Abstract
This study retrospectively analyzed the efficacy of hydrogen water in the treatment of neonatal hypoxic-ischemic encephalopathy (HIE) and its effect on serum neuron-specific enolase (NSE), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) levels.
Forty newborns with HIE who received treatment from April 2014 to April 2015 were divided into a conventional care group and a hydrogen water group according to the different treatment methods applied. Twenty healthy full-term newborns comprised the control group. In the hydrogen water group, 5-mL/kg hydrogen water was orally administered two days after birth daily for 10 days in addition to conventional treatment. After 10 days, efficacy indicators were examined in the HIE groups. The NSE, IL-6, and TNF-α levels were compared among all three groups.
The efficacy indicators were significantly lower in the hydrogen water group compared with the conventional group. Before treatment, the serum NSE, IL-6, and TNF-α levels in the HIE groups were higher than those in the control group. After treatment, these levels in the hydrogen water group were lower than those in the conventional group.
Hydrogen water lowers serum NSE, IL-6, and TNF-α levels in HIE newborns, thereby exerting a protective effect.
Keywords: Hydrogen water; Hypoxic-ischemic; IL-6, TNF-α; Newborn
1. Introduction
Hypoxic-ischemic encephalopathy (HIE) refers to partial or complete anoxia caused by perinatal asphyxia and fetal or neonatal brain damage that results from cerebral blood flow reduction or suspension. HIE is a severe illness that has a high mortality and can cause permanent neurological dysfunction [1], such as mental retardation, epilepsy, cerebral palsy, spasticity, and ataxia. HIE is a major cause of full-term neonatal deaths and neurological sequelae worldwide, with an incidence of 0.77%-4.0% in developed countries; however, neonatal HIE is more common in developing countries [1]. China has a high incidence of neonatal HIE, and the reported average annual incidence ranges from 0.77%-8.00% [2]. Therefore, basic and clinical research studies of neonatal HIE are clinically and socially significant.
The pathogenesis of neonatal HIE is complex. A study recently demonstrated that inflammation plays an important role in the development of neonatal HIE [3] and that the neonatal innate immune response occurs within a few minutes of cerebral ischemia. Inflammatory cytokines that are synthesized and secreted by nerve cells promote the accumulation and activation of leukocytes and stimulate the release of inflammatory mediators in the damage area. These cytokines further enhance this inflammatory effect by inducing the synthesis and release of adhesion molecules, which results in a series of inflammatory reactions, including vascular inflammatory reactions and platelet adhesion and aggregation [4]. A previous study found that rapidly increased levels of interleukin-6 (IL-6) and other inflammatory cytokines several minutes after cerebral ischemia promoted ischemic cerebral damage by inducing nerve cell apoptosis and by increasing toxic nitrogen oxide (NO) levels [5]. Tumor necrosis factor-α (TNF-α) is mainly produced by mononuclear macrophages and is an important inflammatory mediator in many pathophysiological processes during the inflammatory response. A high concentration of TNF-α leads to local inflammatory responses and causes damage to body tissues, organs and systems [6]. Neuron-specific enolase (NSE) is involved in glycolysis in the cytoplasm of neurons and is mainly expressed in neurons and neuroendocrine cells. Low levels of NSE are present in normal blood, with a small fluctuation range; therefore, these levels remain relatively stable. After cerebral damage, nerve cells are impaired and the blood-brain barrier is damaged. Therefore, NSE is released into cerebrospinal fluid and blood. NSE can be used as an early sensitive indicator of nerve cell damage, which is positively correlated with the severity of HIE. The NSE levels decrease with improvement of the disease [7]. Serum IL-6, TNF-α and NSE levels can be used to assess the severity of HIE.
