Abstract
A well-designed learning curve is essential for the acquisition of laparoscopic skills: but, are there risk factors that can derail the surgical method? From a review of the current literature on the learning curve in laparoscopic surgery, we identified learning curve components in video laparoscopic cholecystectomy; we suggest a learning curve model that can be applied to assess the progress of general surgical residents as they learn and master the stages of video laparoscopic cholecystectomy regardless of type of patient.
Electronic databases were interrogated to better define the terms “surgeon”, “specialized surgeon”, and “specialist surgeon”; we surveyed the literature on surgical residency programs outside Italy to identify learning curve components, influential factors, the importance of tutoring, and the role of reference centers in residency education in surgery. From the definition of acceptable error, self-efficacy, and error classification, we devised a learning curve model that may be applied to training surgical residents in video laparoscopic cholecystectomy.
Based on the criteria culled from the literature, the three surgeon categories (general, specialized, and specialist) are distinguished by years of experience, case volume, and error rate; the patients were distinguished for years and characteristics. The training model was constructed as a series of key learning steps in video laparoscopic cholecystectomy. Potential errors were identified and the difficulty of each step was graded using operation-specific characteristics. On completion of each procedure, error checklist scores on procedure-specific performance are tallied to track the learning curve and obtain performance indices of measurement that chart the trainee’s progress.
Conclusions. The concept of the learning curve in general surgery is disputed. The use of learning steps may enable the resident surgical trainee to acquire video laparoscopic cholecystectomy skills proportional to the instructor’s ability, the trainee’s own skills, and the safety of the surgical environment. There were no patient characteristics that can derail the methods. With this training scheme, resident trainees may be provided the opportunity to develop their intrinsic capabilities without the loss of basic technical skills.
Keywords: Cholecystectomy, General Surgery, Learning curve, Resident, Surgery
1. Introduction
The concept of a learning curve (LC) was first described by T.P. Wright in 1936 as a function of his theory that the efficiency of airplane component production increased as the experience and skill of the workforce increased [1]. Since then, the concept has spread throughout the manufacturing industry and informatics in software development [2]. The definition of the LC has been variously redefined to compare competitive industrial performance and to evaluate progress and achievement of learning targets [3-4]. In the early 1980s, the LC was introduced in medicine where it is widely recognized as a validated measure of professional responsibility. In recent years, it has evolved from a purely theoretical construct to a parameter for evaluating mortality, morbidity, and postoperative outcomes [1]. According to Wright’s original concept, the time an individual needs to complete a task steadily decreases as production doubles. Applied to surgery, the LC may be defined as the time and/or number of procedures needed to carry out a procedure with a reasonable outcome.1 While the LC in open surgery is based on tactile feedback and direct visualization of anatomic structures, these elements are absent in laparoscopy [5]. In a 2001 study, Figert [6] stated that the LC is essential in laparoscopic surgery for the acquisition of laparoscopic skills even for surgeons experienced in open surgery. Whether the laparoscopic skills necessary for ensuring safe intervention can be acquired through the LC is disputed and numerous studies have attempted to answer this question [7].
The aim of this study was to review the literature on the LC in laparoscopic surgery and to devise a learning curve model for video laparoscopic cholecystectomy (VLC) that may be applied to every patient to evaluate the progress of general surgery residents in laparoscopic skills acquisition.
2. Literature analysis
We interrogated three electronic databases (PubMed, Cochrane, World Wide Science) and consulted a standard Italian language dictionary (Devoto-Oli) to identify learning curve components. The search terms were general surgery, surgeon, specialist, specialty, and resident. Twelve articles and five definitions were selected to delineate the terms general surgery, specialist surgeon, specialized surgeon, and tutor. From this analysis we identified the following learning curve components: total learning curve, influential factors, and importance of the instructor and centers of reference for VLC.
3. Results
3.1. Definition of terms
One of the basic steps to constructing a LC is to identify the roles of the various stakeholders involved [8]. The general surgeon has received training, can execute a wide range of surgical procedures, and handle a reasonably broad caseload of care in general surgery. The specialized surgeon has completed a surgical residency; the specialist surgeon has received further training in a specific procedure, completed the LC for that procedure, and reached an acceptable error rate [9]. The instructor manages, facilitates, and guides the learning process, and serves as a reference person [1,10].
3.2. Self-efficacy
At the center of any learning project is the assessment of a surgeon’s skills, i.e., the demonstrated capability to maintain an adequate level of self-sufficiency [11]. This concept is defined as the intrinsic conviction to be able to reach a determined performance level [12]. Hence, self-sufficiency is the capability to execute a procedure, learn it easily, repeat it, and be able to apply it to other situations. The intrinsic capabilities of a trainee surgeon are considered essential [13].
