Abstract
Modulation of dopamine (DA) released by serotonin-2 (5-HT2) receptors has been implicated in the mechanism of action of antipsychotic drugs. The mesocortical DA system has been implicated particularly in the cognitive deficits observed in schizophrenia. Agonism at 5-HT2A receptors in the prefrontal cortex is associated with increases in cortical DA release. Evidence indicates that 5-HT2A receptors in the cortex regulate mesocortical DA release through stimulation of a “long-loop” feedback system from the PFC to the VTA and back. However, a causal role for VTA glutamate in the 5-HT2-induced increases in PFC DA has not been established. The present study does so by measuring 5-HT2 agonist-induced DA release in the cortex after infusions of glutamate antagonists into the VTA. Infusions of a combination of a NMDA (AP-5: 2-amino-5-phosphopentanoic acid) and an AMPA/kainate (CNQX: 6-cyano-7-nitroquinoxaline-2,3-dione) receptor antagonist into the VTA blocked the increases in cortical DA produced by administration of the 5-HT2 agonist DOI [(±)-2,5-Dimethoxy-4-iodoamphetamine] (2.5 mg/kg s.c.). These results demonstrate that stimulation of glutamate receptors in the VTA is necessary for 5-HT2 agonist-induced increases in cortical DA.
Keywords: antipsychotic, prefrontal cortex, microdialysis, schizophrenia, AMPA, NMDA
Introduction
Multiple studies have demonstrated that dopamine (DA) systems are regulated by serotonin (5-HT) receptors (Alex and Pehek, 2007). The serotonin 5-HT2A receptor is of particular interest due to its role in hallucinogenic drug action and its putative involvement in atypical antipsychotic drug mechanisms. A substantial body of literature has shown that 5-HT2A receptors in the prefrontal cortex (PFC) regulate mesocortical DA release (Bortolozzi et al., 2005; Gobert and Millan, 1999; Pehek et al., 2001; 2006). The mesocortical DA system has been implicated particularly in the cognitive deficits observed in disorders such as schizophrenia (Weinberger, 1987). 5-HT2A receptors are localized, in part, to the apical dendrites of pyramidal cells in the rat PFC (Cornea-Hebert et al., 1999; Hamada et al., 1998; Jakab and Goldman-Rakic, 1998; Willins et al., 1997 Weber and Andrade, 2010). More recent data has found that a large number of pyramidal cells projecting to the midbrain ventral tegmental area (VTA), the DA cell body site of origin of the mesocortical tract, contain 5-HT2A receptors (Vasquez-Borsetti et al., 2009). Furthermore, pyramidal neurons projecting from the PFC innervate DA cells in the VTA (Sesack and Pickel, 1992).
These data suggest that cortical 5-HT2A receptor regulation of mesocortical DA is mediated by actions on a “long-loop” neuronal circuit involving glutamatergic corticotegmental projections to DA cells in the VTA (Vasquez-Borsetti et al., 2009). We have previously published data to support such a circuit (Pehek et al., 2006). In addition to increasing DA in the PFC, injections of the 5-HT2 agonist DOI also increased glutamate efflux in the VTA, which was blocked by intracortical infusions of the selective 5-HT2A antagonist M100907. These findings indicate that the effects of DOI are mediated by 5-HT2A receptors localized in the PFC. Furthermore, they demonstrate that 5-HT2A-induced increases in PFC DA are correlated with increases in VTA glutamate. However, they do not establish causality. A more direct test of the hypothesis is necessary and is the objective of the present work. This experiment determined if increases in glutamatergic tone in the VTA are necessary for 5-HT2A agonist-induced cortical DA release.
Dual probe in vivo microdialysis in conscious rats was employed followed by measurements of dialysate DA with HPLC and electrochemical detection. We tested if concurrent blockade of both AMPA and NMDA receptors in the VTA would attenuate the increase in dialysate DA produced by administration of the 5-HT2 agonist DOI. A mixture of the NMDA antagonist AP-5 and the AMPA/kainate receptor antagonist CNQX was infused by reverse dialysis into the VTA. It was hypothesized that such infusions would block DOI-induced increases in cortical DA.
Experimental Procedures
Animals
Male Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing between 200–350 gms at the time of surgery, were used for all experiments. Rats were housed two per cage in a temperature controlled room with a 12hr/12hr light/dark cycle. Food and water were available ad libitum. All animal procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the local animal care committee.