Many basic and clinical studies of neonatal HIE treatments have recently been conducted; however, compre-hensive therapy is currently recommended for neonatal HIE. Several new regimens are undergoing clinical trials, including hyperbaric oxygen therapy; however, the value of this therapy in neonatal hypoxic-ischemic disease is controversial. The goal of hyperbaric oxygen therapy is to improve brain tissue oxygen content and to enhance the oxygen diffusing capacity in the brain by increasing blood oxygen pressure. This increase in oxygen pressure reduces anaerobic metabolism, improves cerebral blood flow, and removes acid metabolites, thereby reducing brain cell edema and nervous system damage secondary to hypoxia-induced ischemia. An advantage of hyperbaric oxygen therapy is that this treatment promotes neural stem cell proliferation and improves brain damage when accompanied by physiotherapy [8]. However, newborns with HIE often exhibit decreased ATP synthesis, brain energy deficiency, and cerebral ischemia-induced brain cell necrosis. Therefore, it is difficult to achieve a therapeutic benefit by using simple hyperbaric oxygen therapy in neonatal HIE. β-Endorphin levels in plasma and cerebrospinal fluid are significantly increased in newborns with HIE, but this endorphin aggravates brain tissue hypoxia in cerebral fluid [9]. Gangliosides, cerebrolysin, and other pharmacological agents have recently been used in the treatment of neonatal HIE and have achieved a therapeutic benefit; however, these drugs are expensive and may not be widely used in hospitals [10]. Therefore, it is critical to identify a specific, efficacious and cost-effective treatment for neonatal HIE.
Hydrogen water, also known as hydrogen-reduced water, is so named due to its enrichment of hydrogen ions. Hydrogen water has strong antioxidant capacity and can remove excessive reactive oxygen species (oxygen-free radicals) from the body. Many studies have shown that hydrogen water is safe and exhibits no side effects. Hydrogen water has a significant therapeutic effect on senile dementia, cardiovascular diseases, and arteriosclerosis [11-13]. Inflammation plays an important role in neonatal HIE, and the aim of HIE treatment is to enhance the body’s own antioxidant system by reducing oxidation and by fighting against toxic reactive oxygen species. Hydrogen is an ideal scavenging substance that specifically targets free radicals. However, the use of hydrogen water in the treatment of HIE has not been reported.
Since 2014, hydrogen water has been used in the treatment of HIE at the Affiliated Hospital of Taishan Medical University, and satisfactory outcomes have been achieved. This study retrospectively analyzed the therapeutic effect of hydrogen water on neonatal HIE and investigated its protective mechanism by detecting serum NSE, IL-6, and TNF-α levels before and after treatment.
2. Materials and methods
2.1. Patients and patient groups
All clinical data were obtained from the Information Center of the Affiliated Hospital of Taishan Medical University. A total of 40 newborns with HIE who were admitted to this hospital from April 2014 to April 2015 were selected to participate in this study based on HIE diagnostic and clinical grading criteria [14]. The included patients met the following criteria: (1) full-term newborns at admission with an age ≤ 24 h and a birth weight ≥ 2,500 g; (2) no serious congenital malformations, such as diaphragmatic hernia, chromosomal abnormalities, or brain hypoplasia; (3) no heavy intracranial hemorrhage caused by birth trauma to the head or skull fracture; (4) no severe obstructive airway disease; (5) no anemia; and (6) patients with complete clinical data. Excluded patients included newborns with a treatment course of less than 7 days and newborns who died before treatment or rejected treatment.
The 40 newborns with HIE were divided into two groups according to the different treatment methods: a conventional treatment group and a hydrogen water treatment group (20 patients/group). Additionally, 20 healthy full-term newborns (1 day after the same period of birth) in the Department of Obstetrics were selected to serve as the control group.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Com-mittee of the Affiliated Hospital of Taishan Medical University. Informed consent was obtained from the guardians of all participants.
2.2. Treatment methods
The conventional treatment group underwent the following comprehensive therapy protocol for neonatal HIE [15]: (1) newborns were kept warm, airways were kept open, and, if necessary, hood oxygen or mechanical ventilation was administered; (2) blood pressure and tissue perfusion were maintained; (3) blood glucose was maintained at a normal high value; (4) convulsions were controlled and cerebral edema was treated; and (5) water and electrolyte balance were maintained and water intake was limited. For the hydrogen water treatment group, hydrogen water was administered on the 2nd day after birth. The hydrogen water was prepared with the assistance of the Diving Medicine Department of The Second Military Medical University. Hydrogen was dissolved in a 0.9% sodium chloride injection at 0.4 Mpa for 6 h to reach saturation. The hydrogen concentration was maintained at approximately 0.6 mmol/L. The hydrogen water treatment was orally administered for 10 days in addition to the comprehensive treatment regimen, with a daily dose of 5 mL/kg. On the 1st day and the 11th day after birth (i.e., before and after the hydrogen water treatment), magnetic resonance imaging (MRI) was performed for the two groups of newborns with HIE.
