Abstract
Background. Chaperone activity of α-crystallin in the lens works to prevent protein aggregation and is important to maintain the lens transparency. This study evaluated the effect of hesperetin on lens chaperone activity in selenite-induced cataracts. Methodology. Thirteen-day-old rats were divided into four groups. Animals were given hesperetin (groups G2 and G4) or vehicle (G1 and G3) on Days 0, 1, and 2. Rats in G3 and G4 were administered selenite subcutaneously 4 hours after the first hesperetin injection. On Days 2, 4, and 6, cataract grades were evaluated using slit-lamp biomicroscopy. The amount of a-crystallin and chaperone activity in water-soluble fraction were measured after animals sacrificed. Results. G3 on day 4 had developed significant cataract, as an average cataract grading of 4.6 ± 0.2. In contrast, G4 had less severe central opacities and lower stage cataracts than G3, as an average cataract grading of 2.4 ± 0.4. The a-crystallin levels in G3 lenses were lower than in G1, but the same as G4. Additionally, chaperone activity was weaker in G3 lenses than G1, but the same as in G4. Conclusions. Our results suggest that hesperetin can prevent the decreasing lens chaperone activity and a-crystallin water solubility by administered of selenite.
Keywords: Cataract, Chaperone activity, Anti-cataract effect, Hesperetin, Selenite-induced cataract
1. Introduction
The lens contains a high concentration of crystallin, which can be separated into three distinct families (α-, β-, and γ-crystallin). α-crystallin consists of two 20 kDa subunits, αA-and αΒ-crystallin, and exists in the lens as a polydisperse multimeric protein with an average molecular mass of 700 kDa [1, 2]. α-crystallin belongs to a small heat shock protein family and acts as a molecular chaperone [3]. This chaperone activity is critical in vivo to the normal function of the lens because lens proteins are long-lived and have negligible turnover. The chaperone activity of α-crystallin in the lens prevents the formation of protein aggregates, lens opacification, and cataract formation [3, 4]. Numerous studies have shown that a-crystallin chaperone activity protects against protein aggregation that occurs during various stress conditions [5-7].
Subcutaneous injection of sodium selenite (Na2SeO3) into suckling rats (10−18 days old) rapidly induces bilateral nuclear cataracts and this animal model has been used to evaluate anti-cataract agents [8]. Sodium selenite-induced cataracts have characteristics similar to those seen clinically, including reduced lens chaperone activity [9, 10]. In selenite-induced cataracts, the reduction in lens chaperone activity can be reversed with antioxidant flavonoids, including curcumin, lutein, and zeaxanthin [9, 11, 12].
Hesperetin, which is a natural flavonoid that is isolated from orange rinds, has a flavone backbone structure and is known to have strong antioxidant activity [13]. We had been previously reported that hespertin prevent cataract formation assessed by observing the cataract lens and measuring the concentration of lens anti-oxidants such as glutathione (GSH) and ascorbate (AsA) [14]. However, the effect of hesperetin on lens chaperone activity in lenses with cataracts remains unknown. Here, we evaluate the effect of hesperetin on lens chaperone activity in rats with selenite-induced cataracts.
