Abstract
The fibrous tumors of the pleura are rare primary tumors, accounting for 5% of malignant pleural neoplasms, which generally originate from sub-mesothelial mesenchymal tissue of the visceral pleura. These tumours generally exhibit clinical benign behavior although 12% of solitary fibrous tumors can be malignant and have worse outcomes. These tumors are considered “giant” when the lesion > 15 cm. Surgical treatment is the best choice for both benign and malignant neoplasms. We retrospectively analyzed the main case series of giant fibrous tumors of the pleura. In addition we report our experience of a 76-year-old woman treated by pre-surgical embolization involving implantation of vascular plugs. Surgery was successfully carried out without complications; imaging and functional assessment 6 months post intervention demonstrated both the absence of recurrence and improvement of lung function parameters.
Keywords: Solitary fibrous tumor of the pleura (SFTP), Surgical treatment, Embolization, Giant tumors
1. Introduction
The solitary fibrous tumor of the pleura (SFTP) is a rare neoplasm that originates from sub-mesothelial mesenchymal tissue of pleura [1], and accounts for only 5% of pleural neoplasms. Due to the slow growth of these types of tumor diagnoses are often incidental. The most frequent symptoms are cough, chest pain and dyspnea that are common to several respiratory diseases [2-10]. Less frequent presentations are hemoptysis, obstructive pneumonia, atelectasis and clubbing; some reports have documented cardiac tamponage [11] and respiratory failure[11]. SFPT may rarely present as Doege-Potter syndrome characterized by hypoglycemia due to inappropriate secretion of insulin-like factor II.
Benign fibrous tumors can be defined as giants when diameter is greater than 15 cm or when tumors occupy more than 40% of the hemithorax[12]. These tumors may originate from sub-mesothelial stromal cells exhibiting fibroblastic or myofibroblastic phenotype whose growth are promoted by an aberrant inflammatory reaction as well as hormonal stimuli [12]. Solitary fibrous tumors have a greyish white surface with areas of soft tissue, necrosis and hemorrhage. Sections are macroscopically composed of dense fibrous tissue nodules, which frequently contain cystic structures; a small vascularized peduncle has been described in about 38-50%of cases.
Histological appearance shows spindle cells with round nuclei immersed in a rich stroma of collagen fibers. Peculiar immune histochemical features are cytokeratin-negativity and positivity to vimentin, a marker of mesenchymal cells. CD34 positivity determines exclusion of other lung cancers. Some malignant forms may be also CD34 negative and exhibit overexpression of Bcl-2, an antiapoptotic proto oncogene [13]. Appearance at CT scans, which is central to diagnosis [14-16] shows these tumors as hypodense or hyperdense compared to muscle density. The attenuation depends on the content of collagen fibers and the possible concomitant presence of hemorrhage, cysts, necrosis or calcification and over half of all benign cases are known to exhibit a heterogeneous enhancement.
At MRI, SFTP are isointense in T1-weighted sequences and exhibit variable intensity on T2. Their rich vascularization and intense enhancement produces a “chocolate chip cookies” appearance. The role of the 18 FDG PET-CT has not been clearly established [15]. Pre-surgical diagnosis requires tissue sampling which is often difficult to achieve using FNAB [17]; tru-cut or core biopsy [18] are therefore also performed.
Surgical excision is gold standard treatment, although intra-operative bleeding is one of the major complications. Preoperative evaluation of vascularity of the tumor is essential in order to optimize surgical management reducing intra and perioperative complications.
Data to support the usage of radiotherapy and chemotherapy in the treatment of SFTPs, which are widely used in thoracic cancers, is insufficiently available [19-23]. Thirty nine case series identifying 82 patients with giant SFTP have been reported in the literature between 1980 and 2014 [17, 24-28]. All cases reported in these series underwent surgical excision. In only 15 patients a preoperative vascularity study was performed. In this subgroup (eight women and seven men), mostly benign tumors were found with an average weight of tumor between 500-4500 g. Age at diagnosis ranged from 38 to 72 years.
