Abstract
The aim of this meta-analysis was to evaluate whether there was an association between glutathione S-transferase M1(GSTM1)gene polymorphism and Parkinson’s disease (PD) susceptibility by pooling published data.
We performed comprehensive electronic database search for articles published between February12,2015 and April30 2016. The published case-control or cohort studies related to GSTM1 gene polymorphism and Parkinson’s disease susceptibility were screened, reviewed, and included in this meta-analysis. The correlation between GSTM1 gene polymorphism and PD susceptibility was expressed by odds ratio (OR) and its corresponding 95% confidence interval (95%CI). Publication bias was evaluated by Begg’s funnel plot and Egger’s line regression test. All analysis was done by stata11.0 software.
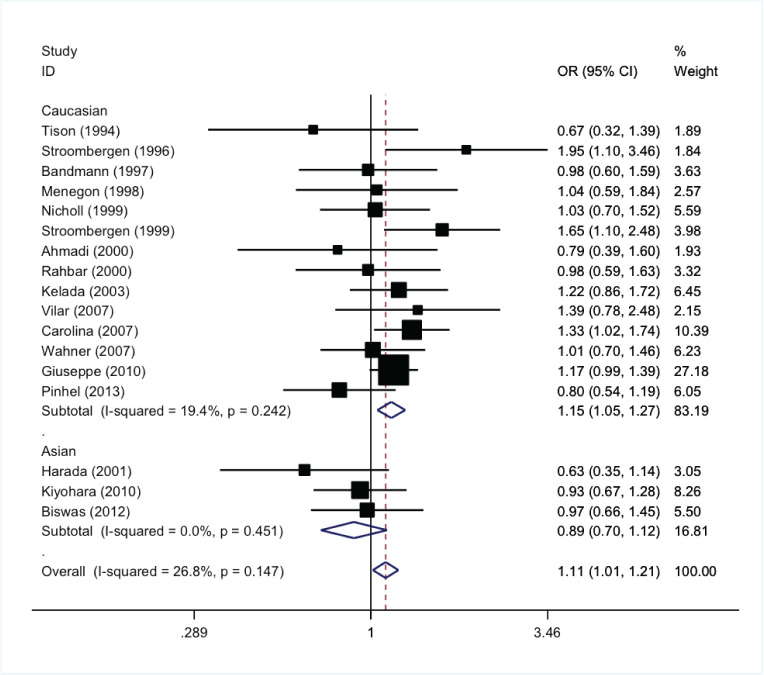
After searching the PubMed, EMBASE, and CNKI databases, seventeen case-control studies with 3,538 PD and 5,180 controls were included in the final meta-analysis. The data was pooled by a fixed-effect model for lack of statistical heterogeneity across the studies; the results showed GSTM1 null expression can significant increase the susceptibility of PD (OR=1.11, 95% CI:1.01-1.21, P<0.05). Subgroup analysis indicated GSTM1 gene polymorphism was associated with PD susceptibility in the Caucasian ethnic group (OR=1.15, 95% CI:1.05-1.27, P<0.05) but not in the Asian ethnic group (OR=0.89, 95% CI:0.70-1.12, P>0.05). Begg’s funnel plot and Egger’s line regression test showed no significant publication bias.
Based on the present evidence, GSTM1 null expression can significant increase the susceptibility of PD in persons of Caucasian ethnicity.
