Abstract
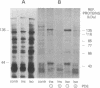
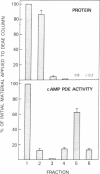
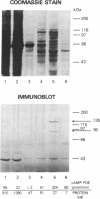
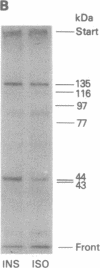
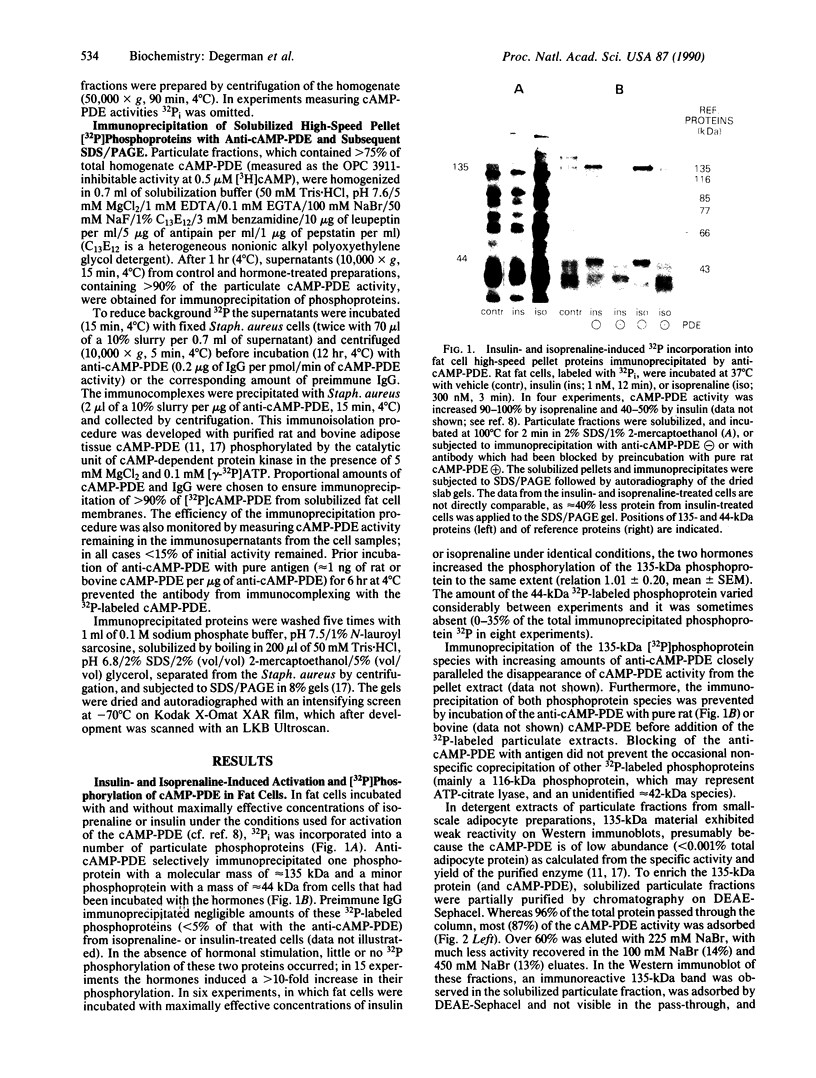
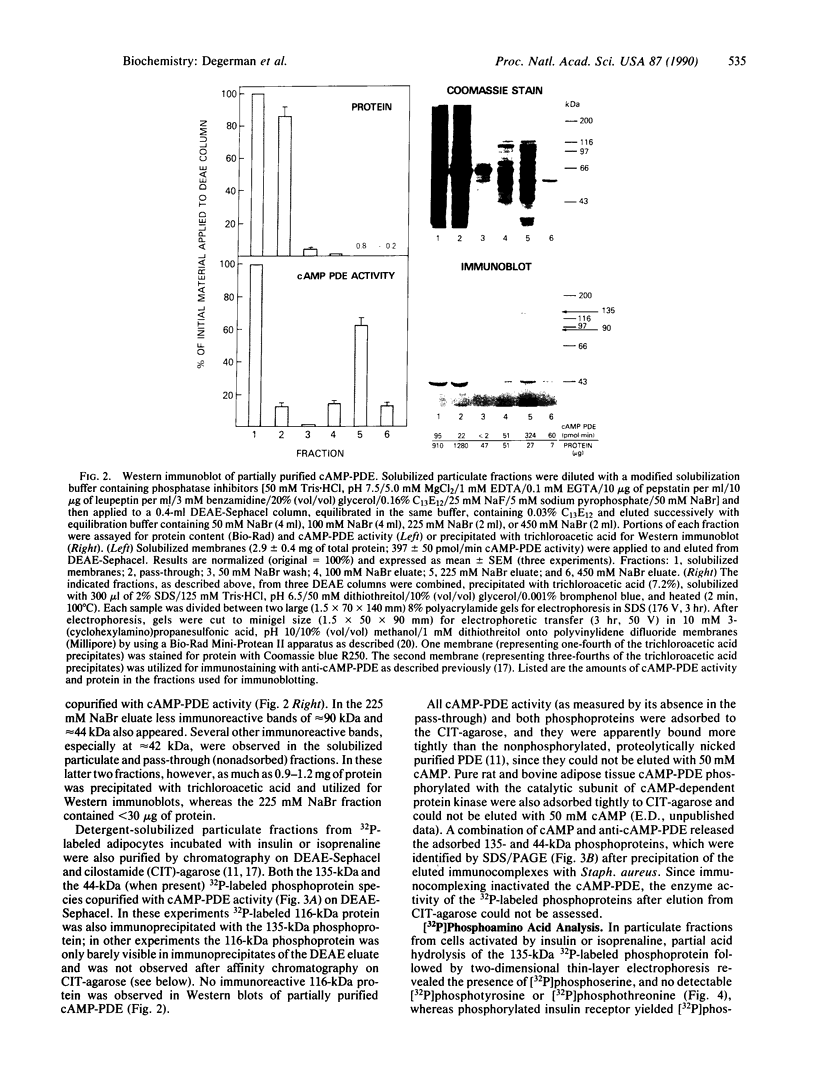
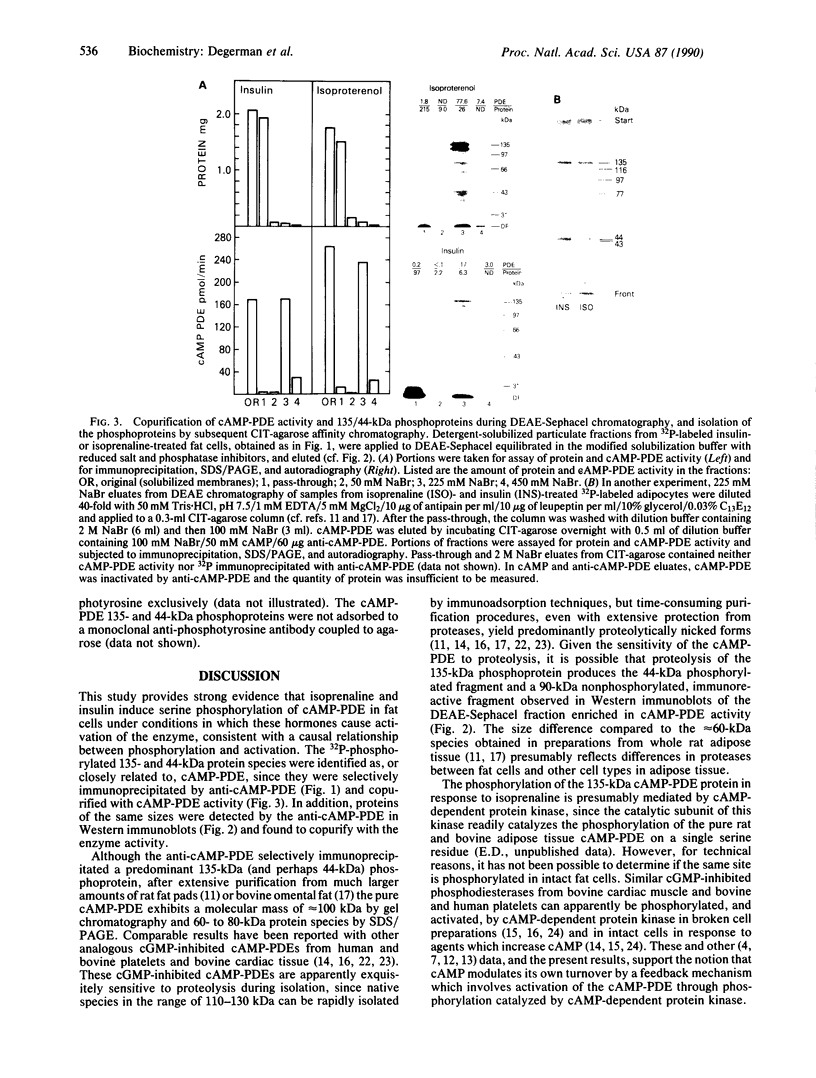
Incubation of intact rat fat cells with maximally effective concentrations of insulin (1 nM, 12 min) or isoprenaline (300 nM, 3 min) increased particulate cGMP- and cilostamide-inhibited, low-Km cAMP phosphodiesterase (cAMP-PDE) activity by about 50% and 100%, respectively. In 32P-labeled cells, these agents induced serine 32P-phosphorylation of a 135-kDa particulate protein and, to a variable and lesser extent, a 44-kDa protein, which were selectively immunoprecipitated by anti-cAMP-PDE, as analyzed by SDS/PAGE and autoradiography. In the absence of hormonal stimulation, little phosphorylation was detected (less than 10% of that with the hormones). The two phosphoproteins were identified as cAMP-PDE or a closely related molecule (in the case of the 44-kDa species, perhaps a proteolytic fragment) since (i) amounts of 32P in the immunoprecipitated 135-kDa protein paralleled enzyme inactivation, (ii) prior incubation of the anti-cAMP-PDE with the pure rat or bovine enzyme selectively blocked the immunoprecipitation of the phosphoproteins, (iii) 135- and 44-kDa proteins reacted with the anti-cAMP-PDE on Western immunoblots, and (iv) the two phosphoproteins copurified with cAMP-PDE activity through DEAE-Sephacel chromatography and were isolated by highly selective affinity chromatography on cilostamide-agarose. Thus, in fat cells, catecholamine- and insulin-induced activation of the cAMP-PDE may be mediated via phosphorylation by cAMP-dependent protein kinase and an insulin-activated serine protein kinase, respectively.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Klarlund J. K., Yagaloff K. A., Bradford A. P., Lewis R. E. Insulin receptor signaling. Activation of multiple serine kinases. J Biol Chem. 1988 Aug 15;263(23):11017–11020. [PubMed] [Google Scholar]