The most common symptoms at presentation were dyspnea (87%), chest pain (67%) and chronic cough (60%). Pleural effusion, syncope and osteoarthrodystrophy were less frequent. In all reported cases a CT-guided percutaneous biopsy was performed preoperatively using fine needle aspiration biopsy (FNAB) or Tru-Cut biopsy, although preoperative diagnosis was not always achieved [17,18]. Angiography with collateral embolization was the preferred technique in 14 cases: micro-coils, polyvinyl alcohol granules, spongiosis granules or intravascular plugs were used to reduce vascular supply alone or in combination. CT-angiogram was carried out only once; to improve safety of the procedure and decrease intraoperative bleeding total circulatory arrest was made using cardiopulmonary by-pass. Interestingly, some authors [17] have pointed out that the low-flow cardiac arrest is not useful in management of blood loss due to possible difficulties in recognition of bleeding sources.
In the Pinedo et al. series [24]five subjects underwent preoperative embolization; one case reported paresis of lower right limb from medullary ischemia without further consequences resulting in 16.7% procedure linked intra-operative complication rate. Average intraoperative bleeding was 1908.3 ml. In contrast, blood leakage was lower in Guo et al. series [25](average value 800 ml); this difference was probably related to timing of surgery after embolization; average delay to surgery was 7 vs 1 day. Other perioperative complications noted were pleural effusion, air leaks and re-dilatation pulmonary edema.
Based on the immunohistochemistry data available, cytokeratins SMA, EMA, S100 and Desmin had a 0% posi-tivity whilst a positivity of 25% was found for BCL2, 62.5% for vimentin and 100% for CD34. In one case D 99 was tested (positive) and CD31 (negative). Malignant lesions, determined by the number of mitosis (4 per microscope field 10X), marked pleomorphism, high cellularity, necrosis and intralesional haemorrhage, accounted for 19% of cases. Amongst 15 cases collected from the literature only one patient in Pinedo et al. series had recurrence resulting in 6.7% overall relapse. Perioperative mortality was 1/15 (6,7%); the only death in this series occurred when low-flow cardiac arrest rather than preoperative embolization was employed. Table 1 summarizes the demographic, clinical and therapeutic features of the cases reported in these series.
2. Case report
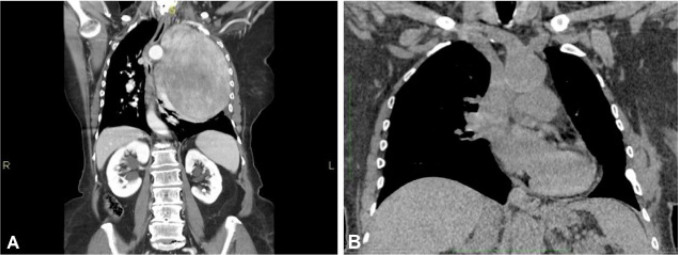
A 76-year old female with a medical history of bilateral keratokonus, presented with a 3-month history of increasing breathlessness and left sided chest pain. Radiographic study showed an extensive opacity in the left hemithorax that displaced the contralateral mediastinum. Chest CT scan (Fig. 1) showed the presence of an abnormal solid mass occupying the left hemithorax (craniocaudal diameter of 15 cm.) with defined margins and consensual pleural and pericardial effusion.
Figure 1.
Giant solitary fibrous tumor of the pleura CT-scan (coronal view): pre-surgical assessment (A); post-surgical view (B).
Pre-surgical histological samples were obtained through true-cut under CT guidance. Tissues were composed of spindle cells with a thin fibrovascular stroma and scattered myxoid-like areas in the absence of necrosis and appreciable mitotic index; immunohistochemistry was positive for vimentin, CD34, and CD99 BLC2. Hematological routine exams showed no relevant abnormalities. Respiratory functional study showed a severe restrictive ventilatory pattern causing a mild hypoxemia.
