Abstract
Background/Aim:
We investigated the association between blood amino acid concentration changes caused by elemental diet (ED) and their relationship to its therapeutic effect.
Patients and Methods:
Patients with active Crohn's disease (CD) followed ED for 12 weeks. Patients not previously treated with ED were defined as new ED, and those with previous ED therapy (≥900 kcal/day) were defined as previous ED. Disease activity markers [Crohn's disease activity index (CDAI) and C-reactive protein (CRP) level], blood biochemistry test results, and plasma amino acid concentrations were measured before and after the treatment.
Results:
Histidine (His), tryptophan (Trp), valine (Val), and methionine (Met) increased after the treatment in the 17 patients with clinical remission, however, no increase occurred in plasma amino acid concentrations in the 8 patients without remission. The multivariate index using AminoIndex™ technology (MIAI) was correlated with the CDAI (r = 0.475, P < 0.001), and it decreased as patients' conditions improved during the treatment. All patients in the new ED group (n = 11) exhibited increases in the nutritional indices, albumin level, and body mass index after treatment, as well as increased levels of His, Trp, Val, and phenylalanine. None of these changes were observed in the previous ED group (n = 14).
Conclusions:
Plasma amino acid concentrations and MIAI may provide useful noninvasive markers for evaluating disease activity and response to treatment. ED was effective in improving disease activity, nutritional status, and plasma amino acid levels, and thus it may be particularly effective for poorly nourished patients with CD who have not previously undergone this treatment.
Key Words: Crohn's disease, elemental diet, multivariate index using AminoIndex™ technology, histidine, tryptophan
Crohn's disease (CD), a type of inflammatory bowel disease (IBD), is a disorder that causes inflammation and ulceration throughout the gastrointestinal tract, particularly in the small and large intestines, producing gastrointestinal symptoms such as diarrhea and abdominal pain, which lead to weight loss and nutritional impairment. Many years of research on the causes of CD have suggested that it arises from immunological abnormalities against a backdrop of both genetic and environmental factors;[1,2,3,4] however, its mechanism is yet to be elucidated in detail. Numerous studies have described the value of treating patients with CD with immunoregulatory therapy employing biological agents such as anti-tumor necrosis factor-α (TNF-α) agents or immunomodulators (IM);[5] however, these immunoregulatory therapies may increase the risk of malignant neoplasm, severe opportunistic infection, bone marrow suppression, or hepatosplenic T-cell lymphoma.[6,7,8,9,10] In addition, the existence of secondary nonresponders whose response to treatment becomes weaker the longer immunoregulatory therapy continues is becoming a clinical issue.[11,12] An elemental diet (ED) has been shown to be extremely safe for patients with CD, which improves their nutritional status, clinical symptoms, and morphological findings; in Japan, it is the first-line therapy for adults and pediatric patients.[13,14] Although the mechanism accounting for its therapeutic effects has not been clarified, recent studies have shown that ED modifies the composition of the intestinal microbiome, which plays a major causative role in CD.[15,16] Furthermore, certain amino acids have been shown to have major involvement in chronic inflammation, and elucidating the therapeutic efficacy of bowel rest and ED other than nutritional supplements is currently underway.[17] Histidine (His) and tryptophan (Trp) in particular have been shown to be strongly correlated with the disease activity of IBD. Statistical analysis and conversion to indices of the balance of amino acid concentrations in blood by means of a multivariate index using AminoIndex technology (MIAI) has been found to be useful for diagnosing CD and assessing disease activity.[18,19,20] Studies using drug-induced epithelial damage colitis models have also indicated that nutritional supplementation with ED and bowel rest and some amino acids, such as His, glutamine, and glycine, directly suppresses colitis.[21,22,23,24] Although attention is already focused on the association between the pathology of CD and amino acid metabolism, no study has investigated the relationship between disease activity associated with nutritional therapy and changes in plasma amino acid concentrations in patients with active CD. Therefore, the objective of the present study was to elucidate the association between changes in the concentration of amino acids, particularly essential amino acids (EAA) that cannot be synthesized in the human body, in the plasma of patients with CD due to ED and its therapeutic efficacy.
