Abstract
Background
Early detection of high-risk population for osteoporosis is the key to preventing this disease.
Methodology
In this cross-sectional study a continuous sample of 270 women and 89 men (age: 20–90 years) was divided into four groups by age (≤ 55 or > 55 years) and sex. Participants completed the IOF test. Low-, medium-, and high-risk grades were defined by an OSTA index of greater than -1, -1 to -4, and less than -4, respectively.
Results
Most participants were categorized in the low-risk group (240 people, 66.9%), followed by the medium-risk (102 people, 28.4%) and high-risk groups (17 people, 4.7%). Compared to women, men in both age groups had significantly higher OSTA index and greater numbers of positive answers on the IOF test. 64.3% individuals were susceptible to osteoporosis risk (≥1 positive answers on the IOF test). Multiple regression analysis demonstrated that family history of fragility fracture (OR: 0.503, 95% CI: 0.26–0.97), height loss exceeding 3 cm (OR: 2.51, 95% CI: 1.55–4.05), and earlier menopause (OR: 0.434, 95% CI: 0.19–0.97) were associated with higher risk grades.
Conclusions
Combined use of the OSTA and IOF test is a simple and effective method for assessing the risk of osteoporosis.
Keywords: Osteoporosis, Osteoporosis Self-Assessment Tool for Asians, One-Minute Osteoporosis Risk Test, Dual-energy X-ray absorptionmetry
1. Introduction
Osteoporosis is a disease characterized by low bone mass and structural deterioration of the bone tissue. Due to the increased incidence of osteoporosis with age, osteoporosis has become a growing healthcare burden. Early detection of high-risk populations for osteoporosis is the key to preventing this disease.
Dual-energy X-ray absorptiometry (DXA) has been regarded as the gold standard to assess the bone mineral density (BMD) and identify individuals at high risk of fracture. However, due to its relatively high cost, DXA is not recommended as a routine screening test for the general population [1]. Most guidelines limit the use of DXA for postmenopausal women aged 65 years or older and for younger adults with known risk factors [2].
Currently available noninvasive diagnostic techniques for detecting osteoporosis and predicting fracture risk include the Osteoporosis Self-Assessment Tool for Asians (OSTA) and the International Osteoporosis Foundation’s (IOF’s) One-Minute Osteoporosis Risk Test (IOF test). The OSTA was developed in a population of community-dwelling participants who visited 21 clinics in eight Asian countries. This age- and weight-based test has been validated in a Japanese population [3], in Asian men [4,5], and in White Americans [6,7]. However, another study reported poor results when validating use of the OSTA for identifying postmenopausal osteoporosis with lumbar spine BMD measurements in a Chinese cohort [8]. The use of OSTA should be validated across diverse populations.
The IOF developed its osteoporosis risk test to improve public awareness of osteoporosis-related risk factors. Although not designed to assess an individual’s absolute risk, the IOF test includes questions from the fracture risk-assessment tool of the World Health Association (FRAX®) and has been officially translated into multiple languages. The Osteoporosis and Bone Miner Research branch of the Chinese Medical Association adopted the IOF test to assess the risk of osteoporosis in 2011. However, although the IOF test is simple and free, it is seldom used in a clinical setting.
The sensitivity and specificity of the OSTA for identifying subjects in the original development dataset with a BMD T-score of 2.5 or less were 74–83% and 63–68%, respectively [3,4,8-10]. Combining the OSTA and the IOF test provided increased sensitivity and specificity for identifying risk of osteoporosis in an Asian population [11].
In this study, the OSTA and IOF test were used to survey risk factors of osteoporosis among community residents in Wuhan, China, over the full age range of adulthood. The aim of this study was to validate the effectiveness of the combined use of these two simple assessment methods of osteoporosis in a poor area.