Surgery
Rats were anesthetized with a mixture of xylazine and ketamine (6 and 70 mg/kg, respectively; administered i.m.) and mounted in a stereotaxic frame. Microdialysis probes were implanted into the PFC (+3.2 AP, ML 0.8, DV −5.5) and the VTA (−5.60 AP, ML 0.6, DV −8.4) (Paxinos and Watson, 1998; see Fig. 1). Placements were ipsilateral and approximately half of the placements were on the right and half on the left. The probes were then secured in place with three set screws covered with cranioplastic cement. Probe locations were verified histologically at the completion of the experiments. If improperly placed, animals were excluded from the experiments.
Fig 1.
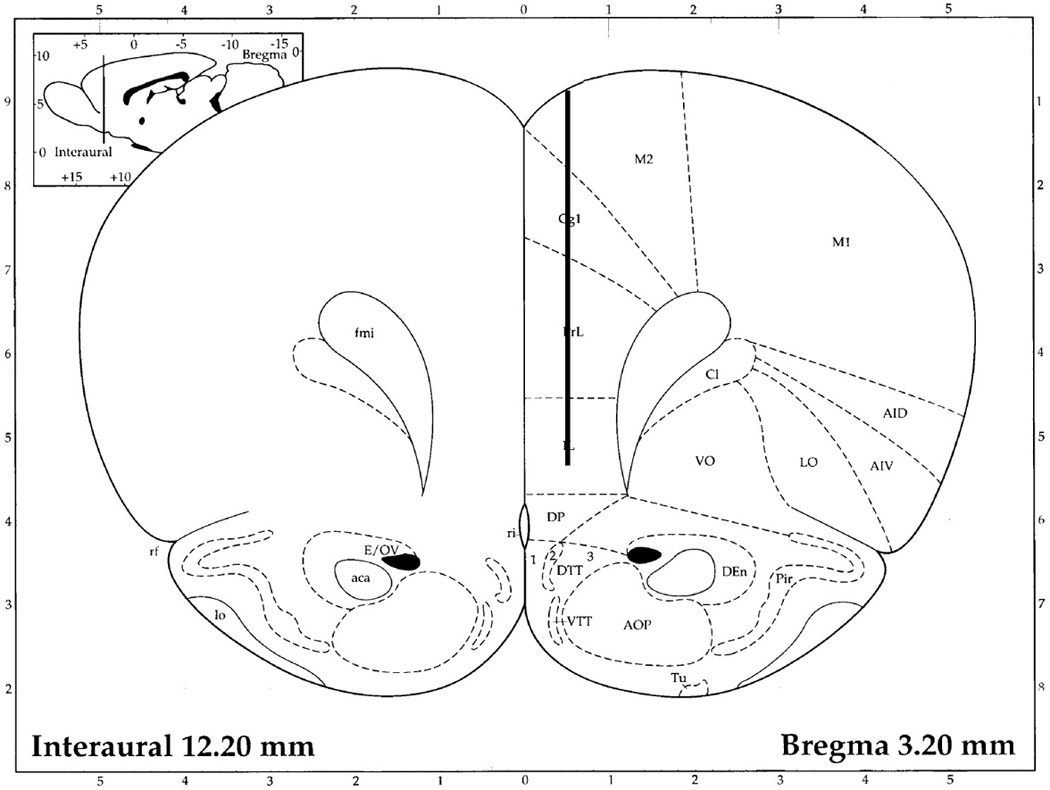
Schematic of the location of the microdialysis probes. The lines in red represent the dialysis membrane. A: prefrontal cortex, B: ventral tegmental area. aca: anterior commissure, anterior; AOP: anterior olfactory nucleus posterior; CA3: field CA3 of the hippocampus; Cg1: cinglulate cortex area 1; Cl: claustrum; csc: commissure sup colliculus; cp: cerebral peduncle; DP: dorsal peduncular cortex; fmi: forceps minor corpus callosum; fr: fasiculus retroflexus; IL: infralimbic cortex; M2: secondary motor cortex; ml: medial lemniscus; mp: mammillary peduncle; PrL: prelimbic cortex; SNCD: substantia nigra, compacta, dorsal tier; SNR: substantia nigra reticulata.