The patient underwent angiography, which showed multiple arterial streams, the mass supplied by two bulky collaterals originating from left side subclavian artery and by a peripheral branch of the internal left mammary artery. During the procedure embolization of the collateral vessels originating from the subclavian was performed using Amplatzer Vascular Plug system device IV 5mm in the first collateral and Amplatzer Vascular Plug IV and 4 mm in the second. The collateral deriving from the left internal mammary artery was embolized by implantation of spiral-controlled detachment Cook 3-PDA-5.
Forty-eight hours following embolization the patient underwent surgical resection: a left lateral thoracotomy approach was employed with dual access to the pleural cavity through the 3rd and 6th intercostal space. The tumour was partially adherent to the parietal pleura, via a large pedunculated and highly vascularized appendix, from where it appeared to originate. Cautious mobilization manoeuvers demonstrated strong adhesions to the upper lung lobe, which was partially torn, and the mediastinum and lower lobe from which it was excised by apical sub-segmentectomy.
The tumor was partially attached to the adventitia of the aortic arch from which it was also excised. A large lymph node station 9 was finally removed. (Fig. 2). The surgical procedure was completed without complications in the peri- and post-operative period. In our case, despite the delay, as opposed to the recommendations in the literature, which suggests a maximum time of 24 hours between collaterals embolization and surgical resection, has not led to the feared risk of bleeding. Imaging and functional reassessment, carried out 90 days after surgery, showed re-expansion of the lung parenchyma and an improvement of all functional parameters (FEV1pre-FEV1po: 41% vs 78% thth; CVpre-CVpo 39% vs 75 th % th; VO2peak pre-VO2peak po: 38% vs 50% th).
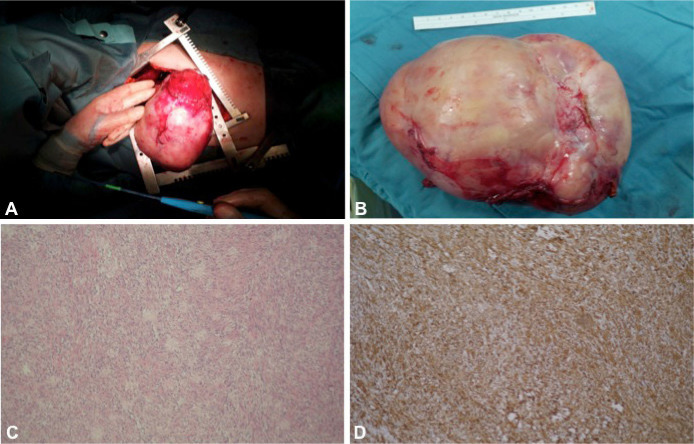
Figure 2.
Giant solitary fibrous tumor of the pleura: Surgical excision (A); Surgical specimen removed (B); H&E Staining, Original Magnification × 200 (C); Immunochemistry CD34 staining, Original Magnification × 200 (D).
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board or equivalent committee.
Informed consent: Informed consent has been obtained from all individuals included in this study.
3. Discussion
Solitary fibrous tumors of the pleura are rare primary tumors with heterogeneous clinical onset, which have represented, over the years, a complex disease entity. Clinical diagnosis is challenging as symptoms may mimic both benign and neoplastic lung disease. Immunohistochemistry techniques and electronic microscopy determine its mesenchymal origin, differentiating it from other diseases of the pleura [11-13].
A precise definition of giant fibrous tumor has not been clarified. Although molecular profiling technologies to assess DNA, RNA, protein and metabolites have led to better understanding of molecular basis of cancers [29-50], research on SFTP has been limited. Complete surgical resection is the mainstay of treatment, which significantly impacts prognosis [51-53]. Local recurrence can occur in malignant cases, but is very rare in solitary benign tumors; it may be a result of an incomplete or conservative surgery, lack of identification of a tumor during the operation or a growth of a synchronous neoplasm independent from that removed [51,52].