2. Methods
2.1. Participants
This cross-sectional study was a field investigation. A total of 400 Wuhan residents who visited the Health Examination Department at our hospital were invited to participate in this study. Potential participants were informed of the aim and procedures and were asked to sign an informed consent form before participation. Inclusion criteria were as follows: age of at least 20 years, able to read and fill out the questionnaire, and willingness to participate. Exclusion criteria were as follows: severe liver, heart, or kidney impairment, and tumor. Procedures of the study were in accordance with the Declaration of Helsinki and approved by the local ethics committee.
The study population was divided into four groups according to age and sex: Younger Women (age ≤ 55 years), Younger Men (age ≤ 55 years), Older Women (age > 55 years), and Older Men (age > 55 years). A research assistant and a nurse distributed the Chinese version of the IOF test to participants. The Chinese version of the IOF test includes 10 questions, two of which are specific to women and one to men. The maximum number of risk factors is 8 for women and 9 for men. Individuals were regarded as susceptible to osteoporosis risk if they answered “yes” to any of the 10 questions.
To assess the grade of osteoporosis risk, the OSTA index was calculated by the equation (body weight (kg) – age (years))*0.2. An OSTA index of -1 to -4 is regarded as medium risk, greater than -1 as low risk, and less than -4 as high risk.
2.2. Sample Size Calculation
Sample size was determined by the formula n = Z2*(1– p)*p/E2, where Z is a statistical value, p is probability (.5), and E is error (5%). When the confidence interval (CI) was set at 95%, Z = 1.96. Therefore, the sample size needed to detect a difference between groups was N = 384. A total of 400 individuals met the inclusion criteria of the study, of which 359 participants completed the questionnaire and were included in the final analysis.
2.3. Statistical analysis
Data analysis was done with the SAS 9.0 software package. Questionnaire data were analyzed with descriptive statistics. The Chi-Squared Test was used to analyze the proportion of positive responses on the IOF test among the four groups. Analysis of Variance (ANOVA) was used to compare differences in age, weight, and OSTA index among the four groups. Multivariate unconditional logistic stepwise regression analysis was used to detect any association between answers on the IOF test and the risk grade according to the OSTA index (forward-step method, p < .1). For all statistical tests, a p-value less than .05 was taken as statistically significant.
3. Results
3.1. Demographic Characteristics
Of the 359 individuals included in the final analysis, 80 were men (22.3%) and 279 were women. The age range was 20 to 90 years, with a mean age of 57.50 ± 15.96 years. The distribution by age groups is shown in Table 1. There was no significant age difference between men and women in either age group. The body weight of men was significantly higher than that of women in both age groups, but there was no difference in weight between groups of the same sex. The number of positive answers on the IOF test was significantly higher for men than for women in the older and younger groups. The number of positive answers on the IOF test was significantly higher for older women than for younger women.
Table 1.
Comparision of Clinical Features in Different Groups
| Variable | Younger Women (n = 100) | Younger Men (n = 32) | Older Women (n = 170) | Older Men (n = 57) |
|---|---|---|---|---|
| Age (year) | 41.15 ± 11.43 | 37.75 ± 11.52 | 67.35 ± 7.41** | 67.93 ± 7.27‡ |
| Weight (kg) | 59.83 ± 11.25 | 70.76 ± 11.21** | 61.49 ± 10.77 | 69.22 ± 10.80∆∆ |
| Number of positive answer(IOF test) | 0.76 ± 0.99 | 1.50 ± 1.76* | 1.21 ± 1.16** | 1.8 ± 1.52∆∆ |
| OSTA index | 3.74 ± 2.98 | 6.60 ± 2.88** | -1.17 ± 2.7** | 0.26 ± 2.55‡∆∆ |
| Low risk | 97 | 32 | 72** | 39‡∆∆ |
| Medium risk | 3 | 0 | 82 | 17 |
| High risk | 0 | 0 | 16 | 1 |
Significant differences are indicates as follows
†p < .05
∆p < .01 compared to Younger Men
p < .01 compared to Younger Women
p < .01 compared to Younger Men
p < .01 compared to Younger Women
p < .01 compared to Older Women.
p < .05
p < .01 compared to Younger Women
p < .01 compared to Older Women.
p < .01 compared to Younger Women
p < .01 compared to Younger Women
p < .01 compared to Younger Men
p < .01 compared to Older Women.
p < .01 compared to Younger Women
p < .01 compared to Younger Men
p < .01 compared to Older Women.