Keywords: Glutathione S-transferase M1gene, Parkinson’s disease, Susceptibility, Meta-analysis
1. Introduction
Parkinson’s disease (PD) is a degenerative disorder of the central nervous system, mainly affecting the motor system. In most patients, it is considered idiopathic. But recent studies have demonstrated that some cases can be attributed to known genetic factors such as single nucleotide polymorphisms. Glutathione S-transferases (GSTs) are a family of cytosolic enzymes involved in the detoxification of various exogenous, as well as endogenous, reactive species [1,2]. The GST family alleviates oxidative stress and can expel such toxicants from the body by increasing watersolubility. The coding region of GSTM1 polymorphisms was considered to confer different catalytic activities [3]. The GSTM1 gene with the homozygous deletion polymorphism is the null genotype. For the null genotype, the protein lacks enzyme activities. Theoretically, theGSTM1 null genotype could cause neuronal demise that may increase PD risk. Several case-control studies have discussed the relationship between GSTM1 polymorphisms and PD susceptibility. However, for the small number of cases and controls included in each of the studies, the statistical power was limited and could not reach a consistent conclusion. Thus, in this study, we searched and included the open published papers related to the association between GSTM1gene polymorphism and PD susceptibility by pooling the published to further discuss their correlation.
1.1. Search strategy
Open published case-control or cohort studies about glutathione S-transferase M1(GSTM1)gene polymorphism and PD risk were searched and screened from the databases of PubMed, EMBASE and CNKI by two reviewers (Chen Weikang and Li Jie) independently from February12 2015 to April 30 2016. The following search terms: “Parkinson’s disease”, “Parkinsons disease”, “Parkinson disease”, “PD”, “gstml”, “Glutathione S-transferase M1” and “polymorphism” were used as free text words in the publication-searching procedure. The studies were restriction to humans, with the language restriction English and Chinese. All potential relevant case-control or cohort studies were evaluated, and additional citations of the included articles were further investigatedto identify additional suitable studies.
1.2. Inclusion criteria and data extraction
The inclusion criteria were: (1) Open published case-control or cohort studies related to GSTM1 gene polymorphism and PD susceptibility (2) PD was confirmed by clinical diagnosis; (3) Single-nucleotide polymorphism of GSTM1 gene was clearly reported or can be calculated from the original included studies; (4) The genotype distributions of controls should be accord with Hardy-Weinberg equilibrium (HWE). (5) The language was limited to English or Chinese. The exclusion criteria were: (1) Case report or review publications; (2) The original paper did not provide enough data to pool the odds ratio of GSTM1 gene polymorphism and PD susceptibility; (3) The paper was published in other languages.
1.3. Data extraction
Lu Liping and Lan Likang independently extracted the data from all the included studies. If there was disagreement for paper inclusion/exclusion or data extraction, the third reviewer (Chen Weikang) was consulted. The general characteristics of publication year, patients’ ethnicity, age, gender, country the research was done, and genotyping method were extracted from the original studies. The number of patient controls, the distribution of GSTM1 null and positive expression subjects were further carefully extracted, which was important for calculation of the odds ratio.
1.4. Statistical analysis
The data was analyzed by stata11.0 software (StataCorp LP,http://www.stata.com). The odds of null and present genotype in PD patients and healthy controls was showed by odds ratio (OR) and its corresponding 95% confidence intervals (95% CI). Statistical heterogeneity among studies was evaluated byI2 [4]. If significant heterogeneity was found (I2>50%), the random-effect method (Der Simonian-Laird method) was used to pool the data. Inversely, fixed-effect method was applied. The publication bias was evaluated by Begg’s funnel plot and Egger’s line regression test [5].