3.3. Error classification
We also identified common errors that may occur during a surgical procedure [14]. An error is defined as the failure of a planned action or the use of the wrong or impropriate plan to reach an objective [15]. Two methods to classify surgical errors are distinguished: 1) a global rating scale based on observation of an entire surgical procedure and assignment of a total score to performance, and 2) an error checklist in which a procedure is broken down into discrete steps, with a score given to the error enacted at each step, and the error scores added to calculate the total score [16]. Error classification involves evaluation of both the instructor and the trainee [17]. The instructor is evaluated for his/her ability to correctly transfer training in a procedure to a trainee and for his/her ability to recognize an incorrect or hazardous operative maneuver [15,18].
3.4. Acceptable risk
The definition of what constitutes acceptable risk remains debated. The decomposition of a surgical procedure into relevant components has been found to contribute to overall skill performance on a task and enhance patient safety [19]. Step lists are key to risk management and are designed according to a training pathway with proven efficacy. Though breaking a surgical procedure down into basic sequential steps may be considered time wasted, it has been demonstrated to be an effective means to enhance technical proficiency and reduce risks to both trainees and patient [19].
Opinions diverge on how to draw the line between a harmful error and an acceptable error. As a surgical team evolves, the objective is not to eliminate risk, which remains elusive at best, but instead to determine the level of acceptable risk so that procedures can be completed without unwanted incidents [20]. Standardization requires that events be labeled, rather than identified as harmful, so that they can be treated as an occurrence not consistent with routine and classified as “near misses”. Accordingly, acceptable risk may be defined as an acceptable error related to an occurrence that can be managed with a technique of proven efficacy [19].
3.5. Learning curve model
Because cholecystectomy is the most common laparoscopic procedure in general surgery [21-22], we chose it as an example for the learning curve model. On the basis of our surgical experience, tutoring, and didactic learning, we divided the procedure into key procedural steps (Table 1) and defined a learning pathway according to the difficulty of executing these steps, starting from the least to the most complex. Each step was assigned an arbitrary difficulty score (1-3) and a learning pathway was created (Table 2).
Table 1.
Key steps in videolaparoscopic cholecystectomy
| Simple steps | |
|---|---|
| 1. | Establishment of pneumoperitoneum with Veress needle and safe umbilical access |
| 2. | Insertion of trocars and abdominal exploration |
| 3. | Lysis of adherences |
| 4. | Exposure of gallbladder, retraction of Hartmann pouch, and dissection of triangle of Calot |
| 5. | Clipping and section of gallbladder artery and duct |
| 6. | Intraoperative cholangiography |
| 7. | Detachment of gallbladder, removal in endobag, irrigation of abdominal cavity, and placement of abdominal drain |
| 8. | Removal of gallbladder, closure of peritoneum and control, extraction of trocars and closure of port sites |
Table 2.
Key steps in videolaparoscopic cholecystectomy according to degree of difficulty
| Step | Difficulty | |
|---|---|---|
| 1. | Insertion of trocars and abdominal exploration | 1 |
| 2. | Removal of gallbladder, closure of peritoneum and control, extraction of trocars and closure of port sites | 1 |
| 3. | Detachment of gallbladder, removal in endobag, irrigation of abdominal cavity, and placement of abdominal drain | 2 |
| 4. | Clipping and section of gallbladder artery and duct | 2 |
| 5. | Establishment of pneumoperitoneum with Veress needle and safe umbilical access | 2 |
| 6. | Intraoperative cholangiography | 3 |
| 7. | Exposure of gallbladder, retraction of Hartmann pouch, and opening of triangle of Calot | 3 |
| 8. | Lysis of adherences | 3 |
We compared our model to evidence culled from the literature with respect to common errors in VLC [16,20,23-29] and the difficulty inherent to each step (Tables 3,4,5). The instructor responsible for resident training supervises trainees through each step until it has been learned; all without distinction as to the type of patients.
Table 3.
Steps and errors in video laparoscopic cholecystectomy. Part 1.16

Table 4.
Steps and errors in video laparoscopic cholecystectomy. Part 2.16

Table 5.