PATIENTS AND METHODS
Patients
The study included patients with active CD [Crohn's disease activity index (CDAI) of ≥150][25,26] who were treated at the Dokkyo Medical University between October 2010 and December 2011. ED therapy with Elental® (300 kcal/pack) was provided for 12 weeks. Patients who were not previously treated with ED were defined as the new ED group, and those who had previously undergone maintenance therapy with ED (≥900 kcal/day) were defined as the previous ED group. In the previous ED group, the amount of Elental® was increased as far as the patients' sensitivity permitted. No restrictions were placed on the use of anti-TNF-α therapy with adalimumab (ADA) or infliximab (IFX) during the treatment period. The CDAI, blood biochemistry test results, and plasma amino acid concentrations were measured before starting the treatment and after the treatment (12 weeks). The association between changes in the plasma amino acid concentrations before and after the treatment as well as the response to treatment were assessed. We used as standard the median plasma amino acid concentration in healthy controls (HCs) reported by Hisamitsu et al.[20] Patients whose CDAI score was <150 due to treatment were classed as having achieved remission, and those whose score did not reach this point were regarded as not having achieved remission.
Multivariate index using AminoIndex technology analysis
MIAI (CDa/CDr), which is regarded to be valuable for distinguishing between remission and nonremission in CD, was calculated according to the following formula: MIAI (CDa/CDr) =16.474 – 3.342 × [His] – 5.190 × [Trp] + 1.857 × [Tau] +2.715 × [methionine (Met)].[20] Plasma amino acid concentrations were measured using an SRL automated amino acid analysis liquid chromatography–mass spectrometry system (Shimadzu).
Statistical analysis
Data were expressed as mean ± standard deviation, and plasma amino acid concentrations (nonparametric data) were expressed as medians (interquartile ranges). A t-test was used to compare parametric data between the two groups, and a paired t-test was used to determine differences in one-sample analyses. Wilcoxon's rank sum test was used to compare plasma amino acid concentrations between the two groups, and Wilcoxon's signed-rank test was used to determine differences in one-sample analyses. An χ2 test was used to investigate bias in the frequency of data on each scale between the two groups. Pearson's product-moment correlation coefficient was calculated to investigate the correlation between two stochastic variables. Receiver operating characteristic (ROC) curve analysis was used to evaluate the diagnostic accuracy of the MIAI and CRP values, and the area under the ROC curve (ROC AUC) for clinical remission was calculated. A value of P < 0.05 was considered significant. All statistical analyses were performed using JMP software, version 9.0.0 (SAS Institute Inc., Cary, NC, USA).
Ethical considerations
The study protocol was approved by the institutional review board of Dokkyo Medical University, and all participants provided informed written consents. The performance of this study adhered to the principles of the Declaration of Helsinki for medical research involving human participants.
RESULTS
Study participants
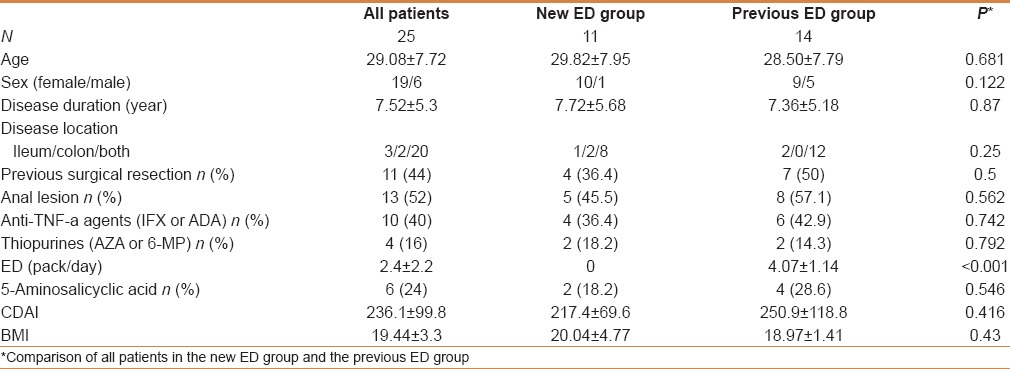
The participants were 25 patients with CD that was active during the study period. Table 1 summarizes the patients' background characteristics before the study started. No patient took systemic corticosteroids during the treatment period, however, 4 (16%) had been given thiopurine before treatment was started, and 1 received azathiopurine during the treatment period with the goal of maintaining remission. Ten patients (40%) were already undergoing maintenance therapy with anti-TNF-α agents (IFX or ADA) at the start of the treatment, and 9 (36%) with high disease activity were started on new anti-TNF-α therapy during the treatment period. There were no significant differences in the characteristics of patients in the new ED group (n = 11) and the previous ED group (n = 14), except for the amount of ED used.
