonal effects in its pathogenesis, although large scale epidemiological studies have not provided unequivocal evidence to prove hormonal factors in pathogenesis, etiology and pathomechanism [10]. Nevertheless, it is well known that during pregnancy a meningioma may undergo rapid growth, which could have a lethal outcome [11]. In breast cancer sufferers there is also modest increase in incidence [12]. Long-term exogenous hormone replacement therapy has also been implicated in tumorigenesis [13]. Hu et al., in a comprehensive analysis, examined the role of various environmental factors in the Chinese population [14]. The results reveal that smoking significantly increases the incidence of meningioma in women. Similarly, lead, tin, cadmium as occupational hazards also increase meningioma incidence in exposed workers. In contrast, a diet rich in fruits and vegetables decreases the chance of having a meningioma [14]. Although alcohol consumption has been implicated as a risk factor for meningioma, there are no convincing evidence to support this hypothesis [15]. With the advent of novel telecommunication techniques, in particular mobile phones, the question has emerged whether there is an association between meningioma development and use of cell phones. The Interphone company conducted a study that concluded there was no such association [16]. Nevertheless, we have to take into account that there are not many research reports on this field and that the follow-up period is relatively short because mobile phones have become widely used only in the past 20 years or so.
2.3. Genetic and epigenetic aspects
Meningioma is also a component of numerous familiar tumour syndromes. It is most frequent in neurofibromatosis type 2 (NF2) and can also occur in other congenital and familial diseases: multiple endocrine neoplasia type 1 (MEN1), Gorlin-, Cowden-, Gardner-, Turcot-, von Hippel– Lindau-(VHL), and Li–Fraumeni syndrome [17]. The NF2 gene is located on the long arm of chromosome 22, coding tumour suppressor protein merlin. In sporadic cases (i.e., in meningiomas unrelated to tumour syndromes) a mutation is found in 60% of cases. The frequency and type of mutation in various meningioma grades is roughly identical [18]. A novel avenue to an understanding of meningioma pathogenesis is research into epigenetic mechanisms. One of the first discovered epigenetic changes in tumours is aberrant DNA methylation. Although NF2 is relatively frequently mutated in meningioma, epigenetic alteration of NF2 is rare. In contrast, TIMP3, TP73, and RASSF1A promoters and HOXA7, HOXA9, HOXA10 homebox genes are often hypermethylated in meningiomas. Another epigenetic change, histone modification, has no proven involvement in meningioma pathogenesis. The third major epigenetic factor, micro RNAs (miRNAs), are important in regulating post-translational silencing. miR-190a is increased, whereas miR-29c-3p and miR-219-5p are decreased in a subset of meningiomas; these tumours involve a higher risk of recurrence [19].
2.4. Imaging
In tumour diagnostics and follow-up, imaging is crucial. The characteristic dural tail detectable on CT and MR images, peritumoral oedema, and calcification are all important features [1]. Significant correlation has been found between tumour size (>50mm), shape (mushroom), localisation (base of skull), presence of brain invasion, and the severity of peritumoral oedema [3, 20-23]. The latter is important in recurrence because oedema increases the chance of brain invasion by the tumour, which is a key factor in recurrence [24]. The large size, basal localisation, and malignant histological features are associated with increased severity of peritumoral edema [25].
2.5. Neuropathological characteristics
2.5.1. Histological grade
The clinical behaviour and risk of recurrence have a very close association with the histological grade of the tumour. The histological grade and the extent of resection appear to be the two most important predictive factors of recurrence. According to the WHO classification, meningiomas are grouped in three grades (grade I, II, III). According to the typical morphological appearances and the mitotic index (assessed by the number of mitosis per 10 high power microscopic fields (HPF), there are 15 histological subtypes [2]. WHO grade I has 9, whereas both WHO II and WHO grade III have 3 subtypes (Table 1). Clear cell meningioma is grade II if intracranial and grade I when it occurs in the vertebral canal.
Table 1.