Errors correlated to steps in video laparoscopic cholecystectomy.[24]
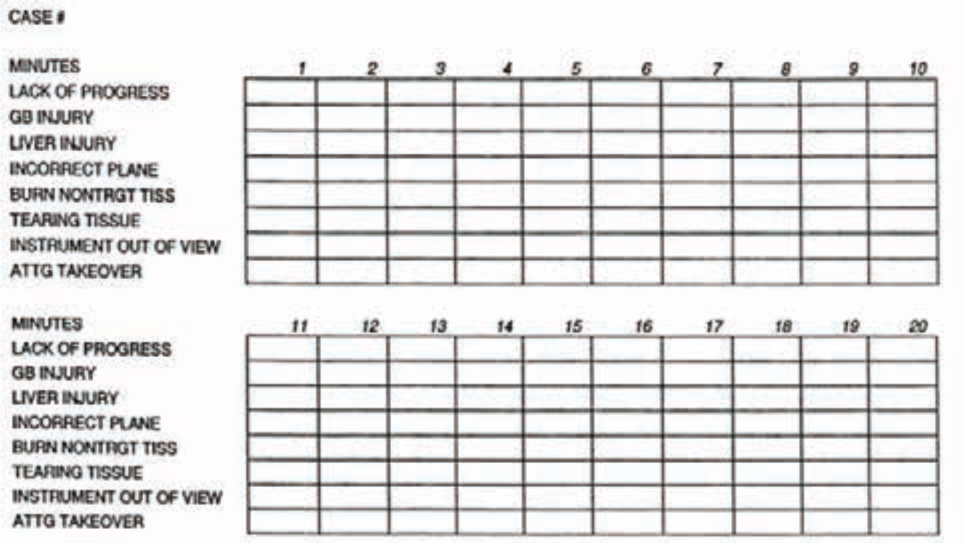
On completion of an operation, an error checklist is compiled and the total performance score is calculated [16] following the surgical error classification system devised by Tang and co-workers [20] (Table 6). These data are recorded for final assessment of the LC objectives, i.e., the trainee’s performance on the key steps of the procedure in the correct manner and order.
Table 6.
Criteria for definition of acceptable error according to Tang et al. [20]

4. Discussion
Surgical training is a gradual process in which the trainee carries out procedures according to standardized maneuvers and learns to manage the difficulties inherent to a given procedure [30]. The arduous LC of laparoscopy results from, and is a measure of, the combined effects of a trainee’s ability to master the skills in the use of complex technologies and the training received to apply them. These factors, coupled with the demand for errorless medicine, have driven the need for training and research worldwide [30-31].
Seems to be no apparent risk factors linked to the type of patient and at his age. In their 2014 study, Kaafarani et al. [32] stated that there is currently no universal approach to the evaluation of the severity of intraoperative adverse events. Therefore, they propose a general evaluation scheme that takes into account the more complex intraoperative maneuvers divided into generic grades of difficulty. The need remains to classify surgical errors through a more accurate analysis of operation-specific characteristics. The present study seeks to meet this need.
The fundamental principle behind a LC in laparoscopy is to provide resident surgeons with the opportunity to carry out as many procedures as possible under the supervision of an expert instructor [30]. The LC for each procedure is complete when the operative time, intraoperative complications, and conversion rate are reduced to a stable level of acceptable error [30,33]
4.1. International context
Controversy surrounds the length of the total LC [34] for resident surgeons to attain an adequate theoretical-practical knowledge base [9]. Organ [35] defines general surgery as the basis for surgical education, research, and patient care [9]. However, fewer and fewer medical students are entering general surgery. In the United States, enrolment in surgical residency programs fell from 12.1% to 6.1% in the 20-year period between 1981 and 2001 [9]. Questionnaire surveys investigating the reasons for the decline in interest cited [36] among other causes of attrition, skepticism toward reference models [37]. USA UCSF Residence Review Committee data show that, starting in the 1990s, the 880 residents in training between 1987 and 1994 performed 948 operations per resident, on average, as principal operating surgeon, a number judged insufficient for gaining adequate training experience [38,39]. The low operative case volume also reflects inadequate involvement of residents in medium and high surgery, as well as in open and laparoscopic surgery [40]. Furthermore, many residency programs fall short of meeting basic training objectives, i.e. to prepare residents for achieving a level equal to that of a specialist surgeon [9].
Moreover, though a surgeon’s skills level is a known predictor for good results, the link between competence and outcome in training modules is tenuous [41]. Data on residency programs in European medical schools are generally scarce. According to a Belgian study, 62% of residents stated that they had received only theoretical training; 66% felt that practical training was inadequate regardless of their residency year [42]; 72% of senior residents postgraduate year (V-VI) stated they had performed fewer than 50 cholecystectomies or laparoscopic appendectomies as principal operator. Few residents had the opportunity to perform advanced procedures. Our analysis also showed that in many reference centers surgical residents have little chance to train laparoscopy skills because of the heterogeneous experience of surgical staff, inadequate learning path definition, and limited case volume of the instructor at the host training institution [9].
Because exposure to technical procedures is often based on available opportunity rather than on standard educational objectives [31], the percentage of operations residents perform as principal operator depends on a center’s case volume. However, while procedures performed by surgical residents take longer on average, as they gain experience through practical training the complications rate turns out to be comparable to that of operations performed by experts [30].