3.2. Estimated Risk Grade
The average OSTA index in each of the four groups is shown in Table 1. The OSTA index decreased and the risk grade increased with increasing age, especially for women. The results of ANOVA illustrated that the OSTA index values were higher for men compared to women in each age group (p < .01) and for younger compared to older individuals in each sex group (p < .001). All individuals in the younger men group were low risk. The low-risk group included 66.9% of all participants, followed by the medium-risk (28.4%) and high-risk (4.7%) groups. Average ages in the low-, medium-, and high-risk groups were 51.23 ± 15.27 years, 68.58 ± 6.83 years, and 79.71 ± 5.25 years, respectively (p < .001).
3.3. IOF Test
Men provided significantly more positive answers to the IOF test than women. There were significant differences between the groups for the number of positive responses to Q1, Q4, Q5, Q6, Q8, and Q10 (Table 2). A total of 225 (64.3%) people gave at least one positive answer and were defined as susceptible to osteoporosis risk. Only 134 people had no positive answers to any of the questions on the IOF test (Figure 1). Almost half of all younger individuals (age ≤ 55 years) had all negative responses to the IOF test, compared to 34.1% of older women and 14.03% of older men. Significant differences were observed for the number of positive answers among the four groups, with men and older women having more positive answers than younger women.
Table 2.
Positive Answers to the IOF Test among the Four Groups
| Question | Younger Women (n =100) | Younger Men(n = 32) | Older Women(n = 170) | Older Men(n = 57) | P |
|---|---|---|---|---|---|
| Q1: Have you ever broken a bone after a minor fall as an adult? | 11 (11%) | 7 (21.9%) | 46 (27.1%) | 12 (21.05%) | .021 |
| Q2: Have either of your parents been diagnosed with osteoporosis or broken a bone after a minor fall (from standing height or less)? | 18 (18%) | 5 (15.6%) | 27 (15.9%) | 11 (19.3%) | .924 |
| Q3: Have you ever taken corticosteroid tablets (cortisone, prednisone, etc.) for more than 3 consecutive months (corticosteroids are often prescribed for conditions like asthma, rheumatoid arthritis, and some inflammatory diseases)? | 2 (2%) | 0 | 11 (6.5%) | 5 (8.77%) | .102 |
| Q4: After the age of 40 years, have you lost > 3 cm in height (just over 1 inch)? | 13 (13%) | 5 (15.6%) | 68 (40%) | 26 (45.6%) | <.001 |
| Q5: Do you regularly drink alcohol in excess of safe drinking limits (>2 units a day)? | 1 (1%) | 9 (28.1%) | 3 (1.76%) | 11 (19.3%) | <.001 |
| Q6: Do you currently, or have you ever, smoked cigarettes (>20 cigarettes/day)? | 1 (1%) | 12 (37.5%) | 2 (1.17%) | 19 (33.3%) | <.001 |
| Q7: Do you often have diarrhea? (due to digestive disease or enterititis?) | 16 (16%) | 5 (15.6%) | 17 (10%) | 4 (7.02%) | .267 |
| Q8: For women over 45: Did your menopause occur before 45 years? | 12 (12%) | 0 | 33 (19.4%) | 0 | <.001 |
| Q9: For women: Have your periods ever stopped for .12 consecutive months (for reasons other than pregnancy, menopause, or hysterectomy)? | 4 (4%) | 0 | 5 (2.94%) | 0 | .343 |
| Q10: For men: Do you suffer from impotence or lack of sexual desire? | 0 | 6 (18.8%) | 2 (1.17%) | 18 (31.6%) | <.001 |
Data are shown as the number (%) of positive responses. Chi-Squared Test was used to compare the proportions of positive responses on the IOF test among the four groups.