Video Assisted Thoracoscopic Surgery (VATS) is indicated for lesions less than 5 cm of diameter. Size of the tumor, relationship with adjacent structures and identification of the vascular peduncle may be challenging also in surgical resection. The blood supply of the tumor is most often guaranteed by collateral branches from phrenic artery, the intercostal arteries, and internal mammary and bronchial arteries [51,52]. Unlike other techniques [54], using micro-coils, polyvinyl alcohol granules or spongiosis granules, the embolization technique used in our case involves implantation of vascular plugs. These consist of a steel wire with strands-filaments of dacron and are able to occlude a vessel through the formation of thrombus and have been shown to reduce procedure time and radiation exposure.
4. Conclusion
Our experience suggests that preoperative embolization has an important role in management of giant chest tumors. Difficulties in diagnosis and therapeutic management of SFTP highlight the importance of a multidisciplinary approach to this disease including pulmonologist, thoracic surgeon, interventional cardiologist, radiologist and pathologist. This integrated approach represents the optimal strategy to ensure best diagnostic and prognostic outcomes for patients suffering from this type of pathology.
Conflict of interest statement
Authors state no conflict of interest.
References
- [1].Kaur A, Singh Gill S, Singh J, Singh A, Mani NS. Giant solitary fibrous tumor pleura: Clinical dilemma and diagnosis. Lung India. 2012 Apr-Jun;29(2):179–181. doi: 10.4103/0970-2113.95338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Mazzarella G, Iadevaia C, Guerra G, Rocca A, Corcione N, Rossi G. et al. Intralobar pulmonary sequestration in an adult female patient mimicking asthma: a case report. Int J Surg. 2014;12:S73–77. doi: 10.1016/j.ijsu.2014.08.376. (Suppl 2) [DOI] [PubMed] [Google Scholar]
- [3].Corbi G, Bianco A, Turchiarelli V, Cellurale M, Fatica F, Daniele A. et al. Potential Mechanisms Linking Atherosclerosis and Increased Cardiovascular Risk in COPD: Focus On Sirtuins. Int J Mol Sci. 2013 Jun 17;14(6):12696–12713. doi: 10.3390/ijms140612696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mazzarella G, Bianco A, Catena E, De Palma R, Abbate GF. Th1/Th2 lymphocyte polarization in asthma. Allergy. 2000;55:6–9. doi: 10.1034/j.1398-9995.2000.00511.x. (Suppl. 61) [DOI] [PubMed] [Google Scholar]
- [5].Esposito V, Lucariello A, Savarese L, Cinelli MP, Ferraraccio F, Bianco A. et al. Morphology changes in human lung epithelial cells after exposure to diesel exhaust micron sub particles (PM₁.₀) and pollen allergens. Environ Pollut. 2012 Dec;171:162–167. doi: 10.1016/j.envpol.2012.07.006. [DOI] [PubMed] [Google Scholar]
- [6].Mazzarella G, Lucariello A, Bianco A, Calabrese C, Thanassoulas T, Savarese L. et al. Exposure to submicron particles (PM1.0) from diesel exhaust and pollen allergens of human lung epithelial cells induces morphological changes of mitochondria tonifilaments and rough endoplasmic reticulum. In Vivo. 2014 Jul-Aug;28(4):557–561. [PubMed] [Google Scholar]
- [7].Mazzarella G, Ferraraccio F, Prati MV, Annunziata S, Bianco A, Mezzogiorno A. et al. Effects of diesel exhaust particles on human lung epithelial cells: an in vitro study. Respir Med. 2007 Jun;101(6):1155–1162. doi: 10.1016/j.rmed.2006.11.011. [DOI] [PubMed] [Google Scholar]