Table 1.
Patients’ characteristics before treatment
Changes after treatment
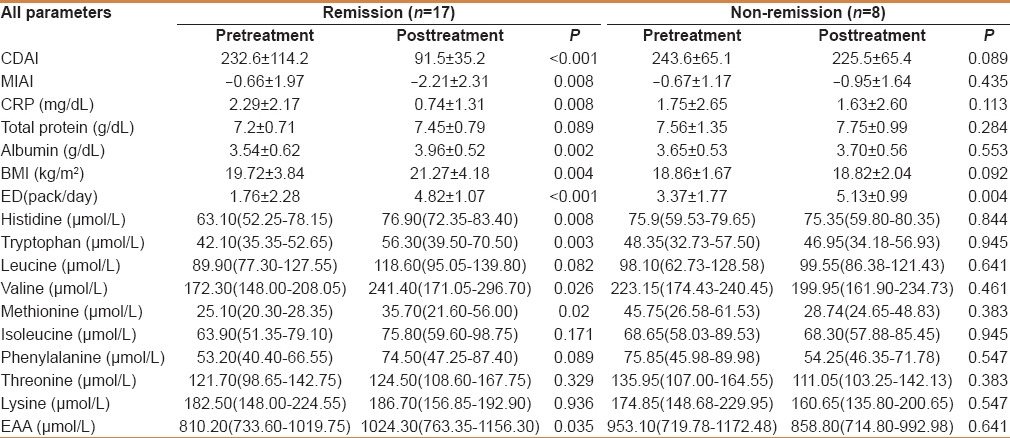
Table 2 shows changes in the disease activity of all 25 patients after the treatment. There were significant decreases in both the CDAI (236.12 to 134.40, P < 0.001) and CRP level (2.12 mg/dL to 1.02 mg/dL, P = 0.007), and 17 of 25 patients (68%) achieved remission (CDAI <150). All physical findings and blood biochemistry test results, except the total protein level, improved in the 17 patients who achieved remission, and the concentrations of the plasma amino acids His, Trp, valine (Val), Met, and EAA increased [Table 3]. There was no change in any of the parameters, including the plasma amino acid concentrations, for the 8 patients who did not achieve remission.
Table 2.
Comparison of all patients in before and after treatment
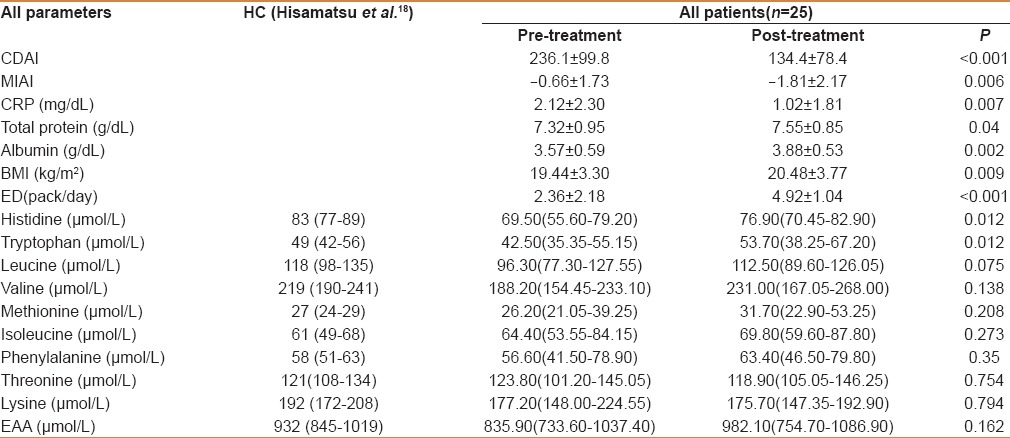
Table 3.