2.3. Efficacy indicators
The efficacy of the different treatment methods were assessed based on the records regarding the anterior fontanel tension, disturbance of consciousness, muscle tone, primitive reflex recovery time, and convulsion control time.
2.4. Magnetic resonance imaging
MRI was performed using the GE Discovery MR750 3.0T, which was equipped with 8-channel head and neck joint neurovascular dedicated coils. Newborns were examined under a natural sleep state or after chloral hydrate enema. Scanning sequences included cross-sectional T1-weighted (T1WI) fluid-attenuated inversion recovery (FLAIR), T2-weighted (T2WI), T2WI FLAIR, diffusion-weighted imaging (DWI) (echo planar imaging, EPI), and sagittal T1WI FLAIR. Scanning images were read by two deputy chief physicians in the MRI room using a double-blind method. Diffusion tensor imaging (DTI) was processed using the Functool software at a random workstation, and the fractional anisotropy (FA) values of the regions of interest (mainly the basal ganglia, the semioval center, the corona radiate, and the corpus callosum area) were measured symmetrically and averaged. The grading criteria for brain damage in the newborns with HIE were obtained from “Neonatal Hypoxic-Ischemic Encephalopathy” and were as follows [16]: (1) mild cases exhibited cortical and subcortical lesions, with or without subarachnoid hemorrhage; (2) moderate cases exhibited deep white matter lesions with focal edema; (3) severe cases exhibited lesions of the posterior limb of the internal capsule, the basal ganglia, and the thalamus in addition to subcortical cystic necrosis and diffuse brain edema.
2.5. Sample collection and ELISA
For the two groups of newborns with HIE, 3 mL of blood was collected from the femoral vein on the 1st and 11th days after birth (i.e., before and after the hydrogen water treatment). In addition, 3 mL of femoral venous blood was obtained from the control group. The blood samples were centrifuged at 3,000 rpm for 10 min, and the serum was separated. Samples with hemolysis were removed. Each serum sample was divided into duplicates, tagged with their group name, and saved in the -70°C freezer for further analyses.
Serum NSE, IL-6, and TNF-α levels were detected using the double antibody sandwich ABC-ELISA method. ELISA kits were provided by Wuhan Jingmei Biotechnology Co., Ltd., China.
2.6. Statistical analysis
The SPSS 20.0 statistical software was used for the statistical analysis. Quantification data were analyzed using the chi-squared test. The measurement data were expressed as the mean ± standard deviation (x±s) and analyzed using the normality test and the homogeneity of variance test. Comparisons between two groups were performed using the paired t-test, and analysis of variance (ANOVA) was used for the comparisons among all the groups. P < 0.05 was considered statistically significant.
3. Results
3.1. Patient data
Newborns with HIE (23 males and 17 females, with a gestational age of 32-42 weeks and an average birth weight of 3,346±258 g) were divided into a conventional treatment group (n=20) and a hydrogen water treatment group (n=20) according to the different treatment methods applied. The conventional treatment group included 7 mild cases, 12 moderate cases, and 1 severe case. The hydrogen water treatment group included 8 mild cases, 10 moderate cases, and 2 severe cases. Therefore, 68% of the HIE cases were moderate to severe. The difference in the severity of HIE between the two groups was not statistically significant (x2=0.613, P=0.578). In addition, the differences in gender, age, birth weight, and gestational age among the three groups were not statistically significant (P>0.05). The patient data for the three groups are shown in Table 1.
Table 1.
Patient data for the three groups.
| Data | Conventional | Hydrogen water | Control | X2 (F) | Pvalue |
|---|---|---|---|---|---|
| Number of cases (N) | 20 | 20 | 20 | ||
| Gender (M/F, number) | 12/8 | 11/9 | 8/12 | 1.814 | 0.416 |
| Age (x±s, h) | 11±6 | 11±5 | 12±5 | 0.975 | 0.413 |
| Weight (x±s, kg) | 3.1±0.3 | 3.2±0.5 | 3.5±0.4 | 0.527 | 0.613 |
| Gestational age (x±s, d) | 277±11 | 276±10 | 279±13 | 0.857 | 0.429 |
| Mode of delivery Eutocia/Caesarean section | 13/7 | 15/5 | 16/4 | 0.375 | 0.477 |
| Degree of severity (mild/moderate-severe) | 7/13 | 8/12 | 0.613 | 0.578 |
3.2. A comparison of the recovery time between the conventional treatment group and the hydrogen water treatment group
Anterior fontanel tension, disturbance of consciousness, muscle tone, primitive reflex recovery time, and con-vulsion control time were significantly decreased in the hydrogen water treatment group compared with the con-ventional treatment group (P<0.05) (Table 2).