- Degerman E., Belfrage P., Newman A. H., Rice K. C., Manganiello V. C. Purification of the putative hormone-sensitive cyclic AMP phosphodiesterase from rat adipose tissue using a derivative of cilostamide as a novel affinity ligand. J Biol Chem. 1987 Apr 25;262(12):5797–5807. [PubMed] [Google Scholar]
- Degerman E., Manganiello V. C., Newman A. H., Rice K. C., Belfrage P. Purification, properties and polyclonal antibodies for the particulate, low Km cAMP phosphodiesterase from bovine adipose tissue. Second Messengers Phosphoproteins. 1988;12(4):171–182. [PubMed] [Google Scholar]
- Gettys T. W., Blackmore P. F., Corbin J. D. An assessment of phosphodiesterase activity in situ after treatment of hepatocytes with hormones. Am J Physiol. 1988 Apr;254(4 Pt 1):E449–E453. doi: 10.1152/ajpendo.1988.254.4.E449. [DOI] [PubMed] [Google Scholar]
- Gettys T. W., Blackmore P. F., Redmon J. B., Beebe S. J., Corbin J. D. Short-term feedback regulation of cAMP by accelerated degradation in rat tissues. J Biol Chem. 1987 Jan 5;262(1):333–339. [PubMed] [Google Scholar]
- Gettys T. W., Vine A. J., Simonds M. F., Corbin J. D. Activation of the particulate low Km phosphodiesterase of adipocytes by addition of cAMP-dependent protein kinase. J Biol Chem. 1988 Jul 25;263(21):10359–10363. [PubMed] [Google Scholar]
- Grant P. G., Colman R. W. Purification and characterization of a human platelet cyclic nucleotide phosphodiesterase. Biochemistry. 1984 Apr 10;23(8):1801–1807. doi: 10.1021/bi00303a034. [DOI] [PubMed] [Google Scholar]
- Grant P. G., Mannarino A. F., Colman R. W. cAMP-mediated phosphorylation of the low-Km cAMP phosphodiesterase markedly stimulates its catalytic activity. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9071–9075. doi: 10.1073/pnas.85.23.9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. A., Reifsnyder D. H., Gallis B., Cadd G. G., Beavo J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol. 1986 May;29(5):506–514. [PubMed] [Google Scholar]