Comparison of all patients in the remission and non-remission groups before and after treatment
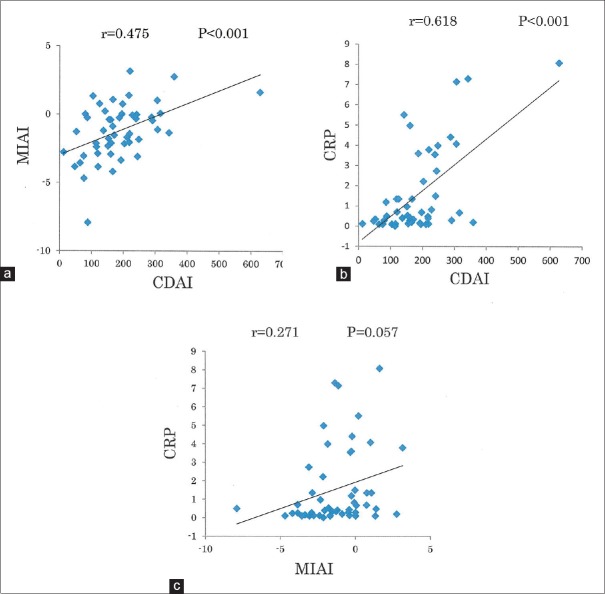
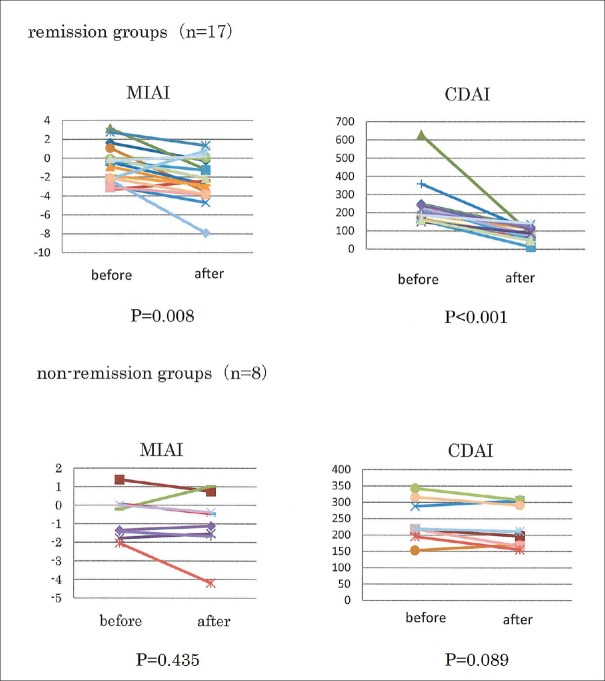
To investigate the value of the MIAI (CDa/CDr) as a CD activity marker, as reported by Hisamatsu et al., we analyzed the correlations between the MIAI and CDAI, and between the MIAI and CRP in all 25 patients before and after treatment (n = 50). There were significant correlations between both the MIAI and CDAI (r = 0.475, P < 0.001), and between the CRP and CDAI (r = 0.618, P < 0.001). There was no significant correlation, however, between the level of CRP (the inflammatory marker) and MIAI (r = 0.271, P = 0.057) [Figure 1]. The ROC AUC of the MIAI for diagnosing remission (CDAI: <150) was 0.688, which was similarly high compared to that of the CRP level (ROC AUC = 0.699). The MIAI also decreased as disease activity improved with treatment [Figure 2].
Figure 1.
(a) Analysis of the correlation between the Crohn's disease activity index (CDAI) and multivariate index using AminoIndex™ (MIAI) technology in 25 patients before and after treatment (n = 50). (b) Analysis of the correlation between the CDAI and C-reactive protein (CRP) level in 25 patients before and after treatment (n = 50). (c) Analysis of the correlation between the MIAI and CRP level in 25 patients before and after treatment (n = 50)
Figure 2.