Figure 1.
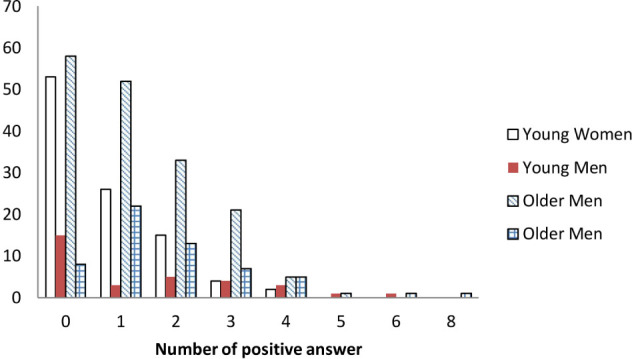
Comparison of number of positive answers to the IOF Test among the four groups.
3.4. Assessment of Association
Results of the multivariate unconditional logistic stepwise regression analysis for the association between the IOF results and OSTA index are shown in Figure 2. The major factors influencing an increased risk grade of osteoporosis were family history of fragility fracture (Q2), height loss greater than 3 cm (Q4), and age at menopause of less than 45 years (Q8).
Figure 2.
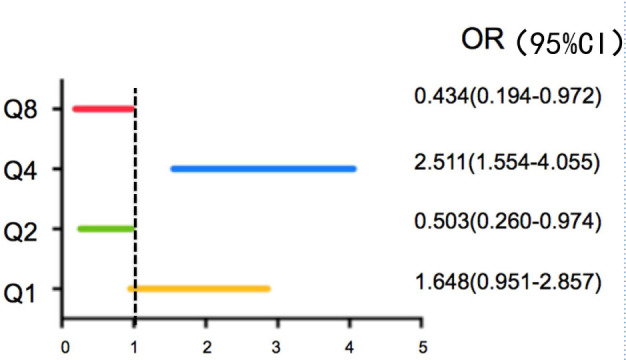
Association between Risk Grade and Risk Factors according to the IOF Test
Multivariate unconditional logistic stepwise regression analysis was used (forward-step method, p < .1). Q1: Have you ever broken a bone after a minor fall as an adult? Q2: Have either of your parents been diagnosed with osteoporosis or broken a bone after a minor fall (from standing height or less)? Q4: After the age of 40 years, have you lost > 3 cm in height (just over 1 inch)? Q8: For women over 45: Did your menopause occur before 45 years?
4. Discussion
We found that the OSTA index decreased and the risk grade increased with increasing age, particularly among women, similar to a previous study.[11] Moreover, the major factors associated with higher risk grade were family history of fragility fracture, loss of height exceeding 3 cm, and age at menopause of less than 45 years.
Sex and age are major factors impacting osteoporosis. Previous studies have also demonstrated that the cortical area and medullary expansion in bones of the lower limbs decrease with age for woman [12]. Increased age and decreased body weight are strongly associated with low BMD and increased fracture risk [9]. As a simple and noninvasive method, the OSTA was found to be the most applicable for identifying people under suspicion of osteoporosis [4]. In this paper, we report that the OSTA may be able to identify subjects with a high risk of osteoporosis.
In both age groups, the OSTA index was significantly higher for men compared to women. One possible explanation for this finding might be that the body weight of men was significantly higher than that of women in both age groups. Almost half of younger participants did not have any “yes” responses on the IOF test. For older people, fewer men than women did not have any “yes” responses. These results are similar to previous study [11] and might be attributed to the higher proportion of men versus women who smoke cigarettes and drink alcohol and less physical activity [13]. Women in the older age group had better knowledge about preventing osteoporosis than men, probably because they perceived more risk from osteoporosis or were the main target for osteoporosis-related health education. Another explanation could be questionnaire bias.