Microdialysis
Microdialysis probes were of a concentric flow design (Yamamoto and Pehek, 1990). Average recovery for DA was 10–15%. PFC probes were constructed with a 5.0 mm active dialyzing surface membrane (Spectra/Por Hollow, MW cutoff = 13,000, diameter = 240 µm) to effectively dialyze from the dorsal anterior cingulate to the most ventral region of the infralimbic PFC. VTA probes were constructed with a 1.0 mm active dialyzing surface at the most ventral extension of the probe to effectively dialyze the mediolateral parabrachial and paranigral VTA (see Fig 1). The tips of the probes (approximately 0.3 mm) were plugged with glue and thus did not recover analyte. The afternoon prior to microdialysis experiments animals were placed in clear Plexiglas microdialysis chambers (Harvard Apparatus, Hollister, MA, USA) with food and water available ad libitum. Animals were tethered to counterbalance arms (Instech, Plymouth Meeting, PA, USA) that permitted free movement about the chamber. 18–24 hrs after probe insertion, a micro-infusion pump (PHD 2000™, Harvard Apparatus) and liquid swivel (Instech) were used to perfuse a modified Dulbecco’s artificial cerebrospinal fluid (aCSF) buffer solution (137 mM NaCl, 3 mM KCl, 1.2 mM MgSO4, 0.4 mM KH2PO4, with 1.2 mM CaCl2 and 10 mM glucose; pH 7.4) through the probes. Samples were collected every 30 min after baseline levels of DA were stable (typically 2–3 hrs.). After baseline collections, drugs dissolved in the aCSF were administered by reverse dialysis. Tubing connections were switched manually while maintaining a constant flow rate and collection volume. Samples were immediately analyzed for DA content by HPLC.
Drugs
The 5-HT2 agonist (±)-DOI hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO, USA). DOI was injected systemically (s.c.) and was dissolved in water (2.5 mg/kg/ml, dose refers to the salt). The AMPA/kainate antagonist CNQX disodium salt was obtained from Sigma-Aldrich. The NMDA antagonist (+/−)AP-5 was obtained from Tocris Bioscience (Ellisville, MO, USA). Since both AMPA and NMDA receptors have been implicated in the regulation of DA release, a combination of CNQX (50 µM) and AP-5 (200 µM) was dissolved in the aCSF and infused into the VTA by reverse dialysis. This drug combination, infused into the VTA at these concentrations, has been used previously by others (Karreman and Moghaddam, 1996; Taber et al., 1995). Empirical evidence indicates that the concentrations of drugs crossing the dialysate membrane during reverse dialysis are extremely small. For example, the amounts of various drugs (DA uptake blockers) that crossed the dialysate membrane in vitro ranged from 2.0 – 8.6% (Nomikos et al., 1990). However, in vitro studies of recovery fail to account for impediments to drug diffusion that normally occur in vivo in the brain tissue. One study that calculated the true in vivo diffusion of an antiviral nucleoside demonstrated that the recovery was one-third of that observed in vitro (Wang et al., 1993). Thus, what appear to be relatively high concentrations of drugs must be used in most microdialysis studies.
The antagonists were initially dissolved in water containing 1.5 – 5 µl of glacial acetic acid to make a 10 mM stock solution. They were then further diluted to the appropriate µM concentrations with aCSF. The pH of all aCSF solutions was adjusted to 7.4. Following the achievement of stable baseline dialysate DA, CNQX + AP-5 were infused for one hr. before the injection of DOI.
Chromatography
DA concentrations in dialysate samples were measured by reverse phase HPLC coupled with electrochemical detection. Twenty µl samples were injected immediately after collection onto a 2 × 100 mm Phenomenex Ultracarb™ (Torrance, CA, USA) column (3 µm particle size, ODS 20). The column was maintained at a temperature of 37°C. The mobile phase consisted of 32 mM anhydrous citric acid, 54 mM sodium acetate trihydrate, 0.074 mM EDTA, 0.215 mM octylsulfonic acid, and 3% methanol (vol/vol), pH 4.2. To maintain separation of DA from its metabolites and 5-hydroxyindoleacetic acid, the pH of the mobile phase and the concentration of the octylsulfonic acid were adjusted as needed. A BAS LC-4C electrochemical detector with a glassy carbon electrode, maintained at a potential of +0.60 V relative to an Ag/AgCl reference electrode, was employed.
Histology
After microdialysis experiments were completed, probe placements were verified histologically. Only animals whose probe placements were verified to be in the PFC and the VTA were used in this study.