Comparison of the multivariate index using AminoIndex™technology and Crohn's disease activity index in the remission and nonremission groups before and after treatment
New ED group vs. previous ED group
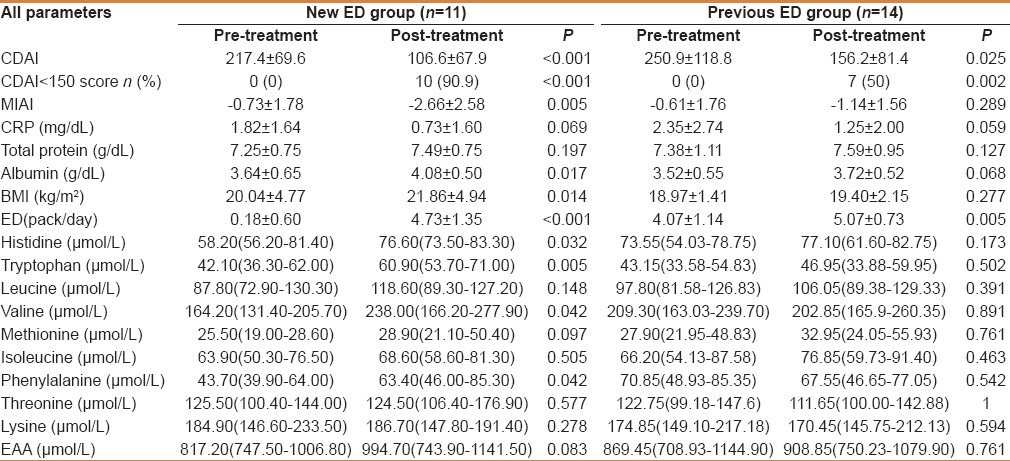
Table 4 shows comparisons of patients in the new ED group (n = 11) and the previous ED group (n = 14) before and after treatment. In the new ED group, the amount of ED increased significantly from 0 packs/day to 4.83 packs/day (P < 0.001), whereas in the previous ED group, it increased from 4.07 packs/day to 5.07 packs/day, which was a high level; the difference was still significant (P = 0.005) but much smaller than that for the new ED group. Anti-TNF-α therapy was started by 4 patients in the new ED group and 5 in the previous ED group, with no significant difference between both the groups. In terms of the response to treatment of patients in the two groups, 10 (90.9%) patients in the new ED group and 7 (50%) in the previous ED group achieved remission, all of whom exhibited significant decreases in the CDAI and CRP level. Patients in the new ED group also exhibited increase in the nutritional indices, albumin, and body mass index after treatment, as well as increased levels of the plasma amino acids His, Trp, Val, and phenylalanine; however, none of these changes was observed in patients in the previous ED group.
Table 4.
Comparison of patients in the new elemental diet (ED) group and the previous ED group before and after treatment
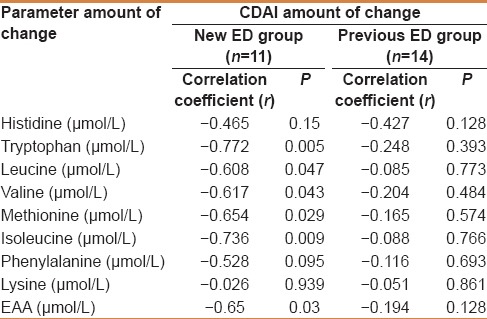
We then analyzed the correlation between the magnitude of changes in the CDAI and the magnitude of changes in plasma amino acid concentrations, and found that the magnitude of changes in the levels of plasma amino acids Trp, leucine, Val, Met, isoleucine, and EAA were also correlated with the magnitude of changes in the CDAI in the new ED group; however, no such correlation was evident in the previous ED group [Table 5].
Table 5.
Analysis of the correlation between the magnitude of changes in Crohn's disease activity index and the magnitude of changes in plasma amino acid concentrations in the new elemental diet (ED) group and the previous ED group
DISCUSSION
In our investigation of changes in the concentrations of plasma amino acids in patients with CD who underwent remission induction therapy with ED, we found that their concentrations varied in accordance with changes in the activity of CD. The MIAI also decreased as disease activity improved. These results reproduced the correlation between the MIAI and CDAI reported by Hisamatsu et al., and suggested that the MIAI may be a noninvasive objective biomarker for assessing disease activity and the response to treatment in patients with CD. In general, CDAI is widely used for evaluating disease activity of CD, however, it has a disadvantage in that its assessment incorporates subjectivity on the parts of both the doctor and patient.[26] It is also known that CDAI does not always correlate with CRP and other inflammatory markers. On the other hand, there are studies showing CDAI to be a marker of disease activity in CD and to correlate with both CRP and fecal calprotectin. Therefore, the correlation between CDAI and CRP remains controversial.[27] In our study, the two showed a correlation; this might be because we minimized interevaluator variability by providing education to our CDAI measurers and ensuring consensus among them; we also instructed the patients fill out the CDAI calculation sheet prospectively for 7 days prior to the medical consultation, consequently achieving accurate evaluations of disease activities in these patients. Both serum CRP and fecal calprotectin have been reported to be useful biomarkers for CD.[27,28,29,30] Specific breakdowns in the balance of plasma amino acid concentrations, which are regulated at a constant level by homeostasis maintenance functions, have been found to occur in diseases such as malignant tumors,[31,32,33,34,35,36] suggesting that the MIAI calculated to evaluate the activity of CD may constitute a disease-specific assessment. The MIAI is also a marker calculated from the viewpoint of nutritional status in terms of amino acid balance, and its combination with the CRP level and fecal calcitonin, which are mainly associated with inflammation, may enable the disease activity of CD to be evaluated from multiple perspectives.