WHO classification of meningiomas (modified from Perry et al. [2])
| WHO grade I | meningothelial microcystic | fibroblastic secretory | transitional lymphoplasmacyte-rich | psammomatous metaplastic | angiomatous clear cell (spinal) |
| WHO grade II | chordoid | clear cell (intracranial) | atypical | ||
| WHO grade III | papillary | rhabdoid | anaplastic |
In WHO grade II, atypical meningioma, a minimum of three of the following histomorphological features are detectable: increased cellularity; small cells with an increased nucleus/cytoplasm ratio; prominent nucleoli, ‘patternless’ or sheet-like growth; focal small, geographic necrosis; 4 or more mitosis/10 HPF. In anaplastic (WHO grade III) meningioma there are obvious malignant cytological features, such as undifferentiated growth, which makes them similar to carcinoma, melanoma, or sarcoma (regarding cytological atypia). In anaplastic meningioma there are 20 or more mitoses/10 HPF.
Several studies have proven the close association between histological grade and risk of recurrence. In higher grade tumours (i.e., grades II, and III) the increased cellularity, higher mitotic rate, and presence of necrotic lesions predict the increased chance of recurrence [3, 21]. However, in anaplastic meningiomas these features are associated with less favourable outcome [26]. Consistent with this, recurrence-free survival and median time to recurrence were also significantly longer in atypical as compared with anaplastic meningiomas. Nevertheless, recurrence is not only a features of higher grade (II and III) meningiomas, because it also occurs in benign (grade I) meningiomas, although less frequently. A study focusing on grade I tumours demonstrated that the chance of recurrence is higher in benign meningiomas that have small atypical areas within the neoplasm [27]. Overall, the most important prognostic factor regarding tumour recurrence by far is the histological grade. In WHO grade I tumours the chance of recurrence is 7%–25%, in grade II 29%–59%, whereas in grade III, it is 60%–94% [27].
2.5.2. Immunohistochemical markers
In immunohistochemical studies the two most frequently examined proteins are Ki-67 and p53; their role in tumour recurrence has been extensively studied. p53 is a transcription factor with an important role in regulation of cell cycle, preservation of genomic integrity, induction of apoptosis, and inhibition of angiogenesis [28]. p53 is activated by a wide range of cell-damaging insults, such as oxidative stress, hypoglycaemia, hypoxia, DNA damage, oncogene expression, ribosomal dysfunction, and telomere damage [29]. p53 is one of the most important tumour suppressor proteins: more than 50% of tumours harbour p53 mutation [15, 16]. Despite the large number of studies, the role of p53 in meningioma development and recurrence remains unclear. Some authors describe significant correlation between p53 immunoreactivity and recurrence [30, 31], whereas other studies could not confirm that correlation [6].
The Ki-67 protein is expressed in mitotic cells. In the routine pathological diagnostics, the protein is detected by the Mib-1 antibody clone. Ki-67 is detectable in all phases of the active cell cycle; however, it is missing in G0. Furthermore, it plays a role in ribosomal-RNA synthesis [32]. The amount of the protein is closely correlated with the proliferative activity of the cell: in the interphase it is localized in the nucleoli, whereas in the mitotic phase it is on the surface of chromosomes. Therefore, the Ki67 protein is an excellent marker for detection of the proliferative pool of a given cell population [33]. Numerous studies have demonstrated a significant correlation between an increased Ki-67 (Mib-1) labelling index and recurrence [31, 34-35]. The Ki-67 labelling index also increases with WHO grade in meningiomas. Interestingly, in recurring WHO grade I tumours, a higher Ki-67 labelling index is present [31]. There is also a significant correlation between extent of peritumoral oedema and expression of the marker [36].
In addition to the markers mentioned previously, there are other important proteins that are recognised in meningiomas. The epithelial membrane antigen (EMA) and the progesterone receptor (PR) are diagnostic markers frequently found in these tumours [37, 38].