2. Methods
2.1. Animals
Sprague-Dawley (SD) rats were obtained from the Sankyo Labo Service Corporation (Tokyo, Japan) and housed in temperature-controlled cages (25 ± 5°C) with a 12-hour light/dark cycle. Rats were fed balanced commercial rat chow (CE-2, Clea Japan, Tokyo, Japan) and allowed water ad libitum. Rats were sacrificed using an overdose of isoflurane (Wako Pure Chemical Industries, Osaka, Japan). The Keio University Animal Research Committee approved all animal procedures performed in this study, and all animals were treated according to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Selenite-induced cataract and hesperetin treatment
Rats were randomized into the following four groups: control group (normal lens without cataract, no hesperetin; G1), hesperetin-treated group (no cataract, hesperetin treated; G2), selenite cataract group (Se-cataract, no hesperetin; G3), and selenite-cataract with hesperetin treatment group (Se-cataract, hesperetin treatment; G4). Hesperetin (Wako Pure Chemical Industries) and sodium selenite (Wako Pure Chemical Industries) were administered to rats according to the methods described by Nakazawa et al. [14], with minor modifications. Briefly, hesperetin was dissolved in the vehicle, which was a 7% ethanol and 93% olive oil solution. Hesperetin was administered to G2 and G4 (0.4 μg/kg body weight) and vehicle (olive oil-ethanol mixture) was administered to G1 and G3 when rats were 13 days old (Day 0). Hesperetin or vehicle was also administered on Days 1 and 2 (post-natal days 14 and 15). Four hours after the first hesperetin administration on Day 0, sodium selenite (Wako Pure Chemical Industries) was administered subcutaneously at a dose of 20 mmol/kg body weight to G2 and G4. A similar volume of phosphate buffered saline (PBS; 130 mM NaCl, 3 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4) was administered to G1 and G3. On Day 2, 4, or 6 (post-natal Day 15, 17, or 19), lenses were observed by slit-lamp biomicroscopy, and measured amount of α-crystallin and chaperone activity in the lens.
2.3. Slit-lamp observation and cataract classification
Lenses were evaluated using slit-lamp biomicroscopy (TOPCON corp., Tokyo, Japan) after mydriasis by administration of tropicamide eye drops (Midorin P, Santen Pharmaceutical Company, Osaka, Japan). Cataracts were classified on a scale of stage 1–6 using the Hiraoka system [15] with minor modifications, as stage 1 indicates a normal transparent lens and stage 6 represents a nuclear mature cataract.
Following slit-lamp examination, rats were sacrificed and lenses were removed for further analyses. The amount of a-crystallin in the water-soluble fraction was used for Western blot and chaperone activity.
2.4. Western blot analysis
Each rat lens was homogenized in 0.1 M Tris buffer (pH 8.0) and centrifuged at 20,000 g for 20 minutes at 4oC. Supernatant protein concentrations were measured using the Bradford Protein Assay Kit (Bio-Rad, Hercules, CA). Bovine serum albumin (BSA) was used as the standard. One mg protein in supernatant was loaded on a 12.5% polyacrylamide gel. After electrophoresis, the gel was transferred to a nitrocellulose membrane (Bio-Rad) for Western blot analysis using an anti–αA-crystallin antibody (ab5595; abcam, Cambridge, UK) or an anti-β-actin antibody (C-11 Santa Cruz, Santa Cruz, CA). Proteins were visualized with the horseradish peroxidase/3,3’-diaminobenzidine (DAB) system using DAB tablets (Wako Pure Chemical Industries) [16]. Obtained band intensities were quantified using the National Institutes of Health (Bethesda, MD) image J software.
2.5. Chaperone activity measurement
The chaperone activity of lens water-soluble fractions was measured the light scattering of alcohol dehydrogenase (ALDH) substrate as ∆A360/180 min (Wako Pure Chemical Industries). One mg/ml ALDH in 50 mM sodium phosphate buffer containing 100 mM NaCl (pH = 7.0) was induced to increase the light scattering by adding 100 μM 1,10-phenanthroline (Wako Pure Chemical Industries) at 42°C.
Lenses were homogenized in four volume of PBS and centrifuged at 20.000 g for 20 minutes at 4°C. The supernatant proteins were collected as water-soluble proteins. Lens water-soluble protein (8 mg/ml) was added in a 1:1 (vol:vol) to ALDH solution. The extent of aggregation was estimated by measuring light scattering at 360 nm using the microplate reader (Infinite M200: TECAN LTD, Männedorf, Switzerland). Each experiment was independently performed three times.
2.6. Statistical analysis
All data are reported as mean ± S.E. Statistical analysis of the data was performed using one-way ANOVA followed by Dunnett’s multiple comparison. Student’s t-test was used for comparison between two groups. Differences were considered significant at p < 0.05.