In general, the surgeon’s attitude, starting level, dexterity and anatomic-surgical knowledge all facilitate the learning of laparoscopic skills [43]. Also important is that the reference center conducts both research and training, combining clinical experience and the application of standardized techniques [30].
Importantly, it is neither ethically warranted nor educationally useful that resident surgeons be allowed to enact errors so that they learn not to repeat them; instead, instructors should point out and correct an erroneous maneuver and provide an explanation for the error [44]. We agree with authors who state that surgical errors do not necessarily stem from the individual surgeon executing a particular step in a given procedure but rather from the lack of work organization. When standardization is lacking, overall vision is lost [45].
Contrary to published opinion [9,46], we believe that a training institution need not necessarily be a reference center with a high case volume of a specific procedure. Ideally, besides having a case volume and selection framework in place, such centers will be highly motivated in carrying out training and education programs. In this context, surgical instructors will have demonstrated experience in a specific procedure and a verified record of surgical results in line with the literature. In addition, the instructor should possess adequate motivation for helping residents attain their training objectives and be supported by an organizational structure with access to teachers who provide ongoing summative and formative education. In this way, education is fostered through the shared commitment of the reference center and not dependent on the availability of a single instructor [47].
Our model may enable resident surgeons to attain the skills necessary for executing a procedure at a level of proficiency directly proportional to the tutor’s ability, the resident’s capabilities, and the safety of the surgical environment [48].
Because of the difficulties of the minimally invasive surgery [49-60], the sensitivity of some surgical procedures [61-66] and because of the advancement in medical - surgical setting, [67-73] we believe that a proper learning curve, associated with a correct diagnosis [74-75], is crucial [76-80].
A well-defined surgical residency program that provides for evaluable feedback on performance will enable the resident to be aware of his/her position on the training pathway and the objectives to be achieved [10]. This process can help to reduce the performance anxiety residents face when executing a procedure, or a part thereof, occasionally and in the absence of an adequate training plan [38]. The responsibility of the instructor and the training institution is to include training in the right context so that patient safety is not diminished. The instructor mentoring the resident should be attuned to the resident’s attitudes and know which procedures the resident may safely execute and what level of difficulty the resident can cope with [10].
Application of the training model we propose will give the resident surgeon an opportunity for growth in which to develop his/her own intrinsic capabilities without the loss of basic surgical skills regardless of type of patient. Learning simple procedures sets the path for progressing to more complex ones with greater expertise and awareness of the risk of error.
Glossary
Abbreviations
- LC
learning curve
- VLC
video laparoscopic cholecystectomy
Footnotes
Conflict of interest statement: Authors state no conflict of interest.
References
- [1].Perticone F, Hollands C, Esposito C.. Learning curve. Videochirurgia Pediatrica - Principi di tecnica in laparoscopia, toracoscopia e retroperitoneoscopia pediatrica, 1st ed. Springer Italia. 2010:541–544. Reference to a chapter in an edited book. [Google Scholar]
- [2].Hopper AN, Jamison MH, Lewis WG.. Learning curves in surgical practice. Postgrad Med J. 2007;83:777–779. doi: 10.1136/pgmj.2007.057190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ezey MD, Rubinovitz J.. Using Learning Theory in Assembly Lines for New Products. International Journal of Production Economics. 1991;25:103–109. [Google Scholar]
