Abstract
Autoimmune hemolytic anemia (AIHA) is a rare hematologic disease, primarily affecting adults or children with immunodeficiency disease. First-line therapy consists of long course of steroids administration, with an early complete response rate (CRr) of 75-80%, but up to 20-30% of patients requires a second-line therapy. Rituximab is the first choice in refractory old AIHA patients, because of its safety and efficacy (early CRr at 80-90% and at 68% at 2-3 years). For this reason, splenectomy is even less chosen as second-line therapy in elderly, even though laparoscopic technique decreased complication and mortality rates. However, splenectomy can be still considered a good therapeutic option with a CRr of 81% at 35.6 months in patients older than 60 year-old, when rituximab administration cannot be performed.
Keywords: Autoimmune hemolytic anemia, Splenectomy, Elderly
1. Introduction
Autoimmune hemolytic anemia (AIHA) is a rare hematologic disease characterized by the presence of erythrocyte-specific autoantibodies in peripheral blood causing extraor intra-vascular hemolysis [1-25]. AIHA can be divided in primary or idiopathic and secondary to underlying diseases as lymphoproliferative disorders, infections or hematologic and solid tumors [14]. Another classification, based on the direct antiglobulin test (DAT) positivity and on thermal characteristics of the autoantibodies, AIHA are divided in warm AIHA (wAIHA), the most frequent accounting for half of the cases [23], cold agglutinin disease (CAD), paroxysmal hemoglobinuria (PCH), and mixed type AIHA (Table 1) [2,14,23]. The incidence is relatively low, estimated at 1-3 per 100,000 per year, primarily affecting adults or children with immuno-deficiency disease [20,23]. The diagnosis is based on clinical and serologic evidences of hemolysis, summarized in Table 2 [1-25]. The most direct parameters for the diagnosis of AIHA are the hemoglobin level (Hb, <10 g/dL) and the increase of reticulocytes, the nonnucleated precursors of red blood cells, in peripheral blood (PB) [24]. Reticulocytosis is an important marker for diagnosis and follow-up, but normal values of reticulocytes in PB may vary between laboratories, therefore it should be preferred the bone marrow responsiveness index [BMRI] defined as patient’s absolute reticulocyte count x (patient’s Hb/normal Hb) [24]. Other markers of hemolysis usually increased are the lactate dehydrogenase (LDH) and bilirubin, while haptoglobin is significantly reduced in both intra- and extravascular forms [5,23-24]. But only the DAT positivity allows the diagnosis of AIHA [1-25]. For differential diagnosis between warm, cold and mixed types, monospecific antisera (anti-IgG anti C3d) test is required [23]. In wAIHA, a common finding is the positivity for both IgG and C3d, while the cold types are characterized by isolated positivity for C3d (Table 2) [23].
Table 1.
Classification of AIHA
Warm AIHA:
|
|
Cold reactive AIHA: Cold agglutinin disease:
Paroxysmal cold hemoglobinuria:
|
Mixed type AIHA:
|
Abbreviations. AIHA: autoimmune hemolytic anemia.
Table 2.
Criteria for diagnosis of AIHA
Laboratory findings:
|
Clinical features:
|
Possible underlying diseases (prevalence of AIHA, %):
|
Abbreviations. AIHA: autoimmune hemolytic anemia; CLL: chronic lymphocytic leukemia; NHL: non-Hodgkin lymphomas; CVID: common variable immunodeficiency; ALPD: autoimmune lymphoproliferative disease: EBV: Epstein-Barr virus.
Once the diagnosis of AIHA is made, as for primary immune thrombocytopenia, the first-line therapy consists of long course of steroids administration, as prednisone at 1 mg/Kg/day [5,23]. Corticosteroids induce complete response in 75-80% of wAIHA patients, but not a sustained response because 50% of patients requires maintenance dose of steroids and 20-30% a second-line therapy [5,23]. In last decades, rituximab, a monoclonal antibody against CD20, at standard dose of 375 mg/m2/week for 4 weeks is becoming the preferred choice as second-line treatment with a complete response rate (CRr) of 70-80% at 1-2 years and infection rate of 7%, due to viral reactivation (mainly hepatitis B virus) [5,11,20]. Because of its low complication rate, rituximab is preferred in elderly (>65-year old), in patients with severe comorbidities, or with a disease course < 12 months [23]. A prospective pilot study proposed low-dose rituximab (100 mg/week per 4 administrations) plus short course of steroids as first-line therapy, increasing the early CRr at 80-90% and at 68% at 2-3 years, and reducing the side effects of long-course steroids administrations [26]. Splenectomy is still considered the “curative” option with an early CRr of 70% and of 20-60% at 47-year follow-up. Even though laparoscopic techniques decreased complication and mortality rates, bleedings, early and late infections, thromboembolic events, and overwhelming sepsis are still the most frequent complications after surgery and their incidence increases with age (>60-year old) and when comorbidities are present, as in elderly [23,27].