3. Results
3.1. Characteristics of selenite-induced cataract rat lens
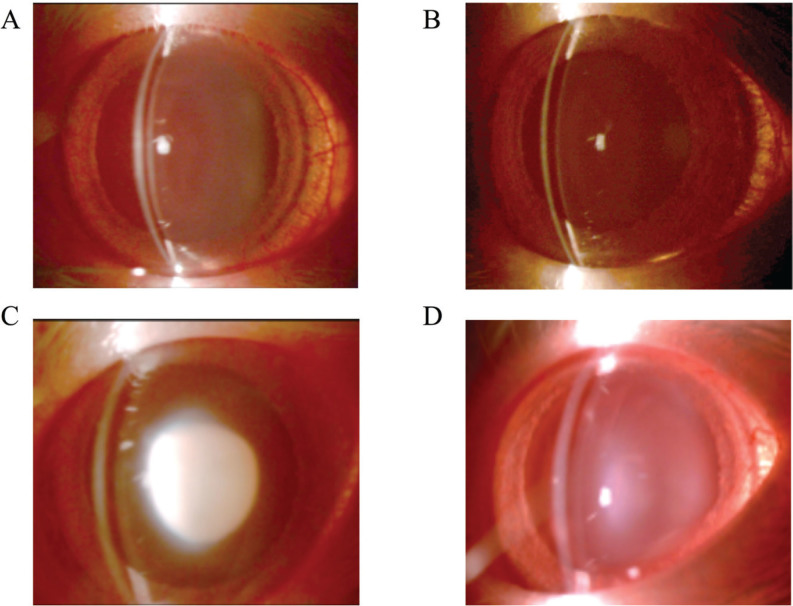
Lenses were observed with slit-lamp biomicroscope on days 2, 4, and 6 and classified into six grades (grade 1 to 6; as grade 1 indicates a transparent lens and stage 6 represents a nuclear mature cataract). After two days following the selenite injection (Day 2), all rats had transparent lenses (grade 1: 100%). On Day 4, all G1 and G2 rats still had transparent lenses, but G3 rats showed significant cataracts (grade 6: 10%, grade 5: 40%, and grade 4: 50%) and the average cataract grade was of 4.6 ± 0.2 (n = 40 lenses). The lenses of G4 rats were less severe cataracts than those of G3 rats (grade 4: 20%, grade 3: 20%, grade 2: 40%, and grade 1: 40%) and had an average cataract grading of 2.4 ± 0.4 (n = 40 lenses). On Day 6, G1 rats (Figure 1A) and G2 rats (Figure 1B) still showed transparent lenses (grade 1: 100%, G1 n = 30 lenses, G2 n = 30 lenses) and all G3 rats (Figure 1C) showed mature nuclear cataracts, characteristic of selenite administration (grade 6: 100%, n = 40 lenses). The lenses of G4 rats (Figure 1D) were also in mature cataracts, but they were significantly less severe than those in G3 rats (grade 6: 20%, grade 5: 30%, grade 4: 20%, grade 2: 10%, and grade 1: 20%; average grade = 3.9 ± 0.6, 40 lenses; Table 1). Figure 1 shows slit-lamp photographs of each group on Day 6.
Figure 1.
Effect of hespertin for cataractgenesis induced selenite administration. Groups G1 and G2 were injected with vehicle (control) and groups G3 and G4 were injected with sodium selenite subcutaneously into 13-day-old rats (Day 0). Hesperetein was administered to groups G2 and G4 subcutaneously in 13-, 14-, and 15-day-old rats (Day 0, 1, and 2, respectively). All lenses are taken 6 days after selenite or vehicle administration. (A) G1: no selenite and no hesperetin treatment. (B) G2: no selenite and hesperetin treatment. (C) G3: selenite treatment and no hesperetin treatment. (D) G4: selenite and hesperetin treatment. Rats in groups G1 and G2 had transparent lenses. Rats in the G3 group had a mature nuclear cataract, while those in group G4 had milder forms of nuclear cataracts.