A comparison of amino acid concentrations in patients who achieved remission (n = 17) and those who did not achieve remission (n = 8) showed particularly large increases in the concentrations of His, Trp, and other plasma amino acids in the remission group, whereas in the nonremission group there was no increase in the level of any EAA. As EAAs cannot be synthesized in the human body, they must be ingested as part of the diet, and unless all of them are eaten in the right quantities, with no excess or deficiency, they cannot be used effectively in the synthesis of proteins needed by the body.[37,38,39,40] This may imply that ED is important in terms of improving the amino acid balance.
A comparison of patients in the new and previous ED groups showed that although a poor nutritional status and EAA balance improved in the new ED group, no such improvement was seen in the previous ED group. An analysis of the correlation between the magnitude of changes in the CDAI and the magnitude of changes in plasma amino acid concentrations also found that these were only correlated in patients in the new ED group. These results suggested that the therapeutic efficacy of ED for CD may lie in improving poor nutrition and amino acid balance, and ED may be particularly effective for poorly nourished patients with CD who have not previously undergone this treatment. For patients such as those in the previous ED group whose caloric intake will probably not be further increased by ED, it may be necessary to consider immunoregulatory therapy with biologics or IM rather than increasing the amount of ED. The advent of biological agents in recent years has contributed to improving therapeutic outcomes for CD, however, the existence of secondary nonresponders whose response to treatment becomes weaker as it continues is becoming a clinical issue.[11] In Europe and North America, attempts are being made to prevent the appearance of secondary nonresponders to IFX by using immunomodulators such as azathiopurine in combination with IFX from the start of therapy. A combination of these two types of drug has been reported to significantly extend the duration for which remission is maintained.[12] However, because this may increase the risk of opportunistic infections and the development of hepatosplenic lymphoma, caution is required.[6,10] ED is an extremely safe therapy, and recent studies have found that the combination of nutritional therapy with IFX maintenance therapy significantly extends the duration of remission, suggesting that ED is a promising therapy when used during the administration of IFX.[41,42,43] In the present study, we assessed whether remission had been achieved after 3 months of therapy, however, the efficacy of ED in improving plasma amino acid concentrations and CD activity suggested that it may also be an effective method of treatment for patients being treated with biologics. However, our results from this study are limited in that this was a small, nonrandomized cohort study; the gastrointestinal mucosa of CD patients was not analyzed; there may be potential bias in the selection of patients; and the treatment method cannot be ruled out. Further multicenter, large-scale randomized controlled trials are required in the future.
CONCLUSIONS
Remission induction therapy with ED resulted in improved CDAI and increases in plasma amino acid concentrations, suggesting that plasma amino acid concentrations may provide a useful noninvasive marker for assessing disease activity in patients with CD and determining their response to treatment. No previous study has observed improvements in amino acid concentrations that parallel the condition of patients with CD after remission induction therapy with ED; thus, ED may be particularly effective for poorly nourished patients with CD who have not previously undergone this treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We are grateful to all the clinicians involved in the management and treatment of the patients.