- [8].Grella E, Paciocco G, Caterino U, Mazzarella G. Respiratory function and atmospheric pollution. Monaldi Arch Chest Dis. 2002 Jun-Aug;57(3-4):196–199. [PubMed] [Google Scholar]
- [9].Grella E, Paciocco G, Caterino U, Mazzarella G. Respiratory function and atmospheric pollution. Monaldi Arch Chest Dis. 2002 Jun-Aug;57(3-4):196–199. [PubMed] [Google Scholar]
- [10].Vatrella A, Montagnani S, Calabrese C, Parrella R, Pelaia G, Biscione GL. et al. Neuropeptide expression in the airways of COPD patients and smokers with normal lung function. J Biol Reg Homeos Ag. 2010 Oct-Dec;24(4):425–432. [PubMed] [Google Scholar]
- [11].Moccia F, Dragoni S, Cinelli M, Montagnani S, Amato B, Rosti V. et al. How to utilize Ca2+ signals to rejuvenate the repairative phenotype of senescent endothelial progenitor cells in elderly patients affected by cardiovascular diseases: a useful therapeutic support of surgical approach? BMC Surg. 2013 Oct;8(13):S46. doi: 10.1186/1471-2482-13-S2-S46. (Suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tamenishi A, Matsumura Y, Okamoto H. Solitary fibrous tumor causing cardiac tamponade. Ann Thorac Surg. 2013 Jule 23816088;96(1):319–321. doi: 10.1016/j.athoracsur.2012.11.062. [DOI] [PubMed] [Google Scholar]
- [13].De Perrot M, Fischer S, Bründler MA, Sekine Y, Keshavjee S. Solitary fibrous tumors of the pleura. Ann Thorac Surg. 2002 Jule;74(1):285–293. doi: 10.1016/s0003-4975(01)03374-4. [DOI] [PubMed] [Google Scholar]
- [14].Bongiovanni M, Viberti L, Pecchioni C, Papotti M, Thonhofer R, Hans Popper H. et al. Steroid hormone receptor in pleural solitary fibrous tumours and CD34+ progenitor stromal cells. J Pathol. 2002 Octe;198(2):252–257. doi: 10.1002/path.1195. [DOI] [PubMed] [Google Scholar]
- [15].Pusiol T, Scialpi M. Role of computed tomography in the preoperative diagnosis of giant benign solitary fibrous tumor pleura. Lung India. 2013 Jane;30(1):82–85. doi: 10.4103/0970-2113.106128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brunese L, Greco B, Setola FR, Lassandro F, Guarracino MR, De Rimini M. et al. Non-small cell lung cancer evaluated with quantitative contrast-enhanced CT and PET-CT: net enhancement and standardized uptake values are related to tumour size and histology. Med Sci Monit. 2013 Feb;7(19):95–101. doi: 10.12659/MSM.883759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yan J, Ahl KL, Manning KA, Mann FA, Lewis DH. Radiology-Pathology Conference: 18F FDG PET-CT imaging of solitary fibrous tumor of the pleura. Clin Imaging. 2013 May-Jun;2337(3):598–601. doi: 10.1016/j.clinimag.2012.09.020. [DOI] [PubMed] [Google Scholar]
- [18].Aydemir B, Celik S, Okay T, Doğusoy I. Intrathoracic giant solitary fibrous tumor. Am J Case Rep. 2013 Apr;2(14):91–93. doi: 10.12659/AJCR.883867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Szkorupa M, Klein J, Bohanes T, Neoral C, Chudàcek J. Solitary fibrous tumor of the pleural cavity. Rozel Chir. 2010;89:750–753. [PubMed] [Google Scholar]