Table 1.
Effect of hesperetin to cataract stage in lens of selenite-induced cataract rats.
G1 = no selenite and no hesperetin treatment; G2 = no selenite and hesperetin treatment; G3 = selenite treatment and no hesperetin treatment; G4 = selenite and hesperetin treatment. Means were calculated using data from 30 – 40 lenses.
| Group | Cataract stage | ||
|---|---|---|---|
| Day 2 | Day 4 | Day 6 | |
| G1 | 1.0 | 1.0 | 1.0 |
| G2 | 1.0 | 1.0 | 1.0 |
| G3 | 1.0 | 4.6 ± 0.2 | 6.0 |
| G4 | 1.0 | 2.4 ± 0.4 | 3.9 ± 0.6 |
3.2. Western blot analyses of α-crystallin
The amount of a-crystallin in the water-soluble fraction was measured using image of a western blot analysis on days 2, 4, and 6. On Day 2, amount of aA-crystallin was not significantly different between groups (Figure 2A). On Days 4 and 6, water-soluble aA-crystallin levels were significantly lower in G3 (Se-cataract) lenses than in G1 (control) lenses (Figure 2B, lane G3; Figure 2C, lane G3). On Day 6, the intensity of the aA-crystallin in G4 (Se-cataract, hesperetin-treated) lenses was not different from G1 or G2 lenses, but was significantly higher than that in G3 (Se-cataract) lenses (Figure 2C, lane G4). aA-crystallin levels were normalized by β-actin levels, as measured with densitometry (Figure 2D). On Day 6, aA-crystallin levels were significantly lower in G3 lenses than in G1 and G2 lenses. To the contrast, hesperetin treatment prevented water-solubility decreasing of this protein in lens with selenite-induced cataracts (G4).
Figure 2.
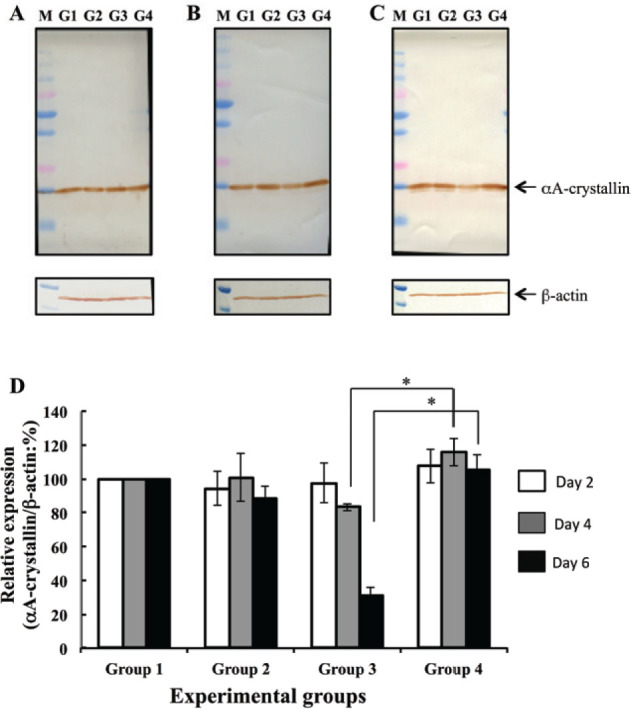
Western blot analyses to determine αA-crystallin levels. aA-crystallin levels were analyzed 2 days (A), 4 days (B), and 6 days (C) following selenite or placebo administration. b-actin was used as an internal control. Lane G1 was loaded water-soluble fraction of G1 lens (no selenite and no hesperetin treatment). Lane G2 was loaded water-soluble fraction of G2 lens (no selenite and hesperetin treatment). Lane G3 was loaded water-soluble fraction of G3 lens (selenite treatment and no hesperetin treatment). Lane G4 was loaded water-soluble fraction of G4 lens (selenite and hesperetin treatment). Lane M shows molecular weight markers. (D) Bar diagraph of aA-crystallin levels, as determined with densitometry-measured band intensity, at each time point examined. All values are relative to that measured in group G1. Data represent mean values and error bars represent one S.E. * indicates P < 0.05 for significant differences.