Immunohistochemical and other molecular studies have rendered electron microscopic analysis less relevant and less important in meningiomas than previously; however, in rare and unusual cases, electron microscopy may be a useful contributor to diagnosis [39]. Molecular and genetic studies establish novel fields of research of meningioma pathogenesis. Immunohistochemistry is considered to be a standard and well established tool to test and analyse novel proteins implicated in tumorigenesis. Therefore, it remains an important research method. The tissue microarray (TMA) technique enables cheap and rapid assessment of candidate proteins in large numbers of tumour samples [40]. A recent article raises the possibility that poly(ADP-ribose) polymerase-1 (PARP1), which is an enzyme involved in DNA repair, may have prognostic implications in meningioma [41].
2.6. Neurosurgical aspects
In meningiomas with clinical symptoms, surgical resection is the primary choice for treatment in the majority of the cases. The aim of the surgical procedure is the complete resection of tumour (if possible) to achieve the best quality of life for the patient and to reduce the risk of recurrence. The extent of resection is graded according to Simpson, using a 5-tier scale [24]. There is close correlation between Simpson grade and risk of recurrence [3]. In higher Simpson grades (III-V) disease-free survival is shorter, quality of life is reduced, and there is an increased chance of early recurrence of the tumour [5, 22, 41, 42] (Table 2). Rarely, ‘seeding metastasis’ may occur along the surgical tract in deep-seated meningiomas with risk of recurrence [43].
Table 2.
Simpson grading system for removal of meningiomas (modified from www.radiopaedia.org [40])
| Extent of macroscopic resection | Removal of dural tail | Risk of recurrence (10 year interval) | |
|---|---|---|---|
| Grade I | Macroscopically complete (tumour, involved bones and venous sinuses) | Macroscopically complete | 9% |
| Grade II | Macroscopically complete (tumour) | Coagulation | 19% |
| Grade III | Macroscopically complete (tumour) | No resection | 29% |
| Grade IV | Partial removal | No resection | 44% |
| Grade V | Simple decompression with or without biopsy | No resection | 100% |
In cranial base meningiomas, surgical removal often presents a higher risk for postoperative complications. In such situations, stereotactic radiosurgery can be successful for tumor control. The main indications for both treatment methods (i.e., surgery and radiosurgery) are the possibility of radiological or neurological progression. However, in a great proportion of cranial base meningiomas – especially in the elderly – follow-up proves no progression; in this case in symptomless patients, a neurosurgical procedure or radiosurgery is not evidently necessary. On the other hand, increasing tumor size can increase the risk of repeat surgical removal or irradiation. Thus, one main challenge for clinicians is to determine factors that correlate with tumor progression in grade I meningiomas. For this reason, some studies have already been published: one such investigates ornithine decarboxylase (ODC) activity and mRNA expression in meningiomas [44]. That report describes a significant correlation between ODC mRNA level in meningiomas with later recurrence and meningiomas without recurrence, despite the same Ki-67 proliferative index in both populations. These findings suggest a role of ODC gene expression in meningiomas recurrence.
3. Conclusion
Although meningiomas are tumours that occur with high frequency, the mechanisms underlying pathogenesis, recurrence, and progression remain poorly understood. With recent advances in diagnostics, treatments, and research methods, there is high hope for reduced recurrence and more efficient treatment. This requires a multidisciplinary approach and will have a major impact on more effective, personalised therapies, and overall, a better clinical outcome for meningioma patients.
Conflict of interest statement
Authors state no conflict of interest.
Acknowledgements
Financial support has been received from the National Brain Research Program, Hungary (KTIA 13 NAP-A-II/7 and KTIA 13 NAP-A-V/3) and AGR_PIAC_13-12013-0008.