- [20].Comella P, Frasci G, De Cataldis G, Panza N, Cioffi R, Curcio C. et al. on behalf of the Gruppo Oncologico Campano: “Cisplatin/ carboplatin + etoposide + vinorelbine in advanced non-small-cell lung cancer: a multicentre randomised trial”. British Journal of Cancer. 1996;74:1805–1811. doi: 10.1038/bjc.1996.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Comella P, Frasci G, Panza N, Manzione L, Lorusso V, Di Rienzo G. et al. “Cisplatin, Gemcitabine, and Vinorelbine Combination therapy in advanced Non-Small-cell Lung Cancer: A Phase II Randomized study of the southern Italy Cooperative oncology group”. Journal of Clinical Oncology. 1999;17(5):1526–1534. doi: 10.1200/JCO.1999.17.5.1526. [DOI] [PubMed] [Google Scholar]
- [22].Frasci G, Lorusso V, Panza N, Comella P, Nicolella G, Bianco A. et al. “Gemcitabine + Vinorelbine vs. Vinorelbine alone in elderly Non-Small-cell Lung Cancer patients with advanced disease. Journal of Clinical Oncology. 2000 Jul;18(13):2529–2536. doi: 10.1200/JCO.2000.18.13.2529. [DOI] [PubMed] [Google Scholar]
- [23].Frasci G, Lorusso V, Panza N, Comella P, Nicolella G, Bianco A. et al. Gemcitabine plus vinorelbine yields better survival outcome than vinorelbine alone in elderly patients with advanced non-small cell lung cancer. A Southern Italy Cooperative Oncology Group (SICOG) phase III trial. Lung Cancer. 2001;4:65–69. doi: 10.1016/s0169-5002(01)00392-0. [DOI] [PubMed] [Google Scholar]
- [24].Piantedosi FV, Caputo F, Mazzarella G, Gilli M, Pontillo A, D’Agostino D. et al. Gemcitabine, ifosfamide and paclitaxel in advanced/metastatic non-small cell lung cancer patients: a phase II study. Cancer Chemother Pharmacol. 2008;61:803–807. doi: 10.1007/s00280-007-0537-1. [DOI] [PubMed] [Google Scholar]
- [25].Pinedo-Onofre JA, Robles-Pérez E, Peña-Mirabal ES, Hernández-Carrillo JA, Téllez-Becerra JL. Giant solitary fibrous tumor of the pleura. Cir. 2010 Jan-Feb;78(1):31–43. [PubMed] [Google Scholar]
- [26].Guo J, Chu X, Sun YE, Zhang L, Zhou N. Giant solitary fibrous tumor of the pleura: an analysis of five patients. World J Surg. 2010 Nov;34(11):2553–2557. doi: 10.1007/s00268-010-0715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Weiss B, Horton DA. Preoperative embolization of a massive solitary fibrous tumor of the pleura. Ann Thorac Surg. 2002 Mar;73(3):983–985. doi: 10.1016/s0003-4975(01)03117-4. [DOI] [PubMed] [Google Scholar]
- [28].Brevetti GR, Sasse KC, Khan JH, Wilson MW, Clary-Macy C, Brevetti LS. et al. Giant tumors of the chest: preoperative embolization and resection. J Cardiovasc Surg (Torino) 2000 Dec;41(6):945–952. [PubMed] [Google Scholar]
- [29].Palleschi A, Cioffi U, De Simone M, Santambrogio L. Preoperative embolization for giant thoracic masses. Interact Cardiovasc Thorac Surg. 2011 Jun;12(6):1065. doi: 10.1510/icvts.2010.259804A. [DOI] [PubMed] [Google Scholar]
- [30].Cattaneo F, Guerra G, Parisi M, Lucariello A, De Luca A, De Rosa N. et al. Expression of Formyl-peptide Receptors in Human Lung Carcinoma. Anticancer Res. 2015 May;35(5):2769–2774. [PubMed] [Google Scholar]
- [31].Cattaneo F, Guerra G, Parisi M, De Marinis M, Tafuri D, Cinelli M. et al. Cell-Surface Receptors Transactivation Mediated by G Protein-Coupled Receptors. Int J Mol Sci. 2014 Oct 29;15(11):19700–19728. doi: 10.3390/ijms151119700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nigro E, Scudiero O, Sarnataro D, Mazzarella G, Sofia M, Bianco A. et al. Adiponectin affects lung epithelial A549 cell viability counteracting TNFα and IL-1ß toxicity through AdipoR1. Int J Biochem Cell Biol. 2013;45(6):1145–1153. doi: 10.1016/j.biocel.2013.03.003. [DOI] [PubMed] [Google Scholar]