3.3. Chaperone activity of lens protein
Lens chaperone activity was evaluated by measuring the time course of the light scattering of ALDH at 360 nm (Figure 3). The light scattering of ALDH was elevated in the absence of lens proteins (Figure 3, curve 1). This elevation of light scattering was suppressed by immixing lens water-soluble protein from G1 (Figure 3, curve 5) and G2 (Figure 3, curve 4). Light scattering was not suppressed in the presence of G3 water-soluble proteins (Figure 3, curve 2), but elevation of light scatting was inhibited in the presence of G4 water-soluble proteins (Figure 3, curve 3). The elevation of light scattering was not occurred in lens water-soluble proteins without ALDH (Figure 3, curve 6).
Figure 3.
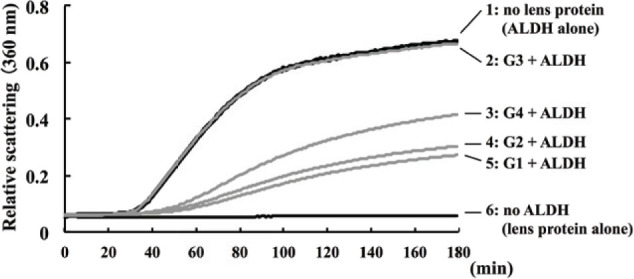
Chaperone activity of lens protein. The chaperone activity of water-soluble lens fractions was measured by a light scattering of ALDH at 360 nm, following the rat lens (day 6) administration of selenite or placebo. Curve 1 represents the light scattering of ALDH in the absence of lens protein. Curve 2 represents the light scattering of mixture of G3 lens protein (selenite treatment and no hesperetin treatment) and ALDH. Curve 3 shows the light scattering of mixture of G4 lens protein (selenite and hesperetin treatment) and ALDH. Curve 4 shows the light scattering of mixture of G2 lens protein (no selenite and hesperetin treatment) and ALDH. Curve 5 was shown the light scattering of mixture of G1 lens protein (no selenite and no hesperetin treatment) and ALDH. Curve 6 was shown the light scattering of lens protein without ALDH. Increasing of light scatting indicates the ALDH aggregation, and inhibition of light scattering depends on chaperone activity.
The depression activity of light scatting in lens watersoluble protein was measured on days 2, 4, and 6. Data were represented relative ∆A360/180 minutes values to light scattering of ALDH without lens protein that was defined as 100% and indicated the bar graph in Figure 4. On Day 2 (white bar), samples from G3 and G4 rats with preclinical cataracts showed similar light scatting as samples from G1 and G2 rats without cataracts (no Se-cataract control groups). On Day 4 (gray bar), the light scattering of ALDH in the presence of G3 was increased compared with in the presence of G1 and G2, but water-soluble proteins from G4 lenses retained protection against light scatting increasing. On Day 6 (black bar), G3 lenses had mature nuclear cataracts and completely lacked the inhibition of light scattering decreasing, but G4 lenses (hesperetin treated group) had less severe cataracts and retained protection against light scatting increasing (Figure 4). Increasing of light scatting indicated ALDH aggregation, and inhibition of protein aggregation was considered to depend on chaperone activity. These data suggested that hesperetin treatment prevent protein aggregation by maintaining the chaperone activity in lens water-soluble proteins.
Figure 4.