2. Results
2.1. General characteristics of the included studies
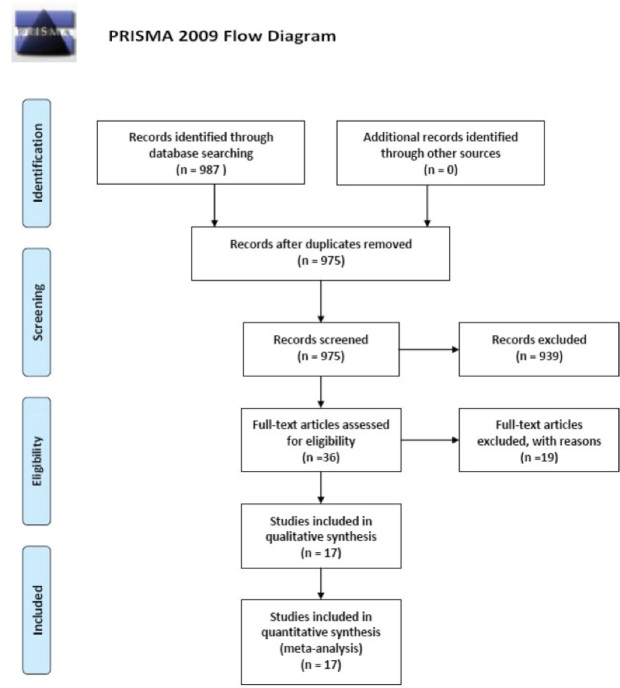
After searching PubMed, EMBASE, and CNKI databases, 987 studies were initially found. Twelve studies were excluded for duplicated publication or duplicated used data. After reading the title and abstract, 914 publications were excluded. And after reading the entire paper, 44 publications were excluded and 17 case-control studies with 3,538 PD and 5,180 controls were finally included in this meta-analysis (Figure 1). For the included 17 case-control studies, 14 papers were conducted on persons of Caucasian ethnicity and other 3 studies were on persons of Asian ethnicity. The general characteristics of the included 17 papers are shown in Table 1.
Figure 1.
The searching strategy flow chart
Table 1.
The general characteristics of the included 17studies
| Author | Year | Area | Genotyping | PD | Control | ||
|---|---|---|---|---|---|---|---|
| Null | Positive | Null | Positive | ||||
| Tison[6] | 1994 | French | NA | 20 | 20 | 60 | 40 |
| Stroombergen[7] | 1996 | Caucasian | PCR | 82 | 40 | 43 | 41 |
| Bandmann[8] | 1997 | Europe | NA | 115 | 85 | 58 | 42 |
| Menegon[9] | 1998 | Caucasian | PCR | 49 | 46 | 48 | 47 |
| Nicholl[10] | 1999 | Caucasian | PCR | 101 | 104 | 100 | 106 |
| Stroombergen[11] | 1999 | Caucasian | PCR | 105 | 62 | 114 | 111 |
| Ahmadi[12] | 2000 | Swedish | PCR-RFLP | 17 | 18 | 154 | 129 |
| Rahbar[13] | 2000 | German | PCR | 76 | 73 | 51 | 48 |
| Harada[14] | 2001 | Japanese | PCR | 32 | 49 | 51 | 49 |
| Kelada[15] | 2003 | Caucasian | NA | 108 | 106 | 149 | 178 |
| Vilar[16] | 2007 | Caucasian | PCR-RFLP | 58 | 36 | 51 | 44 |
| Carolina[17] | 2007 | Amerindian | NA | 164 | 185 | 244 | 367 |
| Wahner[18] | 2007 | Caucasian | NA | 114 | 121 | 106 | 114 |
| Giuseppe[19] | 2010 | Caucasian | NA | 405 | 337 | 973 | 950 |
| Kiyohara[20] | 2010 | Japanese | Taqman assays | 122 | 115 | 197 | 172 |
| Biswas[21] | 2012 | Indian | PCR | 101 | 230 | 55 | 122 |
| Pinhel[22] | 2013 | Brazil | PCR-RFLP | 112 | 130 | 86 | 80 |
2.2. Statistical Heterogeneity evaluation
The statistical heterogeneity among studies was evaluated by I2 test. For all the included studies, the I2=26.8%, which indicated no statistical heterogeneity across the 17 manuscripts. Subgroup analysis also showed no statistical heterogeneity in Caucasian ethnic group (I2=19.4%) and Asian ethnic group (I2=0.00%). Because of no statistical heterogeneity across the studies, all data was pooled by fixed effects model.
2.3. Meta-analysis
The data was pooled by fixed effect model for the lack of statistical heterogeneity across the studies; the result showed GSTM1 null expression can significantly increase susceptibility of PD (OR=1.11, 95% 0:1.01-1.21, P < 0.05), Figure 2.
Figure 2.