Table 2.
A comparison of the clinical recovery time between the conventional treatment group and the hydrogen water treatment group (x±s, d).
| Index | Conventional | Hydrogen water |
|---|---|---|
| N | 20 | 20 |
| Anterior fontanel tension | 2.48±0.26 | 2.76±1.07 |
| Disturbance of consciousness | 4.59±1.48 | 3.36±1.22* |
| Muscle tone | 6.13±1.24 | 4.97±1.31* |
| Primitive reflex recovery time | 6.29±2.31 | 4.23±1.24* |
| Convulsion control time | 2.03±0.62 | 1.85±0.57* |
P<0.05 vs. conventional treatment group.
P<0.05 vs. conventional treatment group.
P<0.05 vs. conventional treatment group.
P<0.05 vs. conventional treatment group.
3.3. MRI
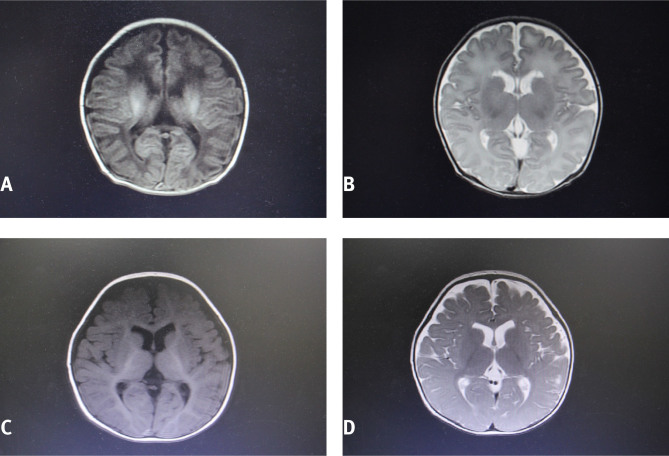
Before treatment, 7 mild cases, 12 moderate cases, and 1 severe case were detected in the conventional treatment group according to MRI, whereas 8 mild cases, 10 moderate cases, and 2 severe cases were identified in the hydrogen water treatment group. After treatment, the MRI examination showed different degrees of improvement in both groups; however, the hydrogen water treatment group significantly improved. The DTI measurement showed that the mean FA value in the hydrogen water treatment group increased from 0.348±0.074 before treatment to 0.583±0.065 after treatment, and this change was significant (P<0.05). Moreover, compared with the conventional treatment group, a significant increase was observed in the mean FA value in the hydrogen water treatment group (P<0.05) (Table 3). MRI images of the hydrogen water treatment group before and after treatment are shown in Figure 1.
Table 3.
A comparison of the MRI findings between the conventional treatment group and the hydrogen water treatment group (x±s).
| Group | MRI findings before treatment | MRI findings after treatment | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | FA value | Mild | Moderate | Severe | FA value | ||
| Conventional | 7 | 12 | 1 | 0.356±0.068 | 11 | 8 | 1 | 0.459±0.072 | <0.05 |
| Hydrogen water | 8 | 10 | 2 | 0.348±0.074 | 14 | 6 | 0 | 0.593±0.065 | <0.05 |
| P value | >0.05 | <0.05 | |||||||
Figure 1.
A case in the hydrogen water treatment group before and after treatment. The male patient weighed 3.1 kg and had a history of choking. A and B: At 12 h after birth, MRI scanning showed symmetrical long T1 and long T2 signals in the white matter near the anterior horns of both lateral ventricles. FLAIR and DWI showed a low signal, and DTI indicated an FA value of 0.316. C and D: After the hydrogen water treatment, the abnormal signal disappeared and DTI indicated an FA value of 0.533.
4. Serum NSE, IL-6, and TNF-α levels
Before treatment, the serum NSE, IL-6, and TNF-α levels in the two groups of newborns with HIE were higher than those in the control group; however, this difference was not statistically significant. On the 11th day after birth, the serum NSE, IL-6, and TNF-α levels in the control group were significantly decreased compared with those on the 1st day after birth (P<0.05). In addition, the serum NSE and S-100B levels in the two groups of newborns with HIE on the 11th day after birth were lower than those on the 1st day after birth, which indicates that NSE may naturally decrease in the neonatal body under normal and disease states. After treatment (on the 9th day after birth), the serum NSE, IL-6, TNF-α levels in the two treatment groups remained higher than those in the control group (P<0.05), whereas the serum NSE, IL-6, and TNF-α levels in the hydrogen water treatment group were lower than those in the conventional treatment group (P<0.05) (Table 4).