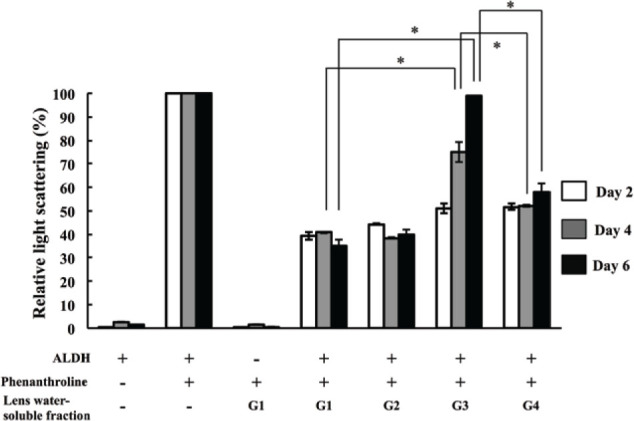
Relative change of light scattering. The change of ALDH scattering during 180 min after adding 1,10-phenanthroline at 42°C was calculated. The change of ALDH scattering in the sample without lens water-soluble fraction indicates for as 100% in the bar graph. The scatterings were measured on day 2 (white bar), day 4 (gray bar), and day 6 (black bar) following selenite or placebo administration. Bar represent mean values (n =9 lenses in each group) and error bars represent S.E. * indicates P < 0.05 for significant differences.
4. Discussion
Alpha-crystallin acts as a molecular chaperone to prevent protein aggregation and cataract formation. In this study, we hypothesized that chaperone activity might be reduced in the presence of a selenite-induced cataract because the water solubility of α-crystallin decreases in selenite-induced cataracts. To test this idea, we assessed lens chaperone activity using heat-aggregated ALDH measured the light scatting of ALDH (∆A360 / 180 minutes).
Chaperone activity was drastically reduced in lenses of selenite-induced cataracts, but hesperetin treatment rescued this activity. Furthermore, decreasing water solubility of a-crystallin by selenite administration was associated with lens chaperone activity. Chaperone activity was known to modulate by antioxidants (e.g., curcumin and cumin) [17, 18]. In agreement, we found that the antioxidant hesperetin also affects chaperone activity, as assessed with heat-aggregated ALDH in this report. Chaperone activity was also evaluated using a-lactalbumin aggregated by DTT and b-crystallin aggregated by heat. a-lactalbumin and b-crystallin aggregation assays also showed that lens chaperone activity reduced in proteins from lenses with mature selenite-induced cataracts and this activity rescued by hesperetin treatment (data not shown). All results showed that lens chaperone activity is correlated with lens transparency.
It has been reported that lens α-crystallin chaperone activity is known to be altered by several posttranslational modifications, including oxidation, racemization, deamidation, glycation, phosphorylation, and kynureniration [19-24]. In particular, α-crystallin in selenite-induced cataracts lenses have been reported to be modified by oxidative stress [11]. In the current study, amount of water soluble α-crystallin apparently decreased in selenite-induced cataract lens (G3, Figure 2). This result indicated that water solubility of α-crystallin was reduced in lenses of selenite-induced cataracts. These results suggest that selenite administration induced oxidative modifications of lens α-crystallin that make the reduction of lens α-crystallin water-solubility in addition to reduced lens chaperone activity. This diminution of water soluble α-crystallin that have chaperone activity ultimately resulted in cataract formation.
The lens contains high concentrations of antioxidants, including GSH and AsA. Both of these compounds maintain a reductive state in the lens and protect lens against photo-oxidative damage and cataract formation. The concentrations of GSH and AsA are frequently used as markers of cataract formation in both humans and animal models [25-27]. Hesperetin administration has been previously shown to prevent selenite-induced cataract formation [14]. This study provided the positive effects of hesperetin on selenite-induced cataracts for GSH and AsA levels and degradation of filensin, a lens-specific beaded filament, in rat lenses. But hesperetin was not detected in the lens within 4 hr after subcutaneous injection using HPLC (data not shown). These results suggested that administration of hesperetin maintained the lens in a reductive state indirectly, inhibited oxidative α-crystallin modification, maintained chaperone activity, keeped the a-crystallin water solubility, and preserved lens transparency. Hesperetin is a cytoprotective agent against oxidative stress and an effective medicine for cataract.