- Heyworth C. M., Wallace A. V., Houslay M. D. Insulin and glucagon regulate the activation of two distinct membrane-bound cyclic AMP phosphodiesterases in hepatocytes. Biochem J. 1983 Jul 15;214(1):99–110. doi: 10.1042/bj2140099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honnor R. C., Dhillon G. S., Londos C. cAMP-dependent protein kinase and lipolysis in rat adipocytes. I. Cell preparation, manipulation, and predictability in behavior. J Biol Chem. 1985 Dec 5;260(28):15122–15129. [PubMed] [Google Scholar]
- Kono T., Barham F. W. The relationship between the insulin-binding capacity of fat cells and the cellular response to insulin. Studies with intact and trypsin-treated fat cells. J Biol Chem. 1971 Oct 25;246(20):6210–6216. [PubMed] [Google Scholar]
- Loten E. G., Assimacopoulos-Jeannet F. D., Exton J. H., Park C. R. Stimulation of a low Km phosphodiesterase from liver by insulin and glucagon. J Biol Chem. 1978 Feb 10;253(3):746–757. [PubMed] [Google Scholar]
- Loten E. G., Sneyd J. G. An effect of insulin on adipose-tissue adenosine 3':5'-cyclic monophosphate phosphodiesterase. Biochem J. 1970 Nov;120(1):187–193. doi: 10.1042/bj1200187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphee C. H., Harrison S. A., Beavo J. A. Immunological identification of the major platelet low-Km cAMP phosphodiesterase: probable target for anti-thrombotic agents. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6660–6663. doi: 10.1073/pnas.83.17.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macphee C. H., Reifsnyder D. H., Moore T. A., Beavo J. A. Intact cell and cell-free phosphorylation and concomitant activation of a low Km, cAMP phosphodiesterase found in human platelets. J Cyclic Nucleotide Protein Phosphor Res. 1986;11(7):487–496. [PubMed] [Google Scholar]