References
- [1].Uduma U.F., Emejulu J.C.. Intracranial meningiomas in the present era of modern neuroimaging: diagnostic and management options, with radiological illustrations. Orient J. Med. 2013;25:67–74. [Google Scholar]
- [2].Perry A, Louis D.N., Scheithauer B.W., Budka H, von Deimling, Meningiomas A., Louis DN, Ohgaki H, Wiestler O.D., cavenee W.K. WHO classification of tumours of the central nervous system. IARC; Lyon: 2007. pp. 164–172. [Google Scholar]
- [3].Ildan F, Erman T, Göçer A.I., Tuna M, Bağdatoğlu H, Cetinalp E. et al. Predicting the probability of meningioma recurrence in the preoperative and early postoperative period: a multivariate analysis in the midterm follow-up. Skull Base. 2007;17:157–171. doi: 10.1055/s-2007-970554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Adegbite A.B., Khan M.I., Paine K.W., Tan L.K.. The recurrence of intracranial meningiomas after surgical treatment. J Neurosurg. 1983;58:51–56. doi: 10.3171/jns.1983.58.1.0051. [DOI] [PubMed] [Google Scholar]
- [5].Mahmood A, Qureshi N.H., Malik G.M.. Intracranial meningiomas: analysis of recurrence after surgical treatment. Acta Neurochir (Wien) 1994;126:53–58. doi: 10.1007/BF01476410. [DOI] [PubMed] [Google Scholar]
- [6].Trott G, Pereira-Lima J.F.S., Leães C.G.S., Ferreira N.P., Barbosa-Coutinho L.M., Oliveira M.C.. Abundant immunohistochemical expression of dopamine D2 receptor and p53 protein in meningiomas: follow-up, relation to gender, age, tumor grade, and recurrence. Brazilian J. Med. Biol. Res. 2015;48:415–419. doi: 10.1590/1414-431X20144163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ron E, Modan B, Boice J.D., Alfandari E, Stovall M, Chetrit A. et al. Tumors of the brain and nervous system after radiotherapy in childhood. N. Engl J. Med. 1988;319:1033–1039. doi: 10.1056/NEJM198810203191601. [DOI] [PubMed] [Google Scholar]
- [8].Umansky F, Shoshan Y, Rosenthal G, Fraifeld S, Spektor S.. Radiation-induced meningioma. Neurosurg. Focus. 2008;24:e7. doi: 10.3171/FOC/2008/24/5/E7. [DOI] [PubMed] [Google Scholar]
- [9].Phillips L.E., Koepsell T.D., van Belle G, Kukull W.A., Gehrels J-A., Longstreth W.T.. History of head trauma and risk of intracranial meningioma: population-based case-control study. Neurology. 2002;58:1849–1852. doi: 10.1212/wnl.58.12.1849. [DOI] [PubMed] [Google Scholar]
- [10].Wiemels J, Wrensch M, Claus E.B.. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99:307–314. doi: 10.1007/s11060-010-0386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lusis E.A., Scheithauer B.W., Yachnis A.T., Fischer B.R., Chicoine M.R., Paulus W. et al. Meningiomas in pregnancy: a clinicopathologic study of 17 cases. Neurosurgery. 2012;71:951–961. doi: 10.1227/NEU.0b013e31826adf65. [DOI] [PubMed] [Google Scholar]
- [12].Custer B.S., Koepsell T.D., Mueller B.A.. The association between breast carcinoma and meningioma in women. Cancer. 2002;94:1626–1635. doi: 10.1002/cncr.10410. [DOI] [PubMed] [Google Scholar]
- [13].Qi Z-Y., Shao C, Huang Y-L., Hui G-Z., Zhou Y-X., Wang Z.. Reproductive and exogenous hormone factors in relation to risk of meningioma in women: a meta-analysis. PLoS One. 2013;8:0083261. doi: 10.1371/journal.pone.0083261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hu J, Little J, Xu T, Zhao X, Guo L, Jia X. et al. Risk factors for meningioma in adults: a case-control study in northeast China. Int J. Cancer. 1999;83:299–304. doi: 10.1002/(sici)1097-0215(19991029)83:3<299::aid-ijc2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- [15].Galeone C, Malerba S, Rota M, Bagnardi V, Negri E, Scotti L. et al. A meta-analysis of alcohol consumption and the risk of brain tumours. Ann. Oncol. 2013;24:514–523. doi: 10.1093/annonc/mds432. [DOI] [PubMed] [Google Scholar]