In summary, our findings demonstrated that hesperetin could delay the progression of lens opacification in selenite-induced cataract rats by maintenance of chaperone activity in lens water-soluble proteins. Hesperetin might be the drug lead compound to prevent the cataract formation.
Conflict of interest
No authors have any conflict of interest to disclose.
Acknowledgments
This work was supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT)-supported Program for Strategic Research Foundation at Private Universities (S1101003).
References
- [1].Spector A, Li LK, Augusteyn RC, Schneider A, Freund T. alpha-Crystallin. Biochem J. The isolation and characterization of distinct macromolecular fractions. 1971;124:337–343. doi: 10.1042/bj1240337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kramps HA, Stols AL, Hoenders HJ. On the quaternary structure of high-molecular-weight proteins from the bovine eye lens. Eur. J. Biochem. 1975;50:503–509. doi: 10.1111/j.1432-1033.1975.tb09889.x. [DOI] [PubMed] [Google Scholar]
- [3].Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hook DW, Harding JJ. Protection of enzymes by alpha-crystallin acting as a molecular chaperone. Int. J. Biol. Macromol. 1998;22:295–306. doi: 10.1016/s0141-8130(98)00027-0. [DOI] [PubMed] [Google Scholar]
- [5].Derham BK, Harding JJ. Alpha-crystallin as a molecular chaperone. Prog. Retin. Eye Res. 1999;18:463–509. doi: 10.1016/s1350-9462(98)00030-5. [DOI] [PubMed] [Google Scholar]
- [6].Horwitz J. Alpha-crystallin. Exp. Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- [7].Sun Y, MacRae TH. Small heat shock proteins: molecular structure and chaperone function. Cell Mol. Life Sci. 2005;62:2460–2476. doi: 10.1007/s00018-005-5190-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ostádalová I, Babický A, Obenberger J. Cataract induced by administration of a single dose of sodium selenite to suckling rats. Experientia. 1978;34:222–223. doi: 10.1007/BF01944690. [DOI] [PubMed] [Google Scholar]
- [9].Kelley MJ, David LL, Iwasaki N, Wright J, Shearer TR. alpha-Crystallin chaperone activity is reduced by calpain II in vitro and in selenite cataract. J. Biol. Chem. 1993;268:18844–18849. [PubMed] [Google Scholar]
- [10].Derham BK, Harding JJ. Effect of aging on the chaperone-like function of human alpha-crystallin assessed by three methods. Biochem. J. 1997;328:763–768. doi: 10.1042/bj3280763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rooban BN, Sasikala V, Sahasranamam V, Abraham A. Analysis on the alterations of lens proteins by Vitex negundo in selenite cataract models. Mol. Vis. 2011;17:1239–1248. [PMC free article] [PubMed] [Google Scholar]
- [12].Sasikala V, Rooban BN, Sahasranamam V, Abraham A. Rutin ameliorates free radical mediated cataract by enhancing the chaperone activity of alpha-crystallin, Graefes. Arch. Clin. Exp. Ophthalmol. 2013;251:1747–1755. doi: 10.1007/s00417-013-2281-z. [DOI] [PubMed] [Google Scholar]
- [13].Hwang SL, Yen GC. Effect of hesperetin against oxidative stress via ER- and TrkA-mediated actions in PC12 cells. J. Agric. Food Chem. 2011;59:5779–5785. doi: 10.1021/jf104632a. [DOI] [PubMed] [Google Scholar]
- [14].Nakazawa Y, Oka M, Bando M, Takehana M. Hesperetin prevents selenite-induced cataract in rats. Mol. Vis. 2015;21:804–810. [PMC free article] [PubMed] [Google Scholar]