In this review, we focused on the role of splenectomy as second-line therapy in AIHA patients and, in particular, on the effectiveness and safety of laparoscopic splenectomy in the management of AIHA in older subjects.
2. Literature analysis
2.1. Search strategy and inclusion and exclusion criteria
Relevant literature was searched in PubMed database, from 1966 to August 2016. The key words for searches were “Splenectomy”, “Autoimmune hemolytic anemia”, and “Elderly”. Limiting factors were “elderly” or “adult”, and “English language”. Two investigators independently scanned, reviewed and chose from a reference list all the potentially eligible abstracts and the full text of articles for the review. After that, eligible articles were reviewed independently for inclusion into the final analysis. Studies were included when they met the following criteria: (1) the data of publication was not older than 1990; (2) the article reported data not older than 1980.
From selected articles, data was collected into a standardized form for basic characteristics including publication year, source and time of cohort enrollment, study design, age, sex, number of enrolled patients divided by age and type of surgery, first-line therapies, time to splenectomy, platelet count and hemoglobin level before splenectomy, and vaccinations (Tables 3 and 4). For the outcome, the following parameters were considered: complete response and relapse, the number of postoperative days, early and late complications, surgery-related mortality, and follow-up time (Tables 5 and 6).
Table 3.
Baseline characteristics of included studies
| Author and year | Study design | Multicentric (number of centers) | Source | Number of splenectomy (Male/Female) | Patient age (years, range) | Date of cohort |
|---|---|---|---|---|---|---|
| Rosen M. et al., 2001 [27] | Retrospective | No (1) | USA | 11 (2/9) | 61 (34-85) | 1995 – 2001 |
| Balagué C. et al., 2004 [28] | Retrospective | No (1) | Spain | 13 (5/8) | 41 (17-65) | 1993 – 2003 |
| Hill J. et al., 2004 [29] | Retrospective | No (1) | USA | 9 (7/2) | 62 (46 – 72) | 1997 – 2001 |
| Patel NY et al., 2012 [30] | Retrospective | No (1) | USA | 15 (6/9) | 57.9 | 1996 – 2010 |
Table 4.
Preoperative characteristics
| Rosen et al. | Balagué et al. | Hill et al. | Patel et al. | |
|---|---|---|---|---|
| Median time to splenectomy (months) | n.r. | n.r. | 62 (25-101) | n.r. |
Prior treatments
|
n.r. | n.r. | 9 5 9 |
15 2 |
Underlying disease
|
None | None | 9 | None |
Operative technique
|
1 (9) 10 (91) |
0 (0) 13 (100) |
0 (0) 9 (100) |
2 (13) 13 (87) |
| Platelet count (x109/L) | 216 (124-308) | n.r. | n.r. | 39 (1-350) |
| Hemoglobin level (g/dL) | n.r. | n.r. | n.r. | 8.6 (5.2-12.2) |
| Median splenic weight (g) | 582 (187-997) | n.r. | 665.5 (245-1300) | 310 g |
n.r.: not reported.
Table 5.
Response after splenectomy
| Rosen et al. | Balagué et al. | Hill et al. | Patel et al. | |
|---|---|---|---|---|
| Postoperative days | 2.6 (1-4) | 4(1-6) | ||
| CR (%) | 10 (91) | 9 (70) | 6 (67) | 14 (93) |
| NR (%) | 4 (30) | 2 (22) | ||
| Not evaluable (%) | 1 (9) | 1 (11) | 1 (7) | |
| Relapse (%) | 1 (9) | 1 (11) | 4 (27) | |
| Median time to CR | n.r. | n.r. | n.r. | 172 days |
| Follow-up (months) | 23 | 40 (22-58) | 24 (9-43) | 54 |
| PFS (days) | n.r. | n.r. | 82 | 31 |
Abbreviations. CR: complete response; R: response; ORR: overall response rate; NR: no response; PFS: progression-free survival; n.r.: not reported.