Data analysis
Microdialysis data were expressed as a percentage of the average of the last three baseline samples prior to drug treatment. The SPSS statistical program was employed to analyze the data by a 2-way repeated measures ANOVA with time as the within factor and drug treatment as the independent factor. This was followed by Simple Effects tests to probe a significant interaction between treatments (Winer, 1971).
Results
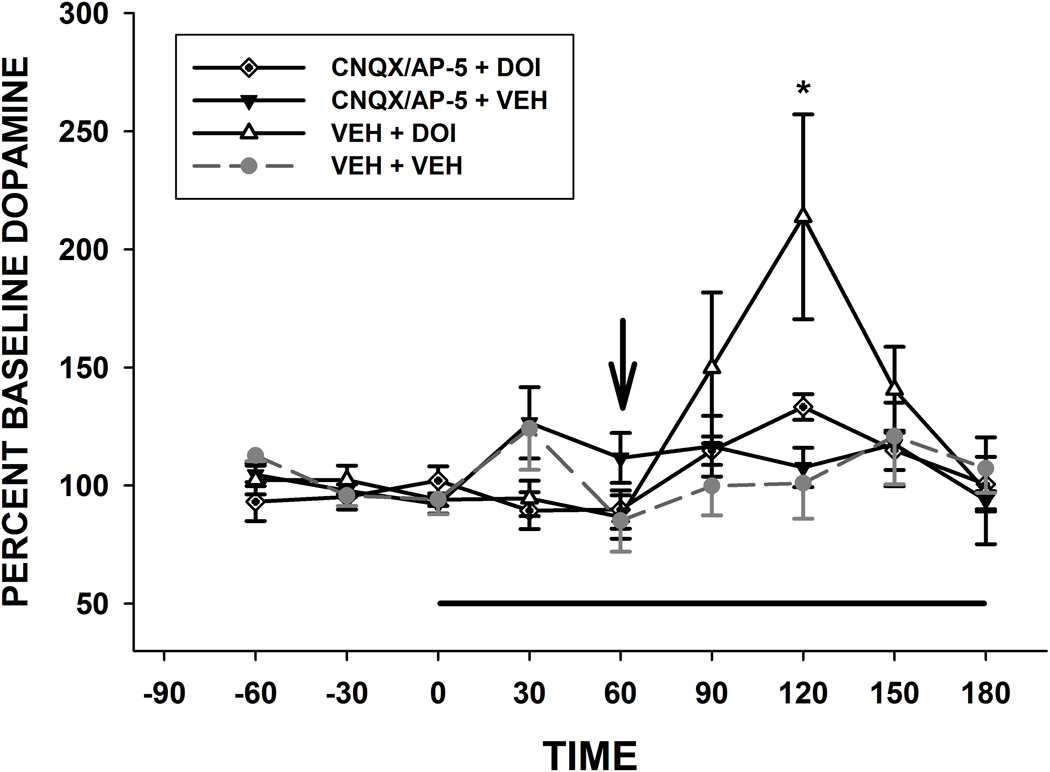
A 2-way ANOVA with time and drug treatment as the factors yielded a significant interaction [F(10,102) = 3.1, p = 0.003] as well as a significant main effect for time [F(6,102) = 7.07, p < 0.001]. (see Fig. 1). Simple Effects Tests revealed that only the vehicle + DOI group changed significantly over time [F(6,59) = 39.32, p < 0.0000]. The only timepoint that showed a difference between groups was at 120 min [F(3,69 )= 7.27, p = 0.0003]. These results show that DOI administration significantly increased DA at the 120 min time point on the graph [60 min after DOI injection) The combination of antagonists (CNQX/AP-5) did not alter basal DA (i.e. did not change over time) but did block the effects of DOI (CNQX/AP-5 + DOI group did not change over time).
Basal levels of DA (pg/20 µl) were: vehicle + vehicle: 0.28 ± 0.08, n = 5; CNQX/AP-5 + DOI: 0.22 + 0.1, n = 5; DOI + vehicle: 0.27 ± 0.07, n = 5; CNQX/AP-5 + vehicle = 0.36 ± 0.08, n = 6.