- [33].Nigro E, Scudiero O, Monaco ML, Palmieri A, Mazzarella G, Costagliola C. et al. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014:658913. doi: 10.1155/2014/658913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nigro E, Imperlini E, Scudiero O, Monaco ML, Polito R, Mazzarella G, Orrù S, Bianco A, Daniele A. Differentially expressed and activated proteins associated with non small cell lung cancer tissues. Respir Res. 2015 Jun;24(16):74. doi: 10.1186/s12931-015-0234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nigro E, Daniele A, Scudiero O, Ludovica Monaco M, Roviezzo F. et al. Adiponectin in asthma: implications for phenotyping. Curr Protein Pept Sci. 2015;16(3):182–187. doi: 10.2174/1389203716666150120095342. [DOI] [PubMed] [Google Scholar]
- [36].Dragoni S, Laforenza U, Bonetti E, Lodola F, Bottino C, Guerra G. et al. Canonical transient receptor potential 3 channel triggers VEGF induced intracellular Ca2+ oscillations in endothelial progenitor cells isolated from umbilical cord blood. Stem Cells Dev. 2013;22:2561–2580. doi: 10.1089/scd.2013.0032. [DOI] [PubMed] [Google Scholar]
- [37].Dragoni S, Laforenza U, Bonetti E, Lodola F, Bottino C, Berra-Romani R. et al. Vascular endothelial growth factor stimulates endothelial colony forming cells proliferation and tubulogenesis by inducing oscillations in intracellular Ca2+ concentration. Stem Cells. 2011;29:1898–1907. doi: 10.1002/stem.734. [DOI] [PubMed] [Google Scholar]
- [38].Mazzarella G, Esposito V, Bianco A, Ferraraccio F, Prati MV, Lucariello A. et al. Inflammatory effects on human lung epithelial cells after exposure to diesel exhaust micron subsubmicron particles (PM₁.₀) and pollen allergens. Environmental PollutionEnviron Poll. 2012;161:64–69. doi: 10.1016/j.envpol.2011.09.046. [DOI] [PubMed] [Google Scholar]
- [39].Mazzarella G, Lucariello A, Bianco A, Calabrese C, Thanassoulas T, Savarese L. et al. Exposure to submicron particles (PM1.0) from diesel exhaust and pollen allergens of human lung epithelial cells induces morphological changes of mitochondria tonifilaments and rough endoplasmic reticulum. In Vivo. 2014 Jul-Aug;28(4):557–561. [PubMed] [Google Scholar]
- [40].Mazzarella G, Ferraraccio F, Prati MV, Annunziata S, Bianco A, Mezzogiorno A. et al. Effects of diesel exhaust particles on human lung epithelial cells: an in vitro study. Respir Med. 2007 Jun;101(6):1155–1162. doi: 10.1016/j.rmed.2006.11.011. [DOI] [PubMed] [Google Scholar]
- [41].Lodola F, Laforenza U, Bonetti E, Lim D, Dragoni S, Bottino C. et al. Store operated Ca2+ entry is remodelled and controls in vivo angiogenesis in endothelial progenitor cells isolated from tumoral patients. PLoS One. 2012;7(9):e42541. doi: 10.1371/journal.pone.0042541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dragoni S, Laforenza U, Bonetti E, Reforgiato M, Poletto V, Lodola F. et al. Enhanced expression of Stim, Orai, and TRPC transcripts and proteins in endothelial progenitor cells isolated from patients with primary myelofibrosis. PLoS One. 2014;9(3):e91099. doi: 10.1371/journal.pone.0091099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Moccia F, Dragoni S, Lodola F, Bonetti E, Bottino C, Guerra G. et al. Store-dependent Ca2+ entry in endothelial progenitor cells as a perspective tool to enhance cell based therapy and adverse tumour vascularisation. Curr Med Chem. 2012;19:5802–5818. doi: 10.2174/092986712804143240. [DOI] [PubMed] [Google Scholar]