Table 6.
Postoperative complications
| Rosen et al. | Balagué et al. | Hill et al. | Patel et al. | |
|---|---|---|---|---|
| Any complications | 0 | 0 | 1 | 3 |
| –Infections | 3 | |||
| –Hematologic disease | 1 | |||
| Operative mortality | 0 | 0 | 0 | 0 |
2.2. Statistical analysis
All data was collected from a computerized database and chart review and was analyzed using GraphPad Prism version 6.
3. Results
3.1. Study selection
A total of 255 articles were screened and 48 of them were identified as eligible for review. We excluded 27 articles because they did not meet the selection criteria. Of the remaining 21 articles, 7 were chosen for relevance and for the use of limiting factors. One article was excluded because the median age of the cohort was less than 55 year-old and two because results were not divided according to the type of surgery or to the type of disease. Figure 1 shows the flow diagram for the selection process. A total of 4 articles were finally included in the analysis [28-31].
Figure 1.
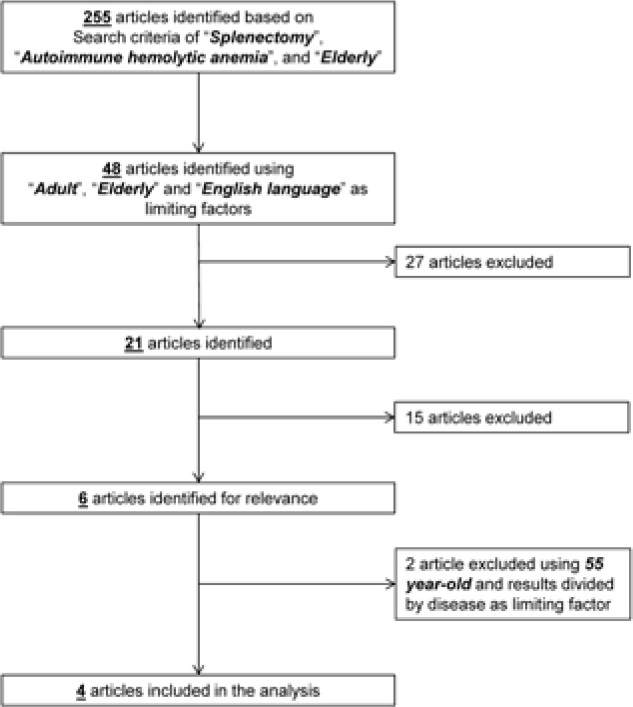
Flow diagram of search strategy
3.2. Pre-operative characteristics
From 1993 to 2010, a total of 48 splenectomies in AIHA patients with a mean age of 55.5 year-old (range, 17-85; male/female, 15/33) were described in selected retrospective studies (Table 3), performed with open technique in 6% of cases (n=3) and laparoscopic procedure in 94% of subjects (n=45) (Table 3). The most common underlying disease was chronic lymphocytic leukemia, registered in two cohorts, one of them excluded from our review because they did not report the type of surgery [32]. The median time from diagnosis was not reported in most of the studies, only Hill et al. reported a median time to splenectomy of 62 months (range, 25–101). For twenty-four patients, prior treatments were not reported, all the remaining 24 patients (50%) received corticosteroids as first-line therapy, 7 of them (14.5%) also received intravenous immunoglobulins (IVIg). Nine patients had previously received chemotherapy as alkylating agents or purine analogues for treatment of their underlying CLL. The median preoperative platelet count was 127.5 x 109/L (range, 1–350 x109/L), while the median preoperative hemoglobin level was reported only for 15 patients at 8.6 g/dL (range, 5.2-12.2). The median splenic weight was 519 g (range, 187-1300) (Table 4).