- [4].Ferrari L, Noe C.. Criteri per l’impiego delle curve di apprendimento.” XX Congresso Nazionale di Impiantistica Industriale”, Capri (NA), Ottobre 1993. Abstract in Congress [Google Scholar]
- [5].Kumar U, Gill IS.. Learning curve in human laparoscopic surgery. Curr Urol Rep. 2006;7:120–124. doi: 10.1007/s11934-006-0070-5. [DOI] [PubMed] [Google Scholar]
- [6].Figert PL, Park AE, Witzke DB, Schwartz RW.. Transfer of training in acquiring laparoscopic skills. J Am Coll Surg. 2001;193:533–537. doi: 10.1016/s1072-7515(01)01069-9. [DOI] [PubMed] [Google Scholar]
- [7].Grantcharov TP, Bardram L, Funch-Jensen P, Rosenberg J.. Assessment of technical surgical skills. Eu J Surg. 2002;168:139–144. doi: 10.1080/110241502320127739. [DOI] [PubMed] [Google Scholar]
- [8].Shrestha BM.. General or specialist surgeons. J Nepal Med Assoc. 2009;48:258–261. [PubMed] [Google Scholar]
- [9].Fernandez-Cruz L.. General surgery as education, not specialization. Ann Surg. 2004;240:932–938. doi: 10.1097/01.sla.0000145966.00037.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gawande AA.. Creating the educated surgeon in the 21st century. Am J Surg. 2001;181:551–556. doi: 10.1016/s0002-9610(01)00638-9. [DOI] [PubMed] [Google Scholar]
- [11].Risucci A, Wolfe KC, Kaul A.. Promoting self-efficacy in minimally invasive surgery training. JSLS. 2009;134 [PMC free article] [PubMed] [Google Scholar]
- [12].Caprara GV, Scabini E, Barbaranelli C. et al. Autoefficacia percepita emotiva e interpersonale e buon funzionamento sociale. Giornale italiano di Psicologia. 1999;4:769–790. [Google Scholar]
- [13].Bandura A.. Self-Efficacy mechanism in human agency. Am Psychol. 1982;37:122–147. [Google Scholar]
- [14].Stajkovic AD, Luthans F.. Self-efficacy and work-related performance: a meta-analysis. Psychol Bull. 1998;124:240–261. [Google Scholar]
- [15].Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human: Building a Safer Health System. National Academy Press; Washington DC: 2000. p. 312. [PubMed] [Google Scholar]
- [16].Eubanks TR, Clements RH, Pohl D. et al. An objective scoring system for laparoscopic cholecystectomy. J Am Coll Surg. 1999;189:566–574. doi: 10.1016/s1072-7515(99)00218-5. [DOI] [PubMed] [Google Scholar]
- [17].Seymour NE, Gallagher AG, Roman SA. et al. Analysis of errors in laparoscopic surgical procedures. Surg Endosc. 2004;18:592–595. doi: 10.1007/s00464-002-8927-2. [DOI] [PubMed] [Google Scholar]
- [18].Gawande AA, Zinner MJ, Studdert DM, Brennan TA.. Analysis of errors reported by surgeons at three teaching hospitals. Surgery. 2003;133:614–621. doi: 10.1067/msy.2003.169. [DOI] [PubMed] [Google Scholar]
- [19].Caprai C.. La pratica chirurgica e il controllo degli errori: una rete di azioni per l’apprendimento riflessivo, paper presented at II Convegno nazionale STS Italia: Catturare Proteo. Tecnoscienza e società della conoscenza in Europa. Università di Genova. 2008; 19th-21st June www.stsitalia.org/papers2008 [Google Scholar]
- [20].Tang B, Hanna GB, Joice P, Cuschieri A.. Identification and categorization of technical errors by Observational Clinical Human Reliability Assessment ( OCHRA) during laparoscopic cholecystectomy. Arch Surg. 2004;139:1215–1220. doi: 10.1001/archsurg.139.11.1215. [DOI] [PubMed] [Google Scholar]
- [21].Grbas H, Kunisek L, Zelic M. et al. Outcome evaluation of 10,317 laparoscopic cholecystectomies: a 17-year experience at a single center. Hepatogastroenterology. 2013;60:1873–1876. [PubMed] [Google Scholar]
- [22].Mattok M, Migaczewski M, Major P. et al. Laparoscopic cholecystectomy in the treatment of gallbladder polypoid lesions – 15 years of experience. Pol Przegl Chir. 2013;85:625–629. doi: 10.2478/pjs-2013-0094. [DOI] [PubMed] [Google Scholar]
- [23].Kala S, Verma S, Dutta G. Difficult Situations in Laparoscopic Cholecystectomy: A Multicentric Retrospective Study. Surg Laparosc Endosc Percutan Tech. 2014;24:484–487. doi: 10.1097/SLE.0b013e31829cebd8. [DOI] [PubMed] [Google Scholar]
- [24].Joice P, Hanna GB, Cuschieri A.. Errors enacted during endoscopic surgery: a human reliability analysis. Appl Ergon. 1998;29:409–414. doi: 10.1016/s0003-6870(98)00016-7. [DOI] [PubMed] [Google Scholar]