REFERENCES
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–57. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 3.Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: Lessons from mouse infection models. Nat Rev Microbiol. 2010;8:564–77. doi: 10.1038/nrmicro2403. [DOI] [PubMed] [Google Scholar]
- 4.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiber S, Colombel JF, et al. Maintenance infliximab for Crohn's disease: The ACCENT I randomised trial. Lancet. 2002;359:1541–9. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 6.Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005;54:1121–5. doi: 10.1136/gut.2004.049460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter MJ, Lobo AJ, Travis SP. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl 5):V1–16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dieren JM, Kuipers EJ, Samsom JN, Nieuwenhuis EE, van der Woude CJ. Revisiting the immunomodulators tacrolimus, methotrexate, and mycophenolate mofetil: Their mechanisms of action and role in the treatment of IBD. Inflamm Bowel Dis. 2006;12:311–27. doi: 10.1097/01.MIB.0000209787.19952.53. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Abreu MT, Cohen R, Tremaine W. Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology. 2006;130:935–9. doi: 10.1053/j.gastro.2006.01.047. [DOI] [PubMed] [Google Scholar]
- 10.Rosh JR, Gross T, Mamula P, Griffiths A, Hyams J. Hepatosplenic T-cell lymphoma in adolescents and young adults with Crohn's disease: A cautionary tale? Inflamm Bowel Dis. 2007;13:1024–30. doi: 10.1002/ibd.20169. [DOI] [PubMed] [Google Scholar]
- 11.Gisbert JP, Panés J. Loss of response and requirement of Infliximab dose intensification in Crohn: A review. Am J Gastroenterol. 2009;104:760–7. doi: 10.1038/ajg.2008.88. [DOI] [PubMed] [Google Scholar]
- 12.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 13.Takagi S, Utsunomiya K, Kuriyama S, Yokoyama H, Takahashi S, Iwabuchi M, et al. Effectiveness of an 'half elemental diet' as maintenance therapy for Crohn's disease: A randomized-controlled trial. Aliment Pharmacol Ther. 2006;24:1333–40. doi: 10.1111/j.1365-2036.2006.03120.x. [DOI] [PubMed] [Google Scholar]
- 14.Okada M, Yao T, Yamamoto T, Takenaka K, Imamura K, Maeda K, et al. Controlled trial comparing an elemental diet with prednisolone in the treatment of active Crohn's disease. Hepatogastroenterology. 1990;37:72–80. [PubMed] [Google Scholar]
- 15.Shah R, Kellermayer R. Microbiome associations of therapeutic enteral nutrition. Nutrients. 2014;6:5298–311. doi: 10.3390/nu6115298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerasimidis K, Bertz M, Hanske L, Junick J, Biskou O, Aguilera M, et al. Decline in presumptively protective gut bacterial species and metabolites are paradoxically associated with disease improvement in pediatric Crohn's disease during enteral nutrition. Inflamm Bowel Dis. 2014;20:861–71. doi: 10.1097/MIB.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 17.Andou A, Hisamatsu T, Okamoto S, Chinen H, Kamada N, Kobayashi T, et al. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology. 2009;136:564–74. doi: 10.1053/j.gastro.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 18.Gupta NK, Thaker AI, Kanuri N, Riehl TE, Rowley CW, Stenson WF, et al. Serum analysis of tryptophan catabolism pathway: Correlation with Crohn's disease activity. Inflamm Bowel Dis. 2012;18:1214–20. doi: 10.1002/ibd.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guloksuz S, Wichers M, Kenis G, Russel MG, Wauters A, Verkerk R, et al. Depressive symptoms in Crohn's disease: Relationship with immune activation and tryptophan availability. PLoS One. 2013;8:e60435. doi: 10.1371/journal.pone.0060435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hisamatsu T, Okamoto S, Hashimoto M, Muramatsu T, Andou A, Uo M, et al. Novel, objective, multivariate biomarkers composed of plasma amino acid profiles for the diagnosis and assessment of inflammatory bowel disease. PLoS One. 2012;7:e31131. doi: 10.1371/journal.pone.0031131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Israeli E, Berenshtein E, Wengrower D, Aptekar L, Kohen R, Zajicek G, et al. Prophylactic administration of topical glutamine enhances the capability of the rat colon to resist inflammatory damage. Dig Dis Sci. 2004;49:1705–12. doi: 10.1023/b:ddas.0000043390.12150.8b. [DOI] [PubMed] [Google Scholar]
- 22.Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487–93. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vicario M, Amat C, Rivero M, Moretó M, Pelegrí C. Dietary glutamine affects mucosal functions in rats with mild DSS-induced colitis. J Nutr. 2007;137:1931–7. doi: 10.1093/jn/137.8.1931. [DOI] [PubMed] [Google Scholar]