3.3. Outcome and complication rate
Splenectomized patients experienced a median postoperative stay of 3.3 days (range, 1–6). The median follow-up was 35.3 months (range, 9–58 months). Complete response (CR) was defined as normal hemoglobin with no additional therapy lasting for at least 6 months after splenectomy or an increase of 2 g/dL with reduction of hemolytic markers and no need of transfusion [24,32], otherwise patients experienced a no response (NR). Using these criteria for definition of response to AIHA treatment, 39 patients (81%) achieved CR after splenectomy, 6 patients (13%) experienced a NR, and in the remaining 3 patients (6%), follow-up was not available. Median time for CR was reported at 172 days in Patel et al. Patients who relapsed after surgery were 13% (n=6) with a median progression-free survival of 57 days; 3 of these patients died because of persistence of AIHA or Hodgkin’s disease development (Tables 5 and 6).
The operative mortality was assessed at 0%. The complication rate was 8% (n=4) and infections (urinary tract infections and pneumonia) were the most common complications (75% of all postoperative events). Late thrombotic events, subphrenic abscess or cardiovascular events were not reported (Table 6).
4. Discussion
AIHA is a rare disease, mostly occurred in adults and treated with corticosteroids as first-line therapy [1-26]. The warm AIHA type (IgG+ and C3d+) accounts for more than half of the cases and the idiopathic wAIHA is the most frequent diagnosed subtype [14]. Of secondary wAIHA, the types associated to lymphoproliferative disorders, especially CLL, are the most common [14]. Regardless the pathophysiology, first-line therapy is based on long course corticosteroid administration [1-25], but 20-30% of AIHA patients requires a second-line treatment, chosen between rituximab administration and splenectomy [20,23]. In the last decades, rituximab has been preferred in elderly, with severe comorbidities or a disease course < 12 months, because of its high CRr (70-80% at 1-2 year follow-up) and low complication rate, mainly due to HBV reactivation, easily handled by anti-viral prophylaxis [23]. This trend was also described during our research, because the last cohort of old AIHA splenectomized patients was from 1996 to 2010, described in a retrospective single-center study of 2012 [31]. In our series, laparoscopic splenectomy allowed the achievement of CR in 81% of patients at 35.3 months, similar to the responses documented for rituximab at standard and low doses (70-80% at 36 months, and 80-90% at 24-36 months, respectively) [5,11,20,23,26]. Relapse rate was assessed at 13%, with fatal evolution in 50% of cases, but as Gonzales-Porras et al. and Park et al. have documented in immune thrombocytopenia, the higher relapse rate is negatively influenced by older age (>65 year-old), also confirmed by Patel et al. [31,33-34]. For the achievement of CR, patients required at least 6 months, while relapse occurred very shortly after splenectomy (within 2 months from surgery). However, the lack of complete data for all the selected studies did not allow a multivariate analysis in order to define predictors of outcome, as already remarked by Barcellini [5].
Due to their comorbidities and the immunological changes related to splenectomy, older patients more frequently experience postoperative complications, in particular infections and overwhelming sepsis [35-49]. Indeed, in our series, the infection rate was 6%, accounting for the 75% of all postoperative complications. The 30-day mortality was assessed at 0%, similar to the mortality rate observed in laparoscopic splenectomy for ITP (0.2%), and the complication rate in all AIHA splenectomies was assessed at 3-5%, lower than the 8% reported in our series [27].
5. Conclusion
Rituximab is the first choice as second-line therapy in refractory AIHA, especially in older patients with severe comorbidities, because of its safety and in order to avoid a surgical procedure under unfavorable conditions (age >60 year-old or presence of comorbidities). Although the introduction of new target drugs changed the trends in the management of refractory wAIHA in elderly, laparoscopic splenectomy can be still considered a good therapeutic option with a complete remission rate of 81% at 35.6 months in patients older than 60 year-old, when rituximab administration cannot be performed or failed. However, because of limited data in literature, these results require further validation in prospective or randomized larger studies.