- [25].Guerlain S, Adams RB, Turrentine FB. et al. Assessing team performance in the operating room: development and use of a “black-box” reorder and other tools for the intraoperative environment. J Am Coll Surg. 2005;200:29–37. doi: 10.1016/j.jamcollsurg.2004.08.029. [DOI] [PubMed] [Google Scholar]
- [26].Pape-Koehler C, Immenroth M, Sauerland S. et al. Multimedia-based training on internet platforms improves surgical performance: a randomized controlled trial. Surg endosc. 2013;27:1737–1747. doi: 10.1007/s00464-012-2672-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Van Det MJ, Meijerink WJ, Hoff C, Middel B. et al. Effective and efficient learning in the operating theater with intraoperative video-enhanced surgical procedure training. Surg Endosc. 2013;27:2947–2954. doi: 10.1007/s00464-013-2862-2. [DOI] [PubMed] [Google Scholar]
- [28].Vitish-Sharma P, Knowles J, Patel B.. Acquisition of fundamental laparoscopic skills: is a box really as good as virtual reality trainer. Int J Surg. 2011;9:659–661. doi: 10.1016/j.ijsu.2011.08.009. [DOI] [PubMed] [Google Scholar]
- [29].Stanisic V, Milicevic M, Kocev N. et al. Prediction of difficulties in laparoscopic cholecystectomy on the base of routinely available parameters in a smaller reginal hospital. Eur Rev Med Pharmacol Sci. 2014;18:1204–1211. [PubMed] [Google Scholar]
- [30].Pugliese R, Bailey M.. Laparosocpic surgery: the need of training centres to spread knowledge. Journal of medicine and the person. 2008;6:160–163. [Google Scholar]
- [31].Royston CM, Lansdown MR, Brough WA.. Teaching laparoscopic surgery. the need for guidelines. 1994;16:1023–1025. doi: 10.1136/bmj.308.6935.1023. BMJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kaafarani HM, Mavros MN, Hwabejire J. et al. Derivation and validation of a novel severity classification for intraoperative adverse events. J Am Coll Surg. 2014;218:1120–1128. doi: 10.1016/j.jamcollsurg.2013.12.060. [DOI] [PubMed] [Google Scholar]
- [33].Davis SSJ, Husain FA, Lin E. et al. Resident participation in index laparoscopic general surgical cases: impact of the learning environment on surgical outcomes. J Am Coll Surg. 2013;216:96–104. doi: 10.1016/j.jamcollsurg.2012.08.014. [DOI] [PubMed] [Google Scholar]
- [34].Reynolds FD, Goudas L, Zuckerman RS. et al. rural A. community-based program can train surgical residents in advanced laparoscopy. J Am Coll Surg. 2003;197:620–623. doi: 10.1016/S1072-7515(03)00675-6. [DOI] [PubMed] [Google Scholar]
- [35].Organ CHJ.. We are the gatekeepers. Arch Surg. 2003;138:470–474. doi: 10.1001/archsurg.138.5.470. [DOI] [PubMed] [Google Scholar]
- [36].Sanfey H.. General surgery training crisis in America. Br J Surg. 2002;89:132–133. doi: 10.1046/j.0007-1323.2001.02026.x. [DOI] [PubMed] [Google Scholar]
- [37].Schwartz RW, Jarecky RK, Strodel WE. et al. Controllable lifestyle: a new factor in career choice by medical students. Acad Med. 1989;64:606–609. [PubMed] [Google Scholar]
- [38].Moorthy K, Munz Y, Dosis A. et al. The effect of stress-inducing conditions on the performance of a laparoscopic task. Surg Endosc. 2003;17:1481–1484. doi: 10.1007/s00464-002-9224-9. [DOI] [PubMed] [Google Scholar]
- [39].Harness JK, Organ CHJ, Thompson NW.. Operative experience of U.S. general surgery residents in thyroid and parathyroid disease. Surgery. 1995;118:1063–1069. doi: 10.1016/s0039-6060(05)80115-1. [DOI] [PubMed] [Google Scholar]
- [40].Hedrick T, Turrentine F, Sanfey H. et al. Implications of laparoscopy on surgery residency training. Am J Surg. 2009;197:73–75. doi: 10.1016/j.amjsurg.2008.08.013. [DOI] [PubMed] [Google Scholar]
- [41].Patil NG, Cheng SW, Wong J.. Surgical competence. World J Surg. 2003;27:943–947. doi: 10.1007/s00268-003-7098-1. [DOI] [PubMed] [Google Scholar]
- [42].Navez B, Penninck F.. Laparoscopic training: results of a Belgian survey in trainees. Belgian Group for Endoscopic Surgery (BGES) Acta Chir Belg. 1999;99:53–58. [PubMed] [Google Scholar]
- [43].Risucci DA, Lutsky L, Rosati RJ, Tortolani AJ.. Reliability and accuracy of resident evaluations of surgical faculty. Eval Health Prof. 1992;15:313–324. doi: 10.1177/016327879201500304. [DOI] [PubMed] [Google Scholar]
