Abstract
Background/Aim:
This study aims to explore the expression and significance of feces cyclooxygensae-2 (COX-2) mRNA in colorectal cancer and colorectal adenomas.
Materials and Methods:
The expression of feces COX-2 mRNA in colorectal cancer (n = 28), colorectal adenomas (n = 54), and normal control group (n = 11) were examined by reverse transcriptase polymerase chain reaction (RT-PCR). The positive rate of fecal occult blood test (FOBT) were detected in colorectal cancer (n = 30), colorectal adenomas (n = 56), and normal control group (n = 11); the sensitivity of the two methods was also compared.
Results:
The positive rate of feces COX-2 mRNA in colorectal cancer was 82.1% (25/28), which was significantly higher than colorectal adenomas 59.3% (32/54), and normal tissues 18.2% (2/11), the difference being significant between the three groups (χ2= 13.842, P = 0.001). The positive rate of FOBT in colorectal cancer was 73.3% (10/30), which was significantly higher than colorectal adenomas 10.7% (6/56) and normal tissues 9.1% (1/11), the difference being significant between these three groups (χ2= 7.525, P = 0.023). There was no significant association between feces COX-2 expression and various clinical pathological features of colorectal cancer and colorectal adenomas (P > 0.05). The sensitivity of the RT-PCR method is higher than FOBT, however, the specificity of FOBT is slightly higher than RT-PCR.
Conclusions:
High expression of feces COX-2 mRNA in colorectal adenomas and colorectal cancer is a common event; it is an early event in the development of colorectal adenomas to colorectal cancer. Feces COX-2 mRNA has a high sensitivity for detect colorectal cancer; combination with FOBT will be the best alternative. Feces COX-2 can be potentially used in the early diagnosis and screening of colorectal cancer.
Key Words: Colorectal adenomas, colorectal cancer, COX-2 mRNA, feces, RT-PCR
Colorectal cancer (CRC) is one of the high incidence of malignant tumors worldwide, with the morbidity and mortality ranking third among malignant tumors in the Western world.[1] Owing to economic development, air pollution, and changing lifestyle to high protein and high fat diet, the incidence and mortality of CRC has been on a rising trend in China.[2] Since the symptoms are not obvious in the early stages of CRC and the rate of early dictation and diagnosis is low, it is easy to misdiagnosis or miss the diagnosis completely. It has been reported that it takes an average of 19 years from normal to mucosa-dysplasia-adenoma-cancer,[3] and hence there is sufficient time for early screening. Evidence-based medicine statistics showed that the survival rate is as high as 90% in early staged of CRC, however, 5-year survival rate is less than 5% in advanced stages.[4] Early diagnosis of precancerous lesions and early cancer is possible through early CRC screening; the mortality rate of CRC can be significantly reduced after appropriate treatment.[5] Therefore, it is very important to find a noninvasive method to screen early CRC.
Thus far, there are many methods of CRC screening, such as fecal occult blood test (FOBT), colonoscopy and sigmoidoscopy, fecal molecular biology-related antigen and antibody detection, and colonoscopy plus pathological biopsy, the gold standard in the diagnosis of CRC,[6] however, these methods are invasive, expensive, require high technology equipment, and bowel preparation. Therefore, these methods have not yet become the preferred screening methods. So far, FOBT has been widely used as a screening test for CRC.[7,8] The introduction of FOBT in CRC screening reduced the morbidity and mortality associated with CRC.[9] The method has the advantages of being simple and inexpensive,[10] but is also associated with high false positive and false negative rate and low sensitivity and low specificity,[11] limiting its clinical diagnostic. Fecal tumor molecular biology detection is a noninvasive screening technology which will be widely applicable in the future. The method has the advantages of good patient compliance, convenience, repeated operation, and without bowel preparation. RNA-based stool assay detection has become very popular recently; the method has good sensitivity and specificity and is inexpensive. In terms of experimental requirements, the method only requires an ordinary PCR amplifier, and can detect a variety of feces RNA gene at the same time. The sensitivity of the fecal cyclooxygensae-2 COX-2 is 80–90%[12,13] and the specificity is 100%.[14] Research shows that more than 80% of CRC have high COX-2 expression compared with normal mucosa.[15,16] Thus, COX-2 is a good candidate gene for an RNA-based tool assay, and is a promising approach to detect feces COX-2 mRNA for CRC screening, however, it needs more clinical investigation.
According to the above research background, our study was aimed at detecting the expression and significance of feces COX-2 mRNA in CRC and colorectal adenomas by reverse transcriptase polymerase chain reaction (RT-PCR), and compare the sensitivity between RT-PCR and FOBT, in search of a noninvasive screening method for early CRC detection, allowing us to distinguish CRC from controls by feces COX-2 mRNA.