Footnotes
Conflict of interest statement: Authors state no conflict of interest
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- [1].Salama A.. Treatment Options for Primary Autoimmune Hemolytic Anemia: A Short Comprehensive Review. Transfus Med Hemother. 2015;42:294–301. doi: 10.1159/000438731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Barcellini W.. New Insights in the Pathogenesis of Autoimmune Hemolytic Anemia. Transfus Med Hemother. 2015;42:287–293. doi: 10.1159/000439002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Freedman J.. Autoimmune Hemolysis: A Journey through Time. Transfus Med Hemother. 2015;42:278–285. doi: 10.1159/000437195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hill Q.A.. Autoimmune hemolytic anemia. Hematology. 2015;20:553–554. doi: 10.1179/1024533215Z.000000000401. [DOI] [PubMed] [Google Scholar]
- [5].Barcellini W.. Current treatment strategies in autoimmune hemolytic disorders. Expert Rev Hematol. 2015;8:681–691. doi: 10.1586/17474086.2015.1073105. [DOI] [PubMed] [Google Scholar]
- [6].Ungprasert P., Tanratana P., Srivali N.. Autoimmune hemolytic anemia and venous thromboembolism: A systematic review and meta-analysis. Thromb Res. 2015;136:1013–1017. doi: 10.1016/j.thromres.2015.09.004. [DOI] [PubMed] [Google Scholar]
- [7].Berentsen S., Randen U., Tjønnfjord G.E.. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am. 2015;29:455–471. doi: 10.1016/j.hoc.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [8].Naik R.. Warm autoimmune hemolytic anemia. Hematol Oncol Clin North Am. 2015;29:445–453. doi: 10.1016/j.hoc.2015.01.001. [DOI] [PubMed] [Google Scholar]
- [9].Zanella A., Barcellini W.. Treatment of autoimmune hemolytic anemias. Haematologica. 2014;99:1547–1554. doi: 10.3324/haematol.2014.114561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rodrigo C., Rajapakse S., Gooneratne L.. Rituximab in the treatment of autoimmune haemolytic anaemia. Br J Clin Pharmacol. 2015;79:709–719. doi: 10.1111/bcp.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Michel M.. Warm autoimmune hemolytic anemia: advances in pathophysiology and treatment. Presse Med. 2014;43:e97–e104. doi: 10.1016/j.lpm.2014.02.009. [DOI] [PubMed] [Google Scholar]
- [12].Chaudhary R.K., Das S.S.. Autoimmune hemolytic anemia: From lab to bedside. Asian J Transfus Sci. 2014;8:5–12. doi: 10.4103/0973-6247.126681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bass G.F., Tuscano E.T., Tuscano J.M.. Diagnosis and classification of autoimmune hemolytic anemia. Autoimmun Rev. 2014;13:560–564. doi: 10.1016/j.autrev.2013.11.010. [DOI] [PubMed] [Google Scholar]
- [14].Jaime-Pérez J.C., Rodríguez-Martínez M., Gömez-de-León A., Tarín-Arzaga L., Gómez-Almaguer D.. Current approaches for the treatment of autoimmune hemolytic anemia. Arch Immunol Ther Exp. (Warsz) 2013;61:385–395. doi: 10.1007/s00005-013-0232-3. [DOI] [PubMed] [Google Scholar]
- [15].Berentsen S., Tjønnfjord G.E.. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26:107–115. doi: 10.1016/j.blre.2012.01.002. [DOI] [PubMed] [Google Scholar]
- [16].Michel M.. Classification and therapeutic approaches in autoimmune hemolytic anemia: an update. Expert Rev Hematol. 2011;4:607–618. doi: 10.1586/ehm.11.60. [DOI] [PubMed] [Google Scholar]
- [17].Zeerleder S.. Autoimmune haemolytic anaemia – a practical guide to cope with a diagnostic and therapeutic challenge. Neth J Med. 2011;69:177–184. [PubMed] [Google Scholar]
- [18].Berentsen S.. How I manage cold agglutinin disease. Br. J. Haematol. 2011;153:309–317. doi: 10.1111/j.1365-2141.2011.08643.x. [DOI] [PubMed] [Google Scholar]
- [19].Lechner K., Jäger U.. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831–1838. doi: 10.1182/blood-2010-03-259325. [DOI] [PubMed] [Google Scholar]