- [15].Hiraoka T, Clark JI. Inhibition of lens opacification during the early stages of cataract formation. Invest. Ophthalmol. Vis. Sci. 1995;36:2550–2555. [PubMed] [Google Scholar]
- [16].Oka M, Kudo H, Sugama N, Asami Y, Takehana M. The function of filensin and phakinin in lens transparency. Mol. Vis. 2008;14:815–822. [PMC free article] [PubMed] [Google Scholar]
- [17].Kumar PA, Suryanarayana P, Reddy PY, Reddy GB. Modulation of alpha-crystallin chaperone activity in diabetic rat lens by curcumin. Mol. Vis. 2005;11:561–568. [PubMed] [Google Scholar]
- [18].Kumar PA, Reddy PY, Srinivas PN, Reddy GB. Delay of diabetic cataract in rats by the antiglycating potential of cumin through modulation of alpha-crystallin chaperone activity. J. Nutr, Biochem. 2009;20:553–562. doi: 10.1016/j.jnutbio.2008.05.015. [DOI] [PubMed] [Google Scholar]
- [19].Finley EL, Dillon J, Crouch RK, Schey KL. Identification of tryptophan oxidation products in bovine alpha-crystallin. Protein Sci. 1998;7:2391–2397. doi: 10.1002/pro.5560071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Fujii N, Hiroki K, Matsumoto S, Masuda K, Inoue M, Tanaka Y, Awakura M, Akaboshi M. Correlation between the loss of the chaperone-like activity and the oxidation, isomerization and racemization of gamma-irradiated alpha-crystallin, Photochem. Photobiol. 2001;74:477–482. doi: 10.1562/0031-8655(2001)074<0477:cbtlot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- [21].Gupta R, Srivastava OP. Deamidation affects structural and functional properties of human alphaA-crystallin and its oligomerization with alphaB-crystallin. J. Biol. Chem. 2004;279:44258–44269. doi: 10.1074/jbc.M405648200. [DOI] [PubMed] [Google Scholar]
- [22].Kumar PA, Kumar MS, Reddy GB. Effect of glycation on alpha-crystallin structure and chaperone-like function. Biochem. J. 2007;408:251–258. doi: 10.1042/BJ20070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kamei A, Hamaguchi T, Matsuura N, Masuda K. Does post-translational modification influence chaperone-like activity of alpha-crystallin? I. Study on phosphorylation. Biol. Pharm. Bull. 2001;24:96–99. doi: 10.1248/bpb.24.96. [DOI] [PubMed] [Google Scholar]
- [24].Garner B, Shaw DC, Lindner RA, Carver JA, Truscott RJ. Non-oxidative modification of lens crystallins by kynurenine: a novel post-translational protein modification with possible relevance to ageing and cataract, Biochim. Biophys. Acta. 2000;1476:265–278. doi: 10.1016/s0167-4838(99)00234-4. [DOI] [PubMed] [Google Scholar]
- [25].Fernando MR, Satake M, Monnier VM, Lou MF. Thioltranferase mediated ascorbate recycling in human lens epithelial cells, Invest. Ophthalmol. Vis. Sci. 2004;45:230–237. doi: 10.1167/iovs.03-0545. [DOI] [PubMed] [Google Scholar]
- [26].Yoshida M, Takashima Y, Inoue M, Iwasaki M, Otani T, Sasaki S, Tsugane S. Prospective study showing that dietary vitamin C reduced the risk of age-related cataracts in a middle-aged Japanese population. Eur. J. Nutr. 2007;46:118–124. doi: 10.1007/s00394-006-0641-8. [DOI] [PubMed] [Google Scholar]
- [27].Nakazawa Y, Oka M, Bando M, Inoue T, Takehana M. The role of ascorbic acid transporter in the lens of streptozotocin-induced diabetic rat. Biomed. Prev. Nutr. 2011;1:43–48. doi: 10.1016/j.biopha.2010.09.003. [DOI] [PubMed] [Google Scholar]