- [44].Rogers DA.. Ethical and Educational Considerations in Minimally Invasive Surgery Training for Practicing Surgeons. Semin Laparosc Surg. 2002;9:206–211. doi: 10.1053/slas.2002.36467. [DOI] [PubMed] [Google Scholar]
- [45].Aggarwall R, Grantcharov TP, Darzi A.. Framework for systematic trining and assessment of technical skills. J Am Coll Surg. 2007;204:697–705. doi: 10.1016/j.jamcollsurg.2007.01.016. [DOI] [PubMed] [Google Scholar]
- [46].Rattner DW.. The need for training opportunities in advanced laparoscopic surgery. Surg Endosc. 2000;90:410–416. doi: 10.1007/s004640080021. [DOI] [PubMed] [Google Scholar]
- [47].Fowler D.. The impact of a full-time director of minimally invasive surgery. Surg Endosc. 2000;103:323–325. doi: 10.1007/s004640000158. [DOI] [PubMed] [Google Scholar]
- [48].Pahl NG, Cheng SWK, Wong J.. Surgical competence. World J Surg. 2003;27:943–947. doi: 10.1007/s00268-003-7098-1. [DOI] [PubMed] [Google Scholar]
- [49].Allaix ME, Giraudo G, Ferrarese A. et al. 10-Year Oncologic Outcomes After Laparoscopic or Open Total Mesorectal Excision for Rectal Cancer. World J Surg. 2016 Jul;14 doi: 10.1007/s00268-016-3631-x. in press. [DOI] [PubMed] [Google Scholar]
- [50].Eretta C, Ferrarese A, Olcese S. et al. Celiac axis compression syndrome: laparoscopic approach in a strange case of Chronic abdominal pain in 71 years old man. Open Med. 2016;11 doi: 10.1515/med-2016-0049. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nano M, Martino V, Ferrarese A, Falcone A.. A brief history of laparoscopy. G Chir. 2012;3(33):53–57. [PubMed] [Google Scholar]
- [52].Martino V, Ferrarese A, Bindi M. et al. Abnormal right hepatic artery injury resulting in right hepatic atrophy: diagnosed by laparoscopic cholecystectomy. Open Med. 2015;10:535–537. doi: 10.1515/med-2015-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ferrarese A, Pozzi G, Borghi F. et al. Malfunctions of robotic system in surgery: role and responsibility of surgeon in legal point of view. Open Med. 2016;11 doi: 10.1515/med-2016-0055. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ferrarese A, Pozzi G, Borghi F. et al. Informed consent in robotic surgery: quality of information and patient perception. Open Med. 2016;11 doi: 10.1515/med-2016-0054. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ferrarese A, Solej M, Enrico S. et al. Elective and emergency laparoscopic cholecystectomy in the elderly: our experience. BMC Surg. 2013;13(Suppl 2):S21. doi: 10.1186/1471-2482-13-S2-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Ferrarese A, Solej M, Enrico S. et al. Diagnosis of incidental gallbladder cancer after laparoscopic cholecystectomy: our experience. BMC Surg. 2013;13(Suppl 2):S20. doi: 10.1186/1471-2482-13-S2-S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ferrarese A, Enrico S, Solej M. et al. Transabdominal pre-peritoneal mesh in inguinal hernia repair in elderly: end point of our experience. BMC Surg. 2013;13(Suppl 2):S24. doi: 10.1186/1471-2482-13-S2-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ferrarese A, Martino V, Enrico S. et al. Laparoscopic repair of wound defects in the elderly: our experience of 5 years. BMC Surg. 2013;13(Suppl 2):S23. doi: 10.1186/1471-2482-13-S2-S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ferrarese A, Martino V, Enrico S. et al. Laparoscopic appendectomy in the elderly: our experience. BMC Surg. 2013;13(Suppl 2):S22. doi: 10.1186/1471-2482-13-S2-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Solej M, Martino V, Mao P. et al. Early versus delayed laparoscopic cholecystectomy for acute cholecystitis. Minerva Chir. 2012 Oct;67(5):381–387. [PubMed] [Google Scholar]
- [61].Ferrarese A, Martino V, Falcone A. et al. Diverticoli duodenali perforati: caso clinico e breve review della letteratura. Chirurgia. 2014 Aprile;27(2):129–131. [Google Scholar]
- [62].Azoulay D, Castaing D, Dennison A. et al. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology. 1994 Jan;19(1):129–132. [PubMed] [Google Scholar]
- [63].Pozzi G, Ferrarese A, Busso M. et al. Percutaneous drainage and sclerosis of mesenteric cysts: literature overview and report of an innovative approach. Int J Surg. 2014;12(Suppl 2):S90–93. doi: 10.1016/j.ijsu.2014.08.372. [DOI] [PubMed] [Google Scholar]