Table 4.
A comparison of the serum NSE, IL-6, and TNF-α levels among the three groups of newborns.
| Group | MRI findings before treatment | MRI findings after treatment | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | FA value | Mild | Moderate | Severe | FA value | ||
| Conventional | 7 | 12 | 1 | 0.356±0.068 | 11 | 8 | 1 | 0.459±0.072 | <0.05 |
| Hydrogen water | 8 | 10 | 2 | 0.348±0.074 | 14 | 6 | 0 | 0.593±0.065 | <0.05 |
| P value | >0.05 | <0.05 | |||||||
5. Discussion
HIE is one of the most common neurological diseases in newborns; however, no consensus has been reached regarding specific treatment. The incidence of neonatal HIE ranges from 0.2%-0.9%. Mild cases of HIE are self-limiting, whereas severe cases are usually associated with negative sequelae, such as mental retardation, cerebral palsy, epilepsy, and ataxia. HIE consists of a series of complex pathological alterations after hypoxic and ischemic changes in brain tissue, which result in neuronal cell apoptosis. Inflammatory cytokine-mediated responses play an important role in the mechanism of ischemic brain damage [17]. This study retrospectively analyzed the efficacy of hydrogen water in the treatment of neonatal HIE and its effect on serum NSE, IL-6, and TNF-α levels.
The results of our study showed that anterior fontanel tension, disturbance of consciousness, muscle tone, primitive reflex recovery time, and convulsion control time were significantly decreased in the hydrogen water treatment group compared with the conventional treatment group (P<0.05). Before treatment, the serum NSE, IL-6, and TNF-α levels in the two groups of newborns with HIE were higher than those in the control group; however, after treatment, the levels were significantly lower. In addition, the serum NSE, IL-6, and TNF-α levels in the hydrogen water treatment group were lower than those in the conventional treatment group. Preliminary studies have found that hydrogen water can lower blood lipids and blood sugar, reduce the side effects of radiotherapy used for tumors, treat gout (hyperuricemia), and have a protective effect against pulmonary hypertension [18, 19]. Inflammation plays an essential role in neonatal HIE, and the aim of HIE treatment is to enhance the body’s own antioxidant system by reducing oxidation and fighting against toxic reactive oxygen species. Hydrogen is an ideal and specific radical scavenging species. As an antioxidant against hydroxyl radicals, hydrogen exerted a protective effect in a model of cerebral ischemia-reper- fusion injury [20], which is consistent with our findings in this study. Therefore, hydrogen water treatment can improve brain cell function and reduce brain cell damage and inflammation, thereby greatly improving brain function in newborns with HIE.
The neonatal innate immune response occurs within a few minutes of cerebral ischemia. Inflammatory cytokines that are synthesized and secreted by nerve cells promote the accumulation and activation of leukocytes and stimulate the release of inflammatory mediators in the damage area. These cytokines further enhance this inflammatory effect by inducing the synthesis and release of adhesion molecules, which results in a series of inflammatory reactions, including vascular inflammatory reactions and platelet adhesion and aggregation. IL-6 is an important factor in the central nervous system that regulates the body’s defense. Astrocytes and microglia are activated to produce IL-6 within several minutes of cerebral ischemia, and IL-6 and other inflammatory cytokines induce neuronal apoptosis to aggravate ischemic brain damage [21, 22]. TNF-α, which is mainly produced by mononuclear macrophages, is a polypeptide regulatory factor with a wide range of biological activities; however, the biological activities of TNF-α are determined by its concentration. High concentrations of TNF-α can mediate many pathophysiological processes during inflammatory responses, thereby causing damage to the body’s tissues, organs and systems [23, 24]. NSE is involved in glycolysis in the cytoplasm of neurons and has a biological half-life of approximately 24 h. NSE is mainly expressed in neurons and neuroendocrine cells. After cerebral damage, NSE is released into cerebrospinal fluid and blood and can be used as an early sensitive indicator of nerve cell damage. NSE levels are positively correlated with the severity of HIE [25-28]. Therefore, serum NSE levels can be used to assess the severity of HIE in newborns. This study showed that hydrogen water can reduce levels of NSE, which is the most sensitive biochemical marker of neuronal damage in neonatal HIE. In addition, hydrogen water reduced levels of TNF-α and IL-6, which mediate inflammatory responses. Therefore, hydrogen water is efficacious in the treatment of neonatal HIE.