The sum of positive answers on the IOF test indicates a presence of risk factors for osteoporosis. Some studies have shown that the incidence of osteoporotic fracture in 10 years increases with an increasing number of clinical risk factors for the disease. The FRAXTM model is also based on clinical risk factors for computing the 10-year probability of osteoporotic fracture, with or without the use of BMD [14]. Fracture is a common complication of osteoporosis, which is defined by the BMD. However, several clinical risk factors contribute to fracture risk independently of BMD. These factors include age, prior fragility fracture, smoking, excess alcohol consumption, family history of hip fracture, rheumatoid arthritis and the oral glucocorticoids use [15]. These risk factors can be integrated with BMD to estimate the fracture probability by the FRAX tool. In a sample of 619 postmenopausal women, a history of fracture increased the chance of developing osteoporosis by 12.49 times [16]. A gene mutation might be a cause of bone fragility [17]. Postmenopausal woman with fragility fractures have a high risk of subsequent fractures. Treatments could focus on reducing the risk of future fractures for older people.
The IOF test is not intended for the assessment of osteoporosis risk, but to help people become aware of their own risk factors for osteoporosis. Although the OSTA might appear crude because it includes only body weight and age for assessing risk, several studies found that the OSTA was an effective method for identifying people at low risk of osteoporosis [10]. The OSTA is an inexpensive and simple clinical prediction rule in the office setting to select people for further screening, especially in places where DXA is not readily accessible. The assessment of osteoporosis risk factors can help guide early intervention.
Compared to most previous studies, this study has several strengths. First, our study was prospective. Second, this study is the first to analyze the correlation between the OSTA and the IOF test. Although the IOF test identifies few risk factors, this test could be somewhat subjective because some people cannot understand the questions. This study has also several limitations. First, a fewer number of men than women was enrolled in the study. If the number of men and women had been equal, then our results would be more convincing. Second, none of the participants received DXA. Therefore, we could not assess the sensitivity and specificity of the combined assessment method. We expect further cohorts to confirm our findings.
In conclusion, we conclude that the OSTA is a simple and effective age- and weight-based clinical risk assessment tool that demonstrates an intrinsic relationship with the IOF test in a population in China.
Conflicts of Interest of each author/contributor
None declared.
Acknowledgements
We thank the members of the Health Examination Department of the Hubei Integrated Traditional Chinese and Western Medicine Hospital who conducted the survey and everyone who contributed to this project.
References
- [1].Nelson H. D, Haney E. M, Dana T, Bougatsos C, Chou R. Screening for osteoporosis: an update for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2010;153:99–111. doi: 10.7326/0003-4819-153-2-201007200-00262. [DOI] [PubMed] [Google Scholar]
- [2].Lim L. S, Hoeksema L. J, Sherin K, Committee A. P. P. Screening for osteoporosis in the adult U.S. population: ACPM position statement on preventive practice. Am. J. Prev. Med. 2009;36:366–375. doi: 10.1016/j.amepre.2009.01.013. [DOI] [PubMed] [Google Scholar]