- [64].Ferrarese A, Marola S, Surace A. et al. Fibrin glue versus stapler fixation in laparoscopic transabdominal inguinal hernia repair: a single center 5-year experience and analysis of the results in the elderly. Int J Surg. 2014;12(Suppl 2):S94–98. doi: 10.1016/j.ijsu.2014.08.371. [DOI] [PubMed] [Google Scholar]
- [65].Surace A, Marola S, Benvenga R. et al. Difficult abdominal access in laparoscopic cholecystectomy in elderly patients: our experience and literature review. Int J Surg. 2014;12(Suppl 2):S1–3. doi: 10.1016/j.ijsu.2014.08.369. [DOI] [PubMed] [Google Scholar]
- [66].Gentile V, Ferrarese A, Marola S. et al. Perioperative and postoperative outcomes of perforated diverticulitis Hinchey II and III: open Hartmann’s procedure vs. laparoscopic lavage and drainage in the elderly. Int J Surg. 2014;12(Suppl 2):S86–89. doi: 10.1016/j.ijsu.2014.08.373. [DOI] [PubMed] [Google Scholar]
- [67].Martino V, Ferrarese A, Borello A. et al. An unusual evolution of a case of Klippel-Trenaunay Syndrome. Open Med. 2015;10:498–501. doi: 10.1515/med-2015-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sandrucci S, Garrone C, Mobiglia C. et al. Evaluation of the toxicity induced in rat by the intra-arterial cytostatic infusion and by hepatic dearterialization associated with systemic cytostatic therapy. Bollettino e Memorie della Societa Piemontese di Chirurgia. 1989;59(2):65–77. [Google Scholar]
- [69].Muzio S, Cassini P, Martino V. et al. Transcystic videolaparoscopy for choledocholithiasis with holmium: YAG laser lithotripsy. A case report. Chir Ital. 2008 Jan-Feb;60(1):119–123. [PubMed] [Google Scholar]
- [70].Serra R, Grande R, Butrico L. et al. Effects of a new nutraceutical substance on clinical and molecular parameters in patients with chronic venous ulceration. Int Wound J. 2016 Feb;13(1):88–96. doi: 10.1111/iwj.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Surace A, Ferrarese A, Benvenga R. et al. ACTH-secreting neuroendocrine pancreatic tumor: a case report. Int J Surg. 2014;12(Suppl 1):S222–224. doi: 10.1016/j.ijsu.2014.05.035. [DOI] [PubMed] [Google Scholar]
- [72].Ferrarese A, Borello A, Gentile V. et al. Meso-pancreatectomy for pancreatic neuroendocrine tumor. Int J Surg. 2014;12(Suppl 1):S123–125. doi: 10.1016/j.ijsu.2014.05.031. [DOI] [PubMed] [Google Scholar]
- [73].Serra R, Gallelli L, Conti A. et al. The effects of sulodexide on both clinical and molecular parameters in patients with mixed arterial and venous ulcers of lower limbs. Drug Des Devel Ther. 2014 May;13(8):519–527. doi: 10.2147/DDDT.S61770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Priola AM, Priola SM, Volpicelli G. et al. Accuracy of 64-row multidetector CT in the diagnosis of surgically treated acute abdomen. Clin Imaging. 2013 Sep-Oct;37(5):902–907. doi: 10.1016/j.clinimag.2013.02.016. [DOI] [PubMed] [Google Scholar]
- [75].Ferrarese A, Enrico S, Solej M. et al. Laparoscopic management of non-midline incisional hernia: A multicentric study. Int J Surg. 2016 Jun 21 doi: 10.1016/j.ijsu.2016.06.023. pii: S1743–9191(16)30181–9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [76].Surace A, Ferrarese A, Marola S. et al. Endorectal ultrasound in the diagnosis of rectal cancer: accuracy and criticies. Int J Surg. 2014;12(Suppl 2):S99–102. doi: 10.1016/j.ijsu.2014.08.370. [DOI] [PubMed] [Google Scholar]
- [77].Berti S, Ferrarese A, Feleppa C. et al. Laparoscopic perspectives for distal biliary obstruction. Int J Surg. 2015 Sep;21(Suppl 1):S64–67. doi: 10.1016/j.ijsu.2015.04.092. [DOI] [PubMed] [Google Scholar]
- [78].Surace A, Ferrarese A, Marola S. et al. Abdominal compartment syndrome and open abdomen management with negative pressure devices. Ann Ital Chir. 2015 Jan-Feb;86(1):46–50. [PubMed] [Google Scholar]
- [79].Ferrarese A, Falcone A, Solej M. et al. Surgeon’s clinical valuation and accuracy of ultrasound in the diagnosis of acute appendicitis: A comparison with intraoperative evaluation. Five years experience. doi: 10.1016/j.ijsu.2016.05.052. [DOI] [PubMed] [Google Scholar]
- [80].Marola S, Ferrarese A, Solej M. et al. Management of venous ulcers: State of the art. Int J Surg. 2016 Jun 21 doi: 10.1016/j.ijsu.2016.06.015. in press. [DOI] [PubMed] [Google Scholar]