- [44].Moccia F, Lodola F, Dragoni S, Bonetti E, Bottino C, Guerra G. et al. Ca2+ Signalling in endothelial pro genitor cells: a novel means to improve cell-based therapy and impair tumour vascularisation. Curr Vasc Pharmacol. 2014;12:87–105. doi: 10.2174/157016111201140327162858. [DOI] [PubMed] [Google Scholar]
- [45].Dragoni S, Turin I, Laforenza U, Potenza DM, Bottino C, Glasnov TN. et al. Store-operated ca(2+) entry does not control proliferation in primary cultures of human metastatic renal cellular carcinoma. Biomed Res Int. 2014;2014:739494. doi: 10.1155/2014/739494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Moccia F, Zuccolo E, Poletto V, Cinelli M, Bonetti E, Guerra G. et al. Endothelial progenitor cells support tumour growth and metastatisation: implications for the resistance to anti-angiogenic therapy. Tumour Biol. 2015 Aug;36(9):6603–6614. doi: 10.1007/s13277-015-3823-2. [DOI] [PubMed] [Google Scholar]
- [47].Dragoni S, Reforgiato M, Zuccolo E, Poletto V, Lodola F, Ruffinatti FA. et al. Dysregulation of VEGF-induced proangiogenic Ca2+ oscillations in primary myelofibrosis-derived endothelial colony forming cells. Exp Hematol. 2015 Dec;43(12):1019–1030.e3. doi: 10.1016/j.exphem.2015.09.002. [DOI] [PubMed] [Google Scholar]
- [48].Moccia F, Guerra G. Ca2+ Signalling in Endothelial Progenitor Cells: Friend or Foe? J Cell Physiol. 2016 Feb;231(2):314–327. doi: 10.1002/jcp.25126. [DOI] [PubMed] [Google Scholar]
- [49].Zuccolo E, Bottino C, Diofano F, Poletto V, Codazzi AC, Mannarino S. et al. Constitutive store-operated Ca2+ entry leads to enhanced nitric oxide production and proliferation in infantile hemangioma-derived endothelial colony forming cells. Stem Cells Dev. Feb 15;25(4):301–319. doi: 10.1089/scd.2015.0240. [DOI] [PubMed] [Google Scholar]
- [50].Ronco V, Potenza DM, Denti F, Vullo S, Gagliano G, Tognolina M. et al. A novel Ca2+-mediated cross-talk between endoplasmic reticulum and acidic organelles: Implications for NAADP-dependent Ca2+ signalling. Cell Calcium. 2015 Feb;57(2):89–100. doi: 10.1016/j.ceca.2015.01.001. [DOI] [PubMed] [Google Scholar]
- [51].Moccia F, Zuccolo E, Soda T, Tanzi F, Guerra G, Mapelli L. et al. Stim and Orai proteins in neuronal Ca(2+) signaling and excitability. Front Cell Neurosci. 2015 Apr;24(9):153. doi: 10.3389/fncel.2015.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rosado-de-Christenson ML, Abbott GF, McAdams HP, Franks TJ, Galvin JR. From the archives of the AFIP: Localized fibrous tumor of the pleura. Radiographics. 2003 May-Jun;23(3):759–783. doi: 10.1148/rg.233025165. [DOI] [PubMed] [Google Scholar]
- [53].Ginat DT, Bokhari A, Bhatt S, Dogra V. Imaging features of solitary fibrous tumors. AJR Am J Roentgenol. 2011 Mar;196(3):487–495. doi: 10.2214/AJR.10.4948. [DOI] [PubMed] [Google Scholar]
- [54].Rena O, Filosso PL, Papalia E, Molinatti M, Di Marzio P, Maggi G. et al. Solitary fibrous tumour of the pleura: surgical treatment. Eur J Cardiothorac Surg. 2001 Feb;19(2):185–189. doi: 10.1016/s1010-7940(00)00636-9. [DOI] [PubMed] [Google Scholar]
- [55].Morandi U, Stefani A, De Santis M, Paci M, Lodi R. Preoperative embolization in surgical treatment of mediastinal hemangiope-ricytoma. Ann Thorac Surg. 2000 Mar;69(3):937–939. doi: 10.1016/s0003-4975(99)01361-2. [DOI] [PubMed] [Google Scholar]