The forest plot of GSTM1 polymorphism and PD susceptibility
2.4. Subgroup analysis
The association between GSTM1 gene polymorphism and PD susceptibility was further analyzed as related to the patient’s ethnicity. GSTM1 gene polymorphism was associated with PD susceptibility in the Caucasian ethnic group (OR=1.15, 95% CI:1.05-1.27, P<0.05) but not in the Asian ethnic group (OR=0.89, 95% CI:0.70-1.12, P>0.05).
2.5. Publication bias
The Begg’s funnel plot and Egger’s line regression test were used to evaluate publication bias [5]. The Begg’s funnel plot was somewhat asymmetrical at the bottom (Figure 3). However, Egger’s line regression test, which can give exact extent of asymmetry of the funnel plot, did not indicate any statistical evidence for publication bias (t=-1.28, P=0.22).
Figure 3.
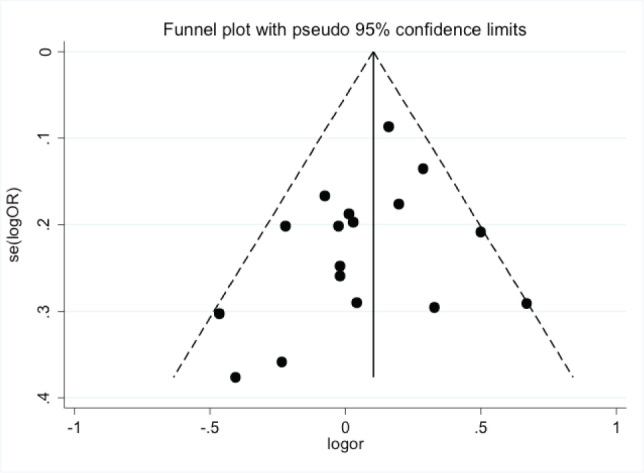
Begg’s funnel plot for GSTM1 polymorphism and PD susceptibility
3. Discussion
Glutathione S-transferases (GSTs) are a family of related isoenzymes protect against xenobiotic chemicals that continuously enter the body, largely through mucous membranes [23]. The GST family alleviates oxidative stress by expelling toxicants from the body by increasing water-solubility. To date, eight types of different GSTs, namely GSTα, GSTκ, GSTδ, GSTω, GSTμ, GSTπ, GSTξ, and GSTθ have been found in humans and other mammals [24]. Several previously published studies have discussed the relationship between GST family polymorphisms and PD susceptibility [18-20]. Pinhel and his colleagues performed a population-based case-control study to evaluate the influence of genetic variants of GSTT1/GSTM1 and their association with PD [22]. In this study, the authors included 254 cases with PD and 169 healthy controls. The genotype of GSTT1/GSTM1 was tested by PCR assay. They found that the present and deletion of GSTT1/GSTM1 were not statistical different in PD and healthy controls which indicating that there was no significant association between GSTT1/GSTM1 present/absence polymorphism and PD susceptibility. However, another case-control study performed by Stroombergen et al found the opposite results [11]. In their study, the authors included 167 PD and 225 non-neurological disease subjects. The association between GSTM1 present/absence polymorphism and PD was investigated by PCR, using primers specific for both genes. The authors found that males with a deletion of the GSTM1or GSTT1gene were more susceptible to PD. Therefore, the results for the above two independent case-control studies were not in agreement with each other. Because of the small number of cases and controls included in each of the individual study, the statistical power was limited and could not reach a consistent conclusion. Thus, in this study, we searched and included the open published papers related to the correlation between GSTM1gene polymorphism and PD susceptibility by pooling the published data to further discuss their correlation. In the present study, 17 case-control publications with 3,538 PD and 5,180 controls were included in this meta-analysis. All of the included manuscripts were published in English. The pooled results showed persons with the GSTM1 null genotype had an increased risk of developing PD (odds ratio 1.11, 95% CI1.01-1.21). The subgroup analysis indicated GSTM1 gene polymorphism was associated with PD susceptibility in the Caucasian ethnic group but not in the Asian ethnic group. The lack of correlation for GSTM1 gene polymorphism and PD susceptibility in the Asian ethnic group maybe related to the small number of studies (only 3) included in this meta-analysis with limited statistical power. It is believed that the homozygous deletion of GSTM1*0 (null expression) could result in a lack of enzyme activity. For that reason, we considered that the inactivity of a phase II enzyme may contribute to the increased susceptibility to PD. However, the cause of PD is complex and related to a variety of genes. The gene–gene and gene–environment interactions may have more obvious effects on the incidence of PD. We suggest that the entire genome-wide analysis may need to be further evaluated for gene polymorphism and PD susceptibility.