Discussion
Results showed that injections of the 5-HT2 agonist DOI increased DA release in the PFC and this was blocked by infusions of a combination of an AMPA and a NMDA antagonist into the VTA. These findings demonstrate that enhanced glutamatergic tone in the VTA is necessary for 5-HT2 modulation of mesocortical DA release. It agrees with our previous work that DOI administration increases glutamate release in the VTA; an effect that is blocked by infusions, directly into the PFC, of the selective 5-HT2A antagonist M 100,907 (Pehek et al., 2006). Thus the 5-HT receptor subtype regulating glutamate release in the VTA is the 5-HT2A subtype and it is localized cortically. These results agree with other evidence that suggests that cortical 5-HT2A receptors modulate cortical DA release by actions on a “long-loop” feedback loop involving glutamatergic projections to the midbrain VTA (Vasquez-Borsett et al., 2009). However, the present work is the first to show that 5-HT2 modulation of cortical DA is dependent on the stimulation of glutamate receptors in the VTA.
The agonist DOI has affinity for all three of the 5-HT2 receptor subtypes: A, B, and C. However, the present results are unlikely to result from binding to 5-HT2B/C receptors because studies have shown that 5-HT2B/C receptor agonism decreases cortical DA release (Gobert et al., 2000; Millan et al., 1998; Pozzi et al., 2002). Furthermore, our previous study showed that DOI-induced glutamate release in the VTA was blocked by the selective 5-HT2A antagonist M100907 (Pehek et al., 2006).
Putative circuitry underlying 5-HT2/DA interactions
One neuronal circuit is the aforementioned “long-loop” monosynaptic feedback circuit from the PFC to the VTA and back. 5-HT2A receptors are dense in the PFC and are localized there, in part, to the apical dendrites of pyramidal neurons (Cornea-Hebert et al., 1999; Hamada et al., 1998; Jakab and Goldman-Rakic, 1998, Weber and Andrade, 2010; Willins et al., 1997). 5-HT2A receptor stimulation can produce excitation in pyramidal neurons (Araneda and Andrade, 1991; Aghajanian and Marek, 1997; Puig et al., 2003). A large proportion of pyramidal neurons that project to the VTA contain 5-HT2A receptors (Vasquez-Borsett et al., 2009). Furthermore, there are monosynaptic connections between PFC pyramidal neurons and DA cells in the VTA (Sesack and Pickel, 1992). These corticotegmental projections synapse on mesocortical DA, but not mesolimbic DA, cell bodies (Carr and Sesack, 2000). Microinjections of glutamate agonists into the VTA increase in vivo extracellular concentrations of dopamine in the rat PFC (Kalivas et al., 1989). Thus, 5-HT2A receptor stimulation could cause the release of glutamate from corticotegmental projections that in turn excite VTA DA cell firing and subsequent release of DA in the PFC. Systemic administration of the 5-HT2 agonist DOI increases mesocortical DA release that is blocked by intracortical infusions of the selective 5-HT2A antagonist M100907 (Pehek et al., 2006). This latter finding indicates that the effect of DOI is due specifically to stimulation of 5-HT2A receptors within the PFC. Administration of DOI also increased glutamate efflux in the VTA, and this was blocked by intracortical infusions of M100907 (Pehek et al., 2006), suggesting that the increases in cortical DA release may be due to increases in glutamate release in the VTA. This latter study also indicates that the effects of DOI on VTA glutamate are dependent on a circuit involving the PFC. The current study demonstrates that stimulation of glutamate receptors in the VTA is necessary for 5-HT2 agonist-induced increases in cortical DA. Previous work by others has shown that application of DOI to the PFC increases burst firing of VTA DA neurons and DA release in the PFC (Martin-Ruiz et al., 2001; Bortolozzi et al., 2005). Taken together, these findings provide strong support for the hypothesis that cortical 5-HT2A receptor-induced increases in mesocortical DA are causally related to increases in glutamate efflux in the VTA. We suggest that cortical 5HT2A agonism stimulates corticotegmental glutamatergic projections that, in turn, stimulate mesocortical DA neurons.