MATERIALS AND METHODS
Patients and sample
All the fecal samples were collected from the Fifth Affiliated Hospital of Sun Yat-Sen University between April 2009 and April 2010. The patients were divided into colorectal cancer, colorectal adenoma, and normal control group. There were 30 patients with colorectal cancer (28 cases were extracted RNA) who were confirmed by pathology after surgery without radiation, chemotherapy, and other adjuvant treatment before the operation. According to the level of tumor, 10 cases of high level (6 cases of moderately-low differentiated adenocarcinoma, 3 cases of poorly differentiated adenocarcinoma, and 1 case of mucus gland carcinoma), 18 cases of low level (14 cases of high-differentiation adenocarcinoma and 4 cases of moderately differentiated adenocarcinoma), 10 cases of higher than 5c m and 18 cases of less than 5 cm according to the size, 18 cases of negative metastasis and 10 cases of positive metastasis, and 22 cases of no distant metastasis and 6 cases of distant metastasis; according to Dukes stage, there were 3, 8, 11, and 8 cases in stage A, B, C, and D respectively. Fifty-six patients of colorectal adenomas (54 cases were extracted RNA), who were confirmed by pathology after polypectomy. The average age of the 54 patients was 59.8 years (range: 28–75), including 35 males and 19 females; 32 cases of more than 50 years and 22 cases of less than 50 years; 40 cases of colon adenomas and 14 cases of rectum adenomas; 28 cases of tubular adenoma, 11 cases of villous adenoma, and 15 cases of villous tubular adenoma. A total of 13 control patients (11 cases extracted RNA) with no obvious pathological changes through electronic colonoscopy biopsy and no colorectal cancer and colorectal polyps. They were mainly irritable bowel syndrome and colorectal inflammation.
All the feces samples (approximately 4g) were collected 2 or 3 days before colonoscopy examination or surgery (within 1 hour after bowel movements). The patients were required to follow a vegetarian diet 3 days before collection of feces samples. Thirty cases of colorectal cancer feces, 56 cases of colorectal adenomas feces, and 11 cases of normal controls feces were detected by FOBT immediately. Twenty-eight cases of colorectal cancer feces, 54 cases of colorectal adenomas feces, and 11 cases of normal controls feces were stored at −80°C for 3 hours after collection for total RNA extraction.
This study was conducted in accordance with the declaration of Helsinki. This study was conducted after obtaining approval from the Ethics Committee of Sun Yat-Sen University. Written informed consent was obtained from all participants.
Reverse transcriptase–polymerase chain reaction
Total RNA was extracted in feces samples by Trizol reagent (Takala, Dalian, China) following the manufacturer's instructions, and its concentration and purity were measured by ultraviolet spectrophotometry. A total of 1.0 μg fecal RNA was reverse transcribed in complementary DNA (cDNA) by Avian Myeloblastosis Virus (AMV) Reverse Transcriptase Kit (Takala, Dalian, China). The cycling conditions were as follows: 42°C for 1 h and 99°C for 4 min. cDNA was used as the template in PCR amplification with primers for COX-2 (Forward: 5'-CCACCTCTGCGATGCTCTTC-3', and Reverse: 5'-ACATTCCCCACGGTTTTGAC-3') and β-actin (Sangon Bitotech, Shanghai, China). PCR reaction system (50 μl): ddH2O 39 μl, l0 × Reaction Buffer 5 μl, dNTP 1 μl, Taq polymerase 1 μl (2.5U), template cDNA 2 μl, upstream and downstream primer of COX-2 per 1 μl (or upstream and downstream primer of COX-2 per 1 μl). The PCR cycle consisted of the following steps: initial denaturing at 94°C for 5 min, denaturing at 94°C for 30 s, annealing at 55°C for 45 s, and elongation at 72°C for 45 s, which was repeated for 35 cycles. The relative expression level of COX-2 mRNA were interpreted by agarose electrophoresis analysis.
Fecal occult blood test
Approximately 5–10 mg fecal samples were mixed with 0.15 ml distilled water. Then, a test paper (Yikang Biological Technology Co., China) was inserted for 1–5 min and observed to be positive or negative result according to the instruction.
Statistical analysis
Statistical analyses were performed with the Statistical analyses were performed with SSPS 19.0 (Statistical Package for the Social Sciences company) software, and qualitative data was described as frequency and rate; the comparison between groups of qualitative data was made using the χ2 test and χ2 test with Yates' continuity correction; P < 0.05 was considered significant.
RESULTS
The results of reverse transcriptase–polymerase chain reaction and fecal occult blood test
The positive rate of feces COX-2 mRNA in normal control group, colorectal adenoma, and CRC was 18.2% (2/11), 59.3% (32/54), and 82.1% (25/28) respectively. It showed that feces COX-2 mRNA is expressed in CRC, colorectal adenomas, and normal control group, which showed an increasing trend; the difference was statistically significant (χ2= 13.842, P = 0.001) [Table 1].
Table 1.