- [3].Koh L. K, Sedrine W. B, Torralba T. P, Kung A, Fujiwara S, Chan S. P. et al. A simple tool to identify asian women at increased risk of osteoporosis. Osteoporos. Int. 2001;12:699–705. doi: 10.1007/s001980170070. [DOI] [PubMed] [Google Scholar]
- [4].Kung A. W, Ho A. Y, Ross P. D, Reginster J. Y. Development of a clinical assessment tool in identifying Asian men with low bone mineral density and comparison of its usefulness to quantitative bone ultrasound. Osteoporos. Int. 2005;16:849–855. doi: 10.1007/s00198-004-1778-z. [DOI] [PubMed] [Google Scholar]
- [5].Li-Yu J. T, Llamado L. J, Torralba T. P. Validation of OSTA among Filipinos. Osteoporos. Int. 2005;16:1789–1793. doi: 10.1007/s00198-005-1929-x. [DOI] [PubMed] [Google Scholar]
- [6].Adler R. A, Tran M. T, Petkov V. I. Performance of the Osteoporosis Self-assessment Screening Tool for osteoporosis in American men. Mayo. Clin. Proc. 2003;78:723–727. doi: 10.4065/78.6.723. [DOI] [PubMed] [Google Scholar]
- [7].Skedros J. G, Sybrowsky C. L, Stoddard G. J. The osteoporosis self-assessment screening tool: a useful tool for the orthopaedic surgeon. J. Bone. Joint. Surg. Am. 2007;89:765–772. doi: 10.2106/JBJS.F.00347. [DOI] [PubMed] [Google Scholar]
- [8].Lu C, Chen D, Cai Y, Wei S. Concordane of OSTA and lumbar spine BMD by DXA in identifying risk of osteoporosis. J. Orthop. Surg. Res. 2006;1:14. doi: 10.1186/1749-799X-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nguyen T. V, Center J. R, Pocock N. A, Eisman J. A. Limited utility of clinical indices for the prediction of symptomatic fracture risk in postmenopausal women. Osteoporos. Int. 2004;15:49–55. doi: 10.1007/s00198-003-1511-3. [DOI] [PubMed] [Google Scholar]
- [10].Park H. M, Sedrine W. B, Reginster J. Y, Ross P. D. Osta. Korean experience with the OSTA risk index for osteoporosis: a validation study, J. Clin. Densitom. 2003;6:247–250. doi: 10.1385/jcd:6:3:247. [DOI] [PubMed] [Google Scholar]
- [11].Wong C. P, Lok M. K, Wun Y. T, Pang S. M. Chinese men’s knowledge and risk factors of osteoporosis: compared with women’s. Am. J. Mens. Health. 2014;8:159–166. doi: 10.1177/1557988313503981. [DOI] [PubMed] [Google Scholar]
- [12].Prevrhal S, Fuerst T, Fan B, Njeh C, Hans D, Uffmann M. et al. Quantitative ultrasound of the tibia depends on both cortical density and thickness, Osteoporos. Int. 2001;12:28–34. doi: 10.1007/s001980170154. [DOI] [PubMed] [Google Scholar]
- [13].Carroll S, Dollman J, Daniel M. Sex-specific correlates of adult physical activity in an Australian rural community. Aust. J. Rural. Health. 2014;22:15–22. doi: 10.1111/ajr.12081. [DOI] [PubMed] [Google Scholar]
- [14].Kanis J. A, Oden A, Johansson H, Borgstrom F, Strom O, McCloskey E. FRAX and its applications to clinical practice. Bone. 2009;44:734–743. doi: 10.1016/j.bone.2009.01.373. [DOI] [PubMed] [Google Scholar]
- [15].Pedrazzoni M, Girasole G, Giusti A, Barone A, Pioli G, Passeri G. et al. Assessment of the 10-year risk of fracture in Italian postmenopausal women using FRAX(R): a north Italian multicenter study. J. Endocrinol. Invest. 2011;34:e386–391. doi: 10.3275/7862. [DOI] [PubMed] [Google Scholar]
- [16].Schnatz P. F, Marakovits K. A, O’Sullivan D. M. Assessment of postmenopausal women and significant risk factors for osteoporosis, Obstet. Gynecol. Surv. 2010;65:591–596. doi: 10.1097/OGX.0b013e3181fc6d30. [DOI] [PubMed] [Google Scholar]
- [17].Fahiminiya S., Majewski J., Al-Jallad H., Moffatt P., Mort J., Glorieux F. H.. et al. Osteoporosis caused by mutations in PLS3: clinical and bone tissue characteristics. J. Bone. Miner. Res. 2014;29:1805–1814. doi: 10.1002/jbmr.2208. [DOI] [PubMed] [Google Scholar]