- [20].Lambert J.F., Nydegger U.E.. Geoepidemiology of autoimmune hemolytic anemia. Autoimmun Rev. 2010;9:A350–354. doi: 10.1016/j.autrev.2009.11.005. [DOI] [PubMed] [Google Scholar]
- [21].Sève P., Philippe P., Dufour J.F., Broussolle C., Michel M.. Autoimmune hemolytic anemia: classification and therapeutic approaches. Expert Rev Hematol. 2008;1:189–204. doi: 10.1586/17474086.1.2.189. [DOI] [PubMed] [Google Scholar]
- [22].Barcellini W.. Immune Hemolysis: Diagnosis and Treatment Recommendations. Semin Hematol. 2015;52:304–312. doi: 10.1053/j.seminhematol.2015.05.001. [DOI] [PubMed] [Google Scholar]
- [23].Barcellini W., Fattizzo B.. Clinical Applications of Hemolytic Markers in the Differential Diagnosis and Management of Hemolytic Anemia. Dis Markers. 2015;2015:635–670. doi: 10.1155/2015/635670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Roumier M., Loustau V., Guillaud C., Languille L., Mahevas M., Khellaf M.. et al. Characteristics and outcome of warm autoimmune hemolytic anemia in adults: New insights based on a single-center experience with 60 patients. Am J Hematol. 2014;89:E150–155. doi: 10.1002/ajh.23767. [DOI] [PubMed] [Google Scholar]
- [25].Barcellini W., Zaja F., Zaninoni A., Imperiali F.G., Di Bona E., Fattizzo B.. et al. Sustained response to low-dose rituximab in idiopathic autoimmune hemolytic anemia. Eur J Haematol. 2013;91:546–551. doi: 10.1111/ejh.12199. [DOI] [PubMed] [Google Scholar]
- [26].Kojouri K., Vesely S.K., Terrell D.R., George J.N.. Splenectomy for adult patients with idiopathic thrombocytopenic purpura: a systematic review to assess long-term platelet count responses, prediction of response, and surgical complications. Blood. 2004;104:2623–2634. doi: 10.1182/blood-2004-03-1168. [DOI] [PubMed] [Google Scholar]
- [27].Rosen M., Brody F., Walsh R.M., Tarnoff M., Malm J., Ponsky J.. Outcome of laparoscopic splenectomy based on hematologic indication. Surg Endosc. 2002;16:272–279. doi: 10.1007/s00464-001-8150-6. [DOI] [PubMed] [Google Scholar]
- [28].Balagué C., Targarona E.M., Cerdán G., Novell J., Montero O., Bendahan G.. et al. Long-term outcome after laparoscopic splenectomy related to hematologic diagnosis. Surg Endosc. 2004;18:1283–1287. doi: 10.1007/s00464-003-9092-y. [DOI] [PubMed] [Google Scholar]
- [29].Hill J., Walsh R.M., McHam S., Brody F., Kalaycio M.. Laparoscopic splenectomy for autoimmune hemolytic anemia in patients with chronic lymphocytic leukemia: a case series and review of the literature. Am J Hematol. 2004;75:134–138. doi: 10.1002/ajh.10472. [DOI] [PubMed] [Google Scholar]
- [30].Patel N.Y., Chilsen A.M., Mathiason M.A., Kallies K.J., Bottner W.A.. Outcomes and complications after splenectomy for hematologic disorders. Am J Surg. 2012;204:1014–1019. doi: 10.1016/j.amjsurg.2012.05.030. discussion 1019-1020. [DOI] [PubMed] [Google Scholar]
- [31].Akpek G., McAneny D., Weintraub L.. Comparative response to splenectomy in Coombs-positive autoimmune hemolytic anemia with or without associated disease. Am J Hematol. 1999;61:98–102. doi: 10.1002/(sici)1096-8652(199906)61:2<98::aid-ajh4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- [32].Park Y.H., Yi H.G., Kim C.S., Hong J., Park J., Lee J.H.. et al. Clinical Outcome and Predictive Factors in the Response to Splenectomy in Elderly Patients with Primary Immune Thrombocytopenia: A Multicenter Retrospective Study. Acta Haematol. 2016;135:162–171. doi: 10.1159/000442703. [DOI] [PubMed] [Google Scholar]
- [33].Gonzalez-Porras J.R., Escalante F., Pardal E., Sierra M., Garcia-Frade L.J., Redondo S.. et al. Safety and efficacy of splenectomy in over 65-yrs-old patients with immune thrombocytopenia. Eur J Haematol. 2013;91:236–241. doi: 10.1111/ejh.12146. [DOI] [PubMed] [Google Scholar]