The result of RT-PCR and FOBT
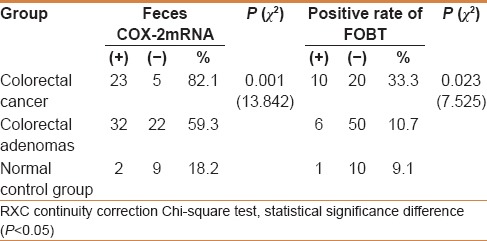
The positive rate of FOBT in normal control group, colorectal adenoma, and CRC was 9.1% (1/11), 10.7% (6/56), and 33.3% (10/30), respectively, which showed an increasing trend; the difference was statistically significant (χ2= 7.525, P = 0.023) [Table 1].
The relationship between feces COX-2 mRNA and clinical pathological factors
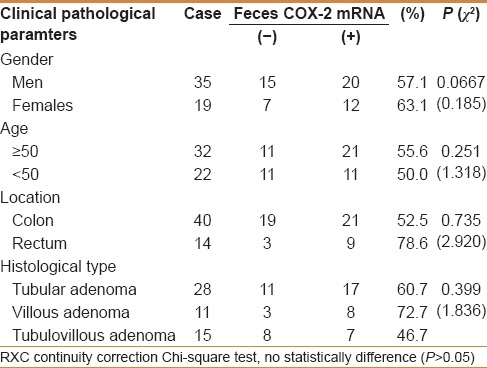
There was no significant association between feces COX-2 mRNA expression with clinical pathological factors in CRC, including differentiation, tumor size, Dukes stage, and distant metastasis (P > 0.05) [Table 2]. There were also no significant association between feces COX-2 mRNA expression with clinical pathological factors in colorectal adenoma, such as age, gender, tumor location, and histological type (P > 0.05) [Table 3], which indicated that is a common event of feces COX-2 mRNA in colorectal cancer and colorectal adenomas.
Table 2.
Relationship between COX-2mRNA and clinical pathological parameters of colorectal cancer
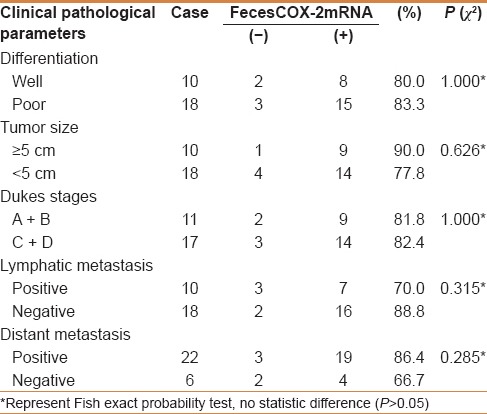
Table 3.
Relationship between COX-2mRNA and clinical pathological parameters of colorectal adenomas
Comparison of reverse transcriptase–polymerase chain reaction with fecal occult blood test
The positive rate of COX-2 mRNA in CRC, colorectal adenomas, and normal control group by RT-PCR were higher than the positive of FOBT [Table 1]. The sensitivity and specificity in colorectal cancer was 82.1% and 81.8% by RT-PCR and 33.3% and 90.9% by FOBT, respectively, which show that the sensitivity of RT-PCR was higher than FOBT but specificity was a slightly lower than FOBT [Table 4].
Table 4.
Sensitivity and specificity of RT-PCR and FOBT

DISCUSSION
CRC is a common malignant tumor in the digestive system, and its mortality is ranked third in malignant tumors. Because its symptoms are not obvious in the early stage, early diagnosis rate is low. During its progress from adenoma to carcinoma, if we can detect precancerous lesions and cancer early, we can prevent the progress of precancerous lesion and reduce the incidence of CRC, because a large number of patients can be treated successfully when metastasis does not occur.[17] Hence, it is very important to find an early screening method to enable an early diagnose.
CRC is developed from the intestinal mucosa; the exfoliated cells contain cancer cells and are abundant in the mucocellular layer overlying the CRC lesion.[18] Because feces are in direct contact with the early lesions of CRC, we believe that it can be detected early by testing the exfoliated cells from the feces during the early stage. Feces examination has the advantage of being convenient and simple and is easily accepted by patients. At present, molecular biological detection technology is continuously improving; RNA-based stool assay is a promising application in the early diagnosis of CRC, although RNA related genes can exist in conditions other than CRC, such as breast cancer, bladder cancer, lung cancer, oral cancer. But it is difficult to find a tumor cell in the feces of those paitents the exfoliated cells from esophageal cancer and gastric carcinoma are almost destroyed by gastric acid and pepsin before discharge, the morbidity of duodenum carcinoma, pancreas cancer, gallbladder carcinoma, bile duct cancer, and small intestinal tumors is extremely low. Therefore, RNA-based stool assay has high specificity in the detection of CRC. At present, fecal RNA gene includes COX-2, MMP-7,[13] c-myc,[19