The present work did not determine the endogenous source of glutamate in the VTA. Our previous work showed that DOI injections increased glutamate in the VTA and this was blocked by infusions of M100907 into the cortex, implicating projections specifically from the PFC. It is likely that the same projections were active in the present study. However, there are 5-HT2A containing pyramidal neurons that project to areas other than the VTA. There is evidence that some of these are callosal/commissural neurons that innervate the contralateral cortex (Avesar and Gulledge, 2012). Thus, in the present study, we cannot rule out a non-PFC source of 5-HT2 regulation of VTA glutamate. There are non-PFC glutamate afferents to the VTA (Yetnikoff et al., 2014) as well as glutamate cell bodies within the VTA (Morales and Root, 2014). There is also evidence for the co-release of glutamate from VTA DA neurons (Broussard, 2012). Recent work has also demonstrated a glutamatergic projection from the raphe to the VTA that is involved in reward (Qi et al., 2014). In addition, while other studies have implicated corticotegmental glutamate projections in the regulation of PFC DA, other non-direct pathways may have been involved. For example, VTA DA neurons may be regulated indirectly through PFC projections to the nucleus accumbens, which projects to the VTA via the ventral pallidum (Floresco et al., 2003; Vasquez-Borsett et al., 2009). Thus, the involvement of projections from the PFC to other brain areas that in turn innervate the VTA cannot be ruled out. 5-HT2A receptors are also localized on DA and GABA cells in the VTA (Nocjar et al., 2002). Thus it is possible that the present effects of DOI are due to direct actions on the VTA. These receptors could directly modulate VTA glutamate by activating glutamate-containing neurons in the VTA. However, we think this is unlikely since our previous research found that DOI-induced increases in glutamate were dependent on a circuit involving the PFC, i.e. blocked by infusions of M100907 into the cortex (Pehek et al., 2006).
In short, 5-HT2A receptor modulation of mesocortical DA release requires the stimulation of glutamate receptors in the VTA. This may be mediated by a “long-loop” feedback system involving 5-HT2A-containing pyramidal cells that project to the VTA and stimulate mesocortical DA neurons. This circuitry may be relevant to the mechanism of atypical antipsychotic and other drugs with 5-HT2A antagonist properties. Knowledge of the underlying neurocircuitry is essential for our understanding of the mechanisms of action of these drugs as well as agents such as hallucinogens that are agonists at the 5-HT2A receptor. Furthermore, DA/5-HT interactions may be generally important in PFC cognitive functions such as working memory and selective attention that are altered in syndromes such as schizophrenia.
Fig 2.
Infusion of the glutamate antagonists CNQX (50 µM) and AP-5 (200 µM) into the VTA blocks 5-HT2 agonist (DOI)-induced mesocortical DA release. A combination of the glutamate antagonists was infused into the VTA at the time indicated by the bar. DOI was injected (2.5 mg/kg s.c.) at the time indicated by the arrow. *p < 0.05 relative to the VEH + VEH group and the CNQX/AP-5 + DOI group, n = 5–6/group.
Acknowledgments
The authors wish to thank Tara Byrd for her technical assistance. This work was funded by a Merit grant to EAP from the Department of Veterans Affairs, USA.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AMPA
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AP-5
2-amino-5-phosphopentanoic acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DA
dopamine
- DOI
(±)-2,5-Dimethoxy-4-iodoamphetamine
- HPLC
high performance liquid chromatography
- 5-HT
serotonin
- NMDA
N-Methyl-D-aspartic acid
- PFC
prefrontal cortex
- 5-HT2
serotonin-2
- VTA
ventral tegmental area
References
- Aghajanian G, Marek G. Serotonin Induces Excitatory Postsynaptic Potentials in Apical Dendrites of Neocortical Pyramidal Cells. Neuropharmacology. 1997;36:589–599. doi: 10.1016/s0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Alex KD, Pehek EA. Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther. 2007;113:296–320. doi: 10.1016/j.pharmthera.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-Hydroxytryptamine1A Receptors Mediate Opposing Responses on Membrane Excitability in Rat Association Cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Avesar D, Gulledge AT. Selective serotonergic excitation of callosal projection neurons. Front Neural Circuits. 2012;6:1–11. doi: 10.3389/fncir.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolozzi A, Diaz-Mataix L, Scorza M, Celada P, Artigas F. The activation of 5-HT receptors in prefrontal cortex enhances dopaminergic activity. J Neurochem. 2005;95:1597–1607. doi: 10.1111/j.1471-4159.2005.03485.x. [DOI] [PubMed] [Google Scholar]