- [16].The INTERPHONE Study Group. Brain tumour risk in relation to mobile telephone use: results of the INTERPHONE international case-control study. Int J. Epidemiol. 2010;39:675–694. doi: 10.1093/ije/dyq079. [DOI] [PubMed] [Google Scholar]
- [17].Murnyák B, Szepesi R, Hortobágyi T.. [Molecular genetics of familial tumour syndromes of the central nervous system] Orv. Hetil. 2015;156:171–177. doi: 10.1556/OH.2015.30092. [DOI] [PubMed] [Google Scholar]
- [18].Murnyák B, Csonka T, Hortobágyi T.. Molecular Pathology of Meningiomas. Ideggyogy. Sz. 2015;68:292–300. doi: 10.18071/isz.68.0292. [DOI] [PubMed] [Google Scholar]
- [19].Murnyák B, Bognár L, Klekner Á.. Hortobágyi T. Epigenetics of Meningiomas. Biomed. Res. Int. 20152015:532451. doi: 10.1155/2015/532451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].McCarthy B.J., Davis F.G., Freels S, Surawicz T.S., Damek D.M., Grutsch J. et al. Factors associated with survival in patients with meningioma. J. Neurosurg. 1998;88:831–839. doi: 10.3171/jns.1998.88.5.0831. [DOI] [PubMed] [Google Scholar]
- [21].Böker D.K., Meurer H, Gullotta F.. Recurring intracranial meningiomas. Evaluation of some factors predisposing for tumor recurrence. J. Neurosurg. Sci. 1985;29:11–17. [PubMed] [Google Scholar]
- [22].Nakasu S, Nakasu Y, Nakajima M, Matsuda M, Handa J.. Preoperative identification of meningiomas that are highly likely to recur. J. Neurosurg. 1999;90:455–462. doi: 10.3171/jns.1999.90.3.0455. [DOI] [PubMed] [Google Scholar]
- [23].Maillo A, Orfao A, Espinosa A.B., Sayagués J.M., Merino M, Sousa P. et al. Early recurrences in histologically benign/ grade I meningiomas are associated with large tumors and coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone. Neuro. Oncol. 2007;9:438–446. doi: 10.1215/15228517-2007-026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mantle R.E., Lach B, Delgado M.R., Baeesa S, Bélanger G.. Predicting the probability of meningioma recurrence based on the quantity of peritumoral brain edema on computerized tomography scanning. J. Neurosurg. 1999;91:375–383. doi: 10.3171/jns.1999.91.3.0375. [DOI] [PubMed] [Google Scholar]
- [25].Bitzer M, Wöckel L, Morgalla M, Keller C, Friese S, Heiss E. et al. Peritumoural brain oedema in intracranial meningiomas: influence of tumour size, location and histology. Acta Neurochir. (Wien) 1997;139:1136–1142. doi: 10.1007/BF01410973. [DOI] [PubMed] [Google Scholar]
- [26].Palma L, Celli P, Franco C, Cervoni L, Cantore G.. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J. Neurosurg. 1997;86:793–800. doi: 10.3171/jns.1997.86.5.0793. [DOI] [PubMed] [Google Scholar]
- [27].Marciscano A.E., Stemmer-Rachamimov A.O., Niemierko A, Larvie M, Curry W.T., Barker F.G.. et al. Benign meningiomas (WHO Grade I) with atypical histological features: correlation of histopathological features with clinical outcomes. J. Neurosurg. 2016;124:106–114. doi: 10.3171/2015.1.JNS142228. [DOI] [PubMed] [Google Scholar]
- [28].Koshland D.E.. Molecule of the year. Science. 1993;262:1953. doi: 10.1126/science.8266084. [DOI] [PubMed] [Google Scholar]