This study has several limitations. This study was retrospective and was conducted at a single center; therefore, the data are limited. Future studies will require an increased sample size and a multi-center collaboration.
Hydrogen water can promote the remission of HIE clinical symptoms and lower serum NSE, IL-6, and TNF-α levels. Hydrogen water has a protective effect against neonatal HIE, which may represent a new method in the treatment of this disease.
Conflict of interest: The authors declare no conflict of interest.
Acknowledgement
This study was supported by the Science and Technology Development Plan of Tai’an City, Shandong Province (201540707).
References
- [1].Kurihara M., Shishido A., Yoshihashi M., Fujita H., Kohagizawa T., Ida H.. No To Hattatsu Brain Dev. 2014;46(4):265–269. [PubMed] [Google Scholar]
- [2].Shao X.M., Ye H.M., Qiu X.S. Practical neonatology. 4th ed. People’s Medical Publishing House; Beijing: 2011. [Google Scholar]
- [3].Liu F., McCullough L.D.. Inflammatory responses in hypoxic ischemic encephalopathy. Acta Pharmacol Sin. 2013;34(9):1121–1130. doi: 10.1038/aps.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Algra S.O., Groeneveld K.M., Schadenberg A.W., Haas F., Evens F.C., Meerding J.. et al. Cerebral ischemia initiates an immediate innate immune response in neonates during cardiac surgery. J. Neuroinflammation. 2013;1024 doi: 10.1186/1742-2094-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Shang Y., Mu L., Guo X., Li Y., Wang L., Yang W.. et al. Clinical significance of interleukin6, tumor necrosis factor-α and high sensitivity Creactive protein in neonates with hypoxic-ischemic encephalopathy. Exp. Ther. Med. 2014;8:1259–1262. doi: 10.3892/etm.2014.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hu J.C., Ke X.J.. Significance of detecting TNF-α and IL-6 in cerebrospinal fluid in patients with cerebral infarction, China. J. Mod. Med. 2012;229:69–71. [Google Scholar]
- [7].Lu J.X., Chen J.G., Chen H.Y.. Diagnostic value of ultrasound and neuron-specific enolase and their correlation in premature infant with white matter damage. J. Clin. Pediatr. 2012;30:836–839. [Google Scholar]
- [8].Wei L., Wang J., Cao Y., Ren Q., Zhao L., Li X.. Hyperbaric oxygenation promotes neural stem cell proliferation and protects the learning and memory ability in neonatal hypoxic- ischemic brain damage. Int. J. Clin. Exp. Pathol. 2015;8(2):1752–1759. [PMC free article] [PubMed] [Google Scholar]
- [9].Li G.Q., Hu H.W., Zhang Y.L.. Changes of plasma and cerebral spinal fluid ß-endorphin in children with febrile convulsion. J. New Med. 2007;31:400–401. [Google Scholar]
- [10].Pang F.Z.. Efficacy of ganglioside in the treatment of neonatal hypoxic-ischemic encephalopathy. China Modern Doctor. 2011;14:41–42. [Google Scholar]
- [11].Chen Y.L., Jiang J., Miao H.B., Chen X.J., Sun X.J., Li Y.J.. Hydrogen-rich saline attenuates vascular smooth muscle cell proliferation and neointimal hyperplasia by inhibiting reactive oxygen species production and inactivating the Ras-L, RKl /2-ML-Kl /2 and Akt pathways. Int. J. Molmed. 2013;31:597–606. doi: 10.3892/ijmm.2013.1256. [DOI] [PubMed] [Google Scholar]
- [12].Manaenko A., Sun X., Kim C.H., Yan J., Ma Q., Zhang J.H.. PAR-1 antagonist SCH79797 ameliorates apoptosis following surgical brain injury through inhibition of ASK1-JNK in rats. Neurobiol. Dis. 2013;50:13–20. doi: 10.1016/j.nbd.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Tan M., Sun X., Guo L., Su C., Sun X., Xu Z.. Hydrogen as additive of HTK solution fortifies myocardial preservation in grafts with prolonged cold ischemia. Int. J. Cardiol. 2013;167(2):383–390. doi: 10.1016/j.ijcard.2011.12.109. [DOI] [PubMed] [Google Scholar]