- Broussard JI. Co-transmission of dopamine and glutamate. J Gen Physiol. 2012;139:93–96. doi: 10.1085/jgp.201110659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacol. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Gobert A, Rivet J, Lejeune F, Newman-Tancredi A, Adhumeau-Auclair A, Nicolas J, Cistarelli L, Melon C, Millan MJ. Serotonin2C receptors tonically suppress the activity of mesocortical dopaminergic and adrenergic, but not serotonergic, pathways: A combined dialysis and electrophysiological analysis in the rat. Synapse. 2000;36:205–221. doi: 10.1002/(SICI)1098-2396(20000601)36:3<205::AID-SYN5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Barrow J. Regulation of the mesocorticolimbic dopamine system by glutamic acid receptor subtypes. J. Pharmacol and Exp Ther. 1989;251:378–387. [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Martin-Ruiz R, Puig MV, Celada P, Shapiro DA, Roth BL, Mengod G, Artigas F. Control of serotonergic function in medial prefrontal cortex by serotonin-2A receptors through a glutamate-dependent mechanism. J. Neurosci. 2001;21:9856–9866. doi: 10.1523/JNEUROSCI.21-24-09856.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Dekeyne A, Gobert A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology. 1998;37:953–955. doi: 10.1016/s0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Morales M, Root DH. Glutamate neurons within the midbrain dopamine regions. Neuroscience. 2014;282:60–68. doi: 10.1016/j.neuroscience.2014.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocjar C, Roth BL, Pehek EA. Localization of 5-HT2A receptors on dopamine cells in subnuclei of the midbrain A10 cell group. Neuroscience. 2002;111:163–176. doi: 10.1016/s0306-4522(01)00593-0. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. In vivo characterization of locally applied dopamine uptake inhibitors by striatal microdialysis. Synapse. 1990;6:106–112. doi: 10.1002/syn.890060113. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Pehek EA, McFarlane HG, Maguschak K, Price B, Pluto CP. M100,907, a selective 5-HT2A antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Res. 2001;888:51–59. doi: 10.1016/s0006-8993(00)03004-3. [DOI] [PubMed] [Google Scholar]
- Pehek E, Nocjar C, Roth B, Byrd T, Mabrouk O. Evidence for the Preferential Involvement of 5-HT2A Serotonin Receptors in Stress- and Drug-Induced Dopamine Release in the Rat Medial Prefrontal Cortex. Neuropsychopharmacology. 2006;31:265–277. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- Pozzi L, Acconcia S, Ceglia I, Invernizzi RW, Samanin R. Stimulation of 5-hydroxytryptamine (5-HT(2C) ) receptors in the ventrotegmental area inhibits stress-induced but not basal dopamine release in the rat prefrontal cortex. J Neurochem. 2002;82:93–100. doi: 10.1046/j.1471-4159.2002.00947.x. [DOI] [PubMed] [Google Scholar]
- Puig MV, Celada P, Diaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- Qi J, Zhang S, Wang H-L, Wang H, de Jesus Aceves Buendia J, Hoffman AF, Lupica CR, Seal RP, Morales M. A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat Commun. 2014;5:1–13. doi: 10.1038/ncomms6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- Vasquez-Borsetti P, Cortes R, Artigas F. Pyramidal neurons in rat prefrontal cortex projecting to ventral tegmental area and dorsal raphe nucleus express 5-HT2A receptors. Cereb Cortex. 2009;19:1678–1686. doi: 10.1093/cercor/bhn204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wong SL, Sawchuck RJ. Microdialysis calibration using retrodialysis and zero-net flux: application to a study of the distribution of zidovudine to rabbit cerebrospinal fluid and thalamus. Pharm Res. 1993;10:1411–1419. doi: 10.1023/a:1018906821725. [DOI] [PubMed] [Google Scholar]
- Weber ET, Andrade R. Htr2a Gene and 5-HT(2A) Receptor Expression in the Cerebral Cortex Studied Using Genetically Modified Mice. Front Neurosci. 2010;4:1–12. doi: 10.3389/fnins.2010.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- Willins DL, Deutch AY, Roth BL. Serotonin 5-HT(2A) receptors are expressed on pyramidal cells and interneurons in the rat cortex. Synapse. 1997;26:1–4. doi: 10.1002/(SICI)1098-2396(199709)27:1<79::AID-SYN8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical principles in experimental design. 2nd. New York: McGraw Hill; 1971. [Google Scholar]
- Yetnikoff L, Lavezzi HN, Reichard RA, Zahm DS. An update on the connections of the ventral mesencephalic dopaminergic complex. Neuroscience. 2014;282:23–48. doi: 10.1016/j.neuroscience.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]