- [29].Bieging K.T., Attardi L.D.. Deconstructing p53 transcriptional networks in tumor suppression. Trends Cell Biol. 2012;22:97–106. doi: 10.1016/j.tcb.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cho H, Ha S.Y., Park S.H., Park K, Chae Y.S.. Role of p53 gene mutation in tumor aggressiveness of intracranial meningiomas. J. Korean Med. Sci. 1999;14:199–205. doi: 10.3346/jkms.1999.14.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ozen O, Demirhan B, Altinörs N.. Correlation between histological grade and MIB-1 and p53 immunoreactivity in meningiomas. Clin. Neuropathol. 2005;24:219–224. [PubMed] [Google Scholar]
- [32].Bullwinkel J, Baron-Lühr B, Lüdemann A, Wohlenberg C, Gerdes J, Scholzen T.. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J. Cell Physiol. 2006;206:624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- [33].Scholzen T, Gerdes J.. The Ki-67 protein: from the known and the unknown. J. Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- [34].Terzi A, Saglam E.A., Barak A, Soylemezoglu F.. The significance of immunohistochemical expression of Ki-67, p53, p21, and p16 in meningiomas tissue arrays. Pathol. Res. Pract. 2008;204:305–314. doi: 10.1016/j.prp.2008.01.013. [DOI] [PubMed] [Google Scholar]
- [35].Perry A, Stafford S.L., Scheithauer B.W., Suman V.J., Lohse C.M.. The prognostic significance of MIB-1, p53, and DNA flow cytometry in completely resected primary meningiomas. Cancer. 1998;82:2262–2269. [PubMed] [Google Scholar]
- [36].Ide M, Jimbo M, Yamamoto M, Umebara Y, Hagiwara S, Kubo O.. MIB-1 staining index and peritumoral brain edema of meningiomas. Cancer. 1996;78:133–143. doi: 10.1002/(SICI)1097-0142(19960701)78:1<133::AID-CNCR19>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- [37].Gabos S, Berkel J.. Meta-analysis of progestin and estrogen receptors in human meningiomas. Neuroepidemiology. 1992;11:255–260. doi: 10.1159/000110938. [DOI] [PubMed] [Google Scholar]
- [38].Artlich A, Schmidt D. Immunohistochemical profile of meningiomas and their histological subtypes. Hum Pathol. 1990;21:843–849. doi: 10.1016/0046-8177(90)90054-9. [DOI] [PubMed] [Google Scholar]
- [39].Bodi I, Hortobágyi T, Buk S.. A 72-year-old woman with right frontal extra-axial mass. Brain Pathol. 2008;18:279–282. doi: 10.1111/j.1750-3639.2008.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jawhar N.M.T.. Tissue Microarray: A rapidly evolving diagnostic and research tool. Ann. Saudi Med. 2009;29:123–127. doi: 10.4103/0256-4947.51806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Csonka T, Murnyák B, Szepesi R, Kurucz A, Klekner Á., Hortobágyi T.. Poly(ADP-ribose) polymerase-1 (PARP1) and p53 labelling index correlates with tumour grade in meningiomas. Folia Neuropathol. 2014;52:111–120. doi: 10.5114/fn.2014.43782. [DOI] [PubMed] [Google Scholar]
- [42]. http://radiopaedia.org/articles/simpson-grade.
- [43].Mahore A, Chagla A, Goel A.. Seeding metastases of a benign intraventricular meningioma along the surgical track. J. Clin. Neurosci. 2010;17:253–255. doi: 10.1016/j.jocn.2009.05.025. [DOI] [PubMed] [Google Scholar]
- [44].Klekner A, Röhn Schillinger G, Schröder R, Klug N, Ernestus R.I.. ODC mRNA as a prognostic factor for recurrence in meningiomas. J. Neuro-oncol. 2001;53:67–75. doi: 10.1023/a:1011878928318. [DOI] [PubMed] [Google Scholar]