- [14].Zhu X.Y., Zhang Q.S.. Re-recognize the diagnosis of neonatal asphyxia. Chin. J. Neonatol. 2011;26:217–220. [Google Scholar]
- [15].Mu D.Z.. Diagnosis and therapy of neonatal hypoxic-ischemic encephalopathy. J. Appl. Clin. Pediatr. 2011;26:1144–1147. [Google Scholar]
- [16].Han Y.K., Yang Y.J., Shao X.M. Neonatal Hypoxic-Ischemic Encephalopathy. People’s Medical Publishing House; Beijing: 2010. [Google Scholar]
- [17].Smith J., Wells L., Dodd K.. The continuing fall in incidence of hypoxic-ischaemic encephalopathy in term infants. BJOG. 2000;107(4):461–166. doi: 10.1111/j.1471-0528.2000.tb13262.x. [DOI] [PubMed] [Google Scholar]
- [18].He B., Zhang Y., Kang B., Xiao J., Xie B., Wang Z.. Protection of oral hydrogen water as an antioxidant on pulmonary hypertension. Mol. Biol. Rep. 2013;40(9):5513–5521. doi: 10.1007/s11033-013-2653-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Schoenfeld M.P., Ansari R.R., Zakrajsek J.F., Billiar T.R., Toyoda Y., Wink D.A.. et al. Hydrogen therapy may reduce the risks related to radiation-induced oxidative stress in space flight. Med. Hypotheses. 2011;76(1):117–118. doi: 10.1016/j.mehy.2010.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ohsawa I., Ishikawa M., Takahashi K., Watanabe M., Nishimaki K., Yamagata K.. et al. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007;13(6):688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- [21].Buono K.D., Goodus M.T., Guardia Clausi M., Jiang Y., Loporchio D., Levison S.W.. Mechanisms of mouse neural precursor expansion after neonatal hypoxia-ischemia. J. Neurosci. 2015;35(23):8855–8865. doi: 10.1523/JNEUROSCI.2868-12.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Xie C., Zhou K., Wang X., Blomgren K., Zhu C.. Therapeutic benefits of delayed lithium administration in the neonatal rat after cerebral hypoxia-ischemia. PLOS ONE. 2014;9:e107192. doi: 10.1371/journal.pone.0107192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Li S.J., Liu W., Wang J.L., Zhang Y., Zhao D.J., Wang T.J.. et al. The role of TNF-α, IL-6, IL-10, and GDNF in neuronal apoptosis in neonatal rat with hypoxic-ischemic encephalopathy. Eur. Rev. Med. Pharmacol. Sci. 2014;18(6):905–909. [PubMed] [Google Scholar]
- [24].Wang Y., Cao M., Liu A., Di W., Zhao F., Tian Y.. et al. Changes of inflammatory cytokines and neurotrophins emphasized their roles in hypoxic-ischemic brain damage. Int. J. Neurosci. 2013;123(3):191–195. doi: 10.3109/00207454.2012.744755. [DOI] [PubMed] [Google Scholar]
- [25].Lv H., Wang Q., Wu S., Yang L., Ren P., Yang Y.. Neonatal hypoxic ischemic encephalopathy-related biomarkers in serum and cerebrospinal fluid. Clin. Chim. Acta. 2015;450:282–297. doi: 10.1016/j.cca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- [26].Merchant N., Azzopardi D.. Early predictors of outcome in infants treated with hypothermia for hypoxic-ischaemic encephalopathy. Dev. Med. Child. Neurol. 2015;57(3):8–16. doi: 10.1111/dmcn.12726. Suppl. [DOI] [PubMed] [Google Scholar]
- [27].Zhang D.S., Bai X.H., Chen D.P., Mu D.Z., Chen J.. Intracerebral transplantation of human umbilical cord-derived mesenchymal stem cells in neonatal rat model of hypoxic-ischemic brain damage: protective effect to injured brain. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16(9):927–932. [PubMed] [Google Scholar]
- [28].Pei X.M., Gao R., Zhang G.Y., Lin L., Wan S.M., Qiu S.Q.. Effects of erythropoietin on serum NSE and S-100B levels in neonates with hypoxic-ischemic encephalopathy. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16(7):705–708. [PubMed] [Google Scholar]