- [34].Zeppa P., Sosa Fernandez L.V., Cozzolino I., Ronga V., Genesio R., Salatiello M.. et al. Immunoglobulin heavy-chain fluorescence in situ hybridization-chromogenic in situ hybridization DNA probe split signal in the clonality assessment of lymphoproliferative processes on cytological samples. Cancer Cytopathol. 2012;120:390–400. doi: 10.1002/cncy.21203. [DOI] [PubMed] [Google Scholar]
- [35].Peluso A.L., Cascone A.M., Lucchese L., Cozzolino I., Ieni A., Mignogna C.. et al. Use of FTA cards for the storage of breast carcinoma nucleic acid on fine-needle aspiration samples. Cancer Cytopathol. 2015;123:582–592. doi: 10.1002/cncy.21577. [DOI] [PubMed] [Google Scholar]
- [36].Zeppa P., Barra E., Napolitano V., Cozzolino I., Troncone G., Picardi M.. et al. Impact of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) in lymph nodal and mediastinal lesions: a multicenter experience. Diagn Cytopathol. 2011;39:723–729. doi: 10.1002/dc.21450. [DOI] [PubMed] [Google Scholar]
- [37].Gallelli L., Busceti M.T., Vatrella A., Maselli R., Pelaia G.. Update on anticytokine treatment for asthma. Biomed Res Int. 2013;2013:104315. doi: 10.1155/2013/104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vatrella A., Montagnani S., Calabrese C., Parrella R., Pelaia G., Biscione G.L.. et al. Neuropeptide expression in the airways of COPD patients and smokers with normal lung function. J Biol Regul Homeost Agents. 2010;24:425–432. [PubMed] [Google Scholar]
- [39].Vatrella A., Ponticiello A., Pelaia G., Parrella R., Cazzola M.. Bronchodilating effects of salmeterol, theophylline and their combination in patients withoderate to severe asthma. Pulm Pharmacol Ther. 2005;18:89–92. doi: 10.1016/j.pupt.2004.09.033. [DOI] [PubMed] [Google Scholar]
- [40].Serio B., Pezzullo L., Giudice V., Fontana R., Annunziata S., Ferrara I.. et al. OPSI threat in hematological patients. Transl Med Uni Sa. 2013;6:2–10. [PMC free article] [PubMed] [Google Scholar]
- [41].Pelaia G., Vatrella A., Busceti M.T., Gallelli L., Calabrese C., Terracciano R.. et al. Cellular mechanisms underlying eosinophilic and neutrophilic air way inflammation in asthma. Mediators Inflamm. 2015:879783. doi: 10.1155/2015/879783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pelaia G., Terracciano R., Vatrella A., Gallelli L., Busceti M.T., Calabrese C.. et al. Application of proteomics and peptidomics to COPD. Biomed Res Int. 2014:764581. doi: 10.1155/2014/764581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].D’Amato G., Stanziola A., Sanduzzi A., Liccardi G., Salzillo A., Vitale C.. et al. Treating severe allergic asthma with anti-IgE monoclonal antibody (omalizumab): a review. Multidiscip Respir Med. 2014;9:23. doi: 10.1186/2049-6958-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Waghorn D.J.. Overwhelming infection in asplenic patients: current best practice preventive measures are not being followed. J Clin Pathol. 2001;54:214–218. doi: 10.1136/jcp.54.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Schwartz P.E., Sterioff S., Mucha P., Melton L.J., Offord K.P.. Postsplenectomy sepsis and mortality in adults. JAMA. 1982;248:2279–2283. [PubMed] [Google Scholar]
- [46].Bisharat N., Omari H., Lavi I., Raz R.. Risk of infection and death among post-splenectomy patients. J Infect. 2001;43:182–186. doi: 10.1053/jinf.2001.0904. [DOI] [PubMed] [Google Scholar]
- [47].Brigden M.L.. Detection, education and management of the asplenic or hyposplenic patient. Am Fam Physician. 2001;63:499–506. [PubMed] [Google Scholar]
- [48].Rodeghiero F., Ruggeri M.. Short- and long-term risks of splenectomy for benign haematological disorders: should we revisit the indications? Br J Haematol. 2012;158:16–29. doi: 10.1111/j.1365-2141.2012.09146.x. [DOI] [PubMed] [Google Scholar]