Footnotes
Conflict of interest statement: Authors state no conflict of interest.
References
- [1].Ketterer B. Protective role of glutathione and glutathione transferases in mutagenesis and carcinogenesis[J] Mutat Res. 1988;202(2):343–361. doi: 10.1016/0027-5107(88)90197-2. [DOI] [PubMed] [Google Scholar]
- [2].Hengstler JG, Arand M, Herrero ME. et al. Polymorphisms of N-acetyltransferases, glutathione S-transferases, microsomal epoxide hydrolase and sulfotransferases: influence on cancer susceptibility[J]. Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer. 1998;154:47–85. doi: 10.1007/978-3-642-46870-4_4. [DOI] [PubMed] [Google Scholar]
- [3].Yang LM, Li XH, Bao CF. Glutathione S-transferase P1 and DNA polymorphisms influence response to chemotherapy and prognosis of bone tumors[J] Asian Pac J Cancer Prev. 2012;13(11):5883–5886. doi: 10.7314/apjcp.2012.13.11.5883. [DOI] [PubMed] [Google Scholar]
- [4].Higgins JP, Thompson SG, Deeks JJ. et al. Measuring inconsistency in meta-analyses[J] BMJ. 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Egger M, Davey SG, Schneider M. et al. Bias in meta-analysis detected by a simple, graphical test[J] BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tison F, Coutelle C, Henry P. et al. Glutathion S-transferase (class mu) phenotype in Parkinson’s disease[J] Mov Disord. 1994;9(1):117–118. doi: 10.1002/mds.870090128. [DOI] [PubMed] [Google Scholar]
- [7].Stroombergen MC, Waring RH, Bennett P. et al. Determination of the GSTM1 gene deletion frequency in Parkinson’s disease by allele specific PCR[J] Parkinsonism Relat Disord. 1996;2(3):151–154. doi: 10.1016/1353-8020(96)00014-4. [DOI] [PubMed] [Google Scholar]
- [8].Bandmann O, Vaughan J, Holmans P, Association of slow acetylator genotype for N-acetyltransferase 2 with familial Parkinson’s disease[J] 9085. Vol. 350. Lancet; London, England: 1997. pp. 1136–1139. [DOI] [PubMed] [Google Scholar]
- [9].Menegon A, Board PG, Blackburn AC, Parkinson’s disease, pesticides, and glutathione transferase polymorphisms[J] 9137. Vol. 352. Lancet; London, England: 1998. pp. 1344–1346. [DOI] [PubMed] [Google Scholar]
- [10].Nicholl DJ, Bennett P, Hiller L. et al. A study of five candidate genes in Parkinson’s disease and related neurodegenerative disorders. European Study Group on Atypical Parkinsonism[J] Neurology. 1999;53(7):1415–1421. doi: 10.1212/wnl.53.7.1415. [DOI] [PubMed] [Google Scholar]
- [11].Stroombergen MC, Waring RH. Determination of glutathione S-transferase mu and theta polymorphisms in neurological disease[J] Hum Exp Toxicol. 1999;18(3):141–145. doi: 10.1177/096032719901800302. [DOI] [PubMed] [Google Scholar]
- [12].Ahmadi A, Fredrikson M, Jerregard H. et al. GSTM1 and mEPHX polymorphisms in Parkinson’s disease and age of onset[J] Biochem Biophys Res Commun. 2000;269(3):676–680. doi: 10.1006/bbrc.2000.2338. [DOI] [PubMed] [Google Scholar]