- 24.Tsune I, Ikejima K, Hirose M, Yoshikawa M, Enomoto N, Takei Y, et al. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology. 2003;125:775–85. doi: 10.1016/s0016-5085(03)01067-9. [DOI] [PubMed] [Google Scholar]
- 25.Best WR, Becktel JM, Singleton JW, Kern F., Jr Development of a Crohn's disease activity index. Gastroenterology. 1976;70:439–4. [PubMed] [Google Scholar]
- 26.Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Löfberg R, Modigliani R, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122:512–30. doi: 10.1053/gast.2002.31072. [DOI] [PubMed] [Google Scholar]
- 27.Vermeire S, Van Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661–5. doi: 10.1097/00054725-200409000-00026. [DOI] [PubMed] [Google Scholar]
- 28.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: Performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–9. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 29.Papamichael K, Karatzas P, Mantzaris GJ. Faecal calprotectin but not C-Reactive Protein (CRP) or Crohn's Disease Activity Index (CDAI) may predict post-operative endoscopic recurrence of Crohn's Disease. J Crohns Colitis. 2013;7:700–1. doi: 10.1016/j.crohns.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ. 2010;341:c3369. doi: 10.1136/bmj.c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ihata Y, Miyagi E, Numazaki R, Muramatsu T, Imaizumi A, Yamamoto H, et al. Amino acid profile index for early detection of endometrial cancer: Verification as a novel diagnostic marker. Int J Clin Oncol. 2014;19:364–72. doi: 10.1007/s10147-013-0565-2. [DOI] [PubMed] [Google Scholar]
- 32.Norton JA, Gorschboth CM, Wesley RA, Burt ME, Brennan MF. Fasting plasma amino acid levels in cancer patients. Cancer. 1985;56:1181–6. doi: 10.1002/1097-0142(19850901)56:5<1181::aid-cncr2820560535>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Naini AB, Dickerson JW, Brown MM. Preoperative and postoperative levels of plasma protein and amino acid in esophageal and lung cancer patients. Cancer. 1988;62:355–60. doi: 10.1002/1097-0142(19880715)62:2<355::aid-cncr2820620221>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 34.Kubota A, Meguid MM, Hitch DC. Amino acid profiles correlate diagnostically with organ site in three kinds of malignant tumors. Cancer. 1992;69:2343–8. doi: 10.1002/1097-0142(19920501)69:9<2343::aid-cncr2820690924>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 35.Maeda J, Higashiyama M, Imaizumi A, Nakayama T, Yamamoto H, Daimon T, et al. Possibility of multivariate function composed of plasma amino acid profiles as a novel screening index for non-small cell lung cancer: A case control study. BMC Cancer. 2010;10:690. doi: 10.1186/1471-2407-10-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holecek M. Three targets of branched-chain amino acid supplementation in the treatment of liver disease. Nutrition. 2010;26:482–90. doi: 10.1016/j.nut.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Millward DJ, Price GM, Pacy PJ, Halliday D. Maintenance protein requirements: The need for conceptual re-evaluation. Proc Nutr Soc. 1990;49:473–87. doi: 10.1079/pns19900056. [DOI] [PubMed] [Google Scholar]
- 38.Nishihira T, Takagi T, Mori S. Amino acid imbalance and intracellular protein synthesis. Nutrition. 1993;9:37–42. [PubMed] [Google Scholar]
- 39.Laidlaw SA, Kopple JD. Newer concepts of the indispensable amino acids. Am J Clin Nutr. 1987;46:593–605. doi: 10.1093/ajcn/46.4.593. [DOI] [PubMed] [Google Scholar]
- 40.Young VR, Bier DM, Pellett PL. A theoretical basis for increasing current estimates of the amino acid requirements in adult man, with experimental support. Am J Clin Nutr. 1989;50:80–92. doi: 10.1093/ajcn/50.1.80. [DOI] [PubMed] [Google Scholar]
- 41.Hirai F, Ishihara H, Yada S, Esaki M, Ohwan T, Nozaki R, et al. Effectiveness of concomitant enteral nutrition therapy and infliximab for maintenance treatment of Crohn's disease in adults. Dig Dis Sci. 2013;58:1329–34. doi: 10.1007/s10620-012-2374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamamoto T, Nakahigashi M, Umegae S, Matsumoto K. Prospective clinical trial: Enteral nutrition during maintenance infliximab in Crohn's disease. J Gastroenterol. 2010;45:24–9. doi: 10.1007/s00535-009-0136-5. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Takahama K, Kimura T, Mizuno T, Nagasaka M, Iwata K, et al. Effect of concurrent elemental diet on infliximab treatment for Crohn's disease. J Gastroenterol Hepatol. 2006;21:1143–9. doi: 10.1111/j.1440-1746.2006.04317.x. [DOI] [PubMed] [Google Scholar]