- Macphee C. H., Reifsnyder D. H., Moore T. A., Lerea K. M., Beavo J. A. Phosphorylation results in activation of a cAMP phosphodiesterase in human platelets. J Biol Chem. 1988 Jul 25;263(21):10353–10358. [PubMed] [Google Scholar]
- Makino H., Kono T. Characterization of insulin-sensitive phosphodiesterase in fat cells. II. Comparison of enzyme activities stimulated by insulin and by isoproterenol. J Biol Chem. 1980 Aug 25;255(16):7850–7854. [PubMed] [Google Scholar]
- Manganiello V., Vaughan M. An effect of insulin on cyclic adenosine 3':5'-monophosphate phosphodiesterase activity in fat cells. J Biol Chem. 1973 Oct 25;248(20):7164–7170. [PubMed] [Google Scholar]
- Pawlson L. G., Lovell-Smith C. J., Manganiello V. C., Vaughan M. Effects of epinephrine, adrenocorticotrophic hormone, and theophylline on adenosine 3', 5'-monophosphate phosphodiesterase activity in fat cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1639–1642. doi: 10.1073/pnas.71.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Characterization of insulin-stimulated microtubule-associated protein kinase. Rapid isolation and stabilization of a novel serine/threonine kinase from 3T3-L1 cells. J Biol Chem. 1988 Sep 5;263(25):12721–12727. [PubMed] [Google Scholar]
- Smith C. J., Manganiello V. C. Role of hormone-sensitive low Km cAMP phosphodiesterase in regulation of cAMP-dependent protein kinase and lipolysis in rat adipocytes. Mol Pharmacol. 1989 Mar;35(3):381–386. [PubMed] [Google Scholar]
- Sommercorn J., Mulligan J. A., Lozeman F. J., Krebs E. G. Activation of casein kinase II in response to insulin and to epidermal growth factor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8834–8838. doi: 10.1073/pnas.84.24.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber H. W., Appleman M. M. Insulin-dependent and insulin-independent low Km cyclic AMP phosphodiesterase from rat adipose tissue. J Biol Chem. 1982 May 25;257(10):5339–5341. [PubMed] [Google Scholar]