- [13].Rahbar A, Kempkes M, Muller T, Glutathione S-transferase polymorphism in Parkinson’s disease[J] 3. Vol. 107. Journal of neural transmission; Vienna, Austria 1996: 2000. pp. 331–334. [DOI] [PubMed] [Google Scholar]
- [14].Harada S, Fujii C, Hayashi A. et al. An association between idiopathic Parkinson’s disease and polymorphisms of phase II detoxification enzymes: glutathione S-transferase M1 and quinone oxidoreductase 1 and 2[J] Biochem Biophys Res Commun. 2001;288(4):887–892. doi: 10.1006/bbrc.2001.5868. [DOI] [PubMed] [Google Scholar]
- [15].Kelada SN, Stapleton PL, Farin FM. et al. Glutathione S-transferase M1, T1, and P1 polymorphisms and Parkinson’s disease[J] Neurosci Lett. 2003;337(1):5–8. doi: 10.1016/s0304-3940(02)01286-7. [DOI] [PubMed] [Google Scholar]
- [16].Vilar R, Coelho H, Rodrigues E. et al. Association of A313 G polymorphism (GSTP1*B) in the glutathione-S-transferase P1 gene with sporadic Parkinson’s disease[J] European journal of neurology. 2007;14(2):156–161. doi: 10.1111/j.1468-1331.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- [17].Perez-Pastene C, Graumann R, Diaz-Grez F. et al. Association of GST M1 null polymorphism with Parkinson’s disease in a Chilean population with a strong Amerindian genetic component[J] Neurosci Lett. 2007;418(2):181–185. doi: 10.1016/j.neulet.2007.03.024. [DOI] [PubMed] [Google Scholar]
- [18].Wahner AD, Glatt CE, Bronstein JM. et al. Glutathione S-transferase mu, omega, pi, and theta class variants and smoking in Parkinson’s disease[J] Neurosci Lett. 2007;413(3):274–278. doi: 10.1016/j.neulet.2006.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].De Palma G, Dick FD, Calzetti S. et al. A case-control study of Parkinson’s disease and tobacco use: gene-tobacco interactions[J] Mov Disord. 2010;25(7):912–919. doi: 10.1002/mds.22980. [DOI] [PubMed] [Google Scholar]
- [20].Kiyohara C, Miyake Y, Koyanagi M. et al. GST polymorphisms, interaction with smoking and pesticide use, and risk for Parkinson’s disease in a Japanese population[J] Parkinsonism Relat Disord. 2010;16(7):447–452. doi: 10.1016/j.parkreldis.2010.04.009. [DOI] [PubMed] [Google Scholar]
- [21].Biswas A, Sadhukhan T, Bose K. et al. Role of glutathione S-transferase T1, M1 and P1 polymorphisms in Indian Parkinson’s disease patients[J] Parkinsonism Relat Disord. 2012;18(5):664–665. doi: 10.1016/j.parkreldis.2011.09.019. [DOI] [PubMed] [Google Scholar]
- [22].Pinhel MA, Sado CL, Longo GS. et al. Nullity of GSTT1/GSTM1 related to pesticides is associated with Parkinson’s disease[J] Arquivos de neuro-psiquiatria. 2013;71(8):527–532. doi: 10.1590/0004-282X20130076. [DOI] [PubMed] [Google Scholar]
- [23].Coles BF, Kadlubar FF. Human alpha class glutathione S-transferases: genetic polymorphism, expression, and susceptibility to disease[J] Methods Enzymol. 2005;401:9–42. doi: 10.1016/S0076-6879(05)01002-5. [DOI] [PubMed] [Google Scholar]
- [24].Salinas AE, Wong MG. Glutathione S-transferases – a review[J] Curr Med Chem. 1999;6(4):279–309. [PubMed] [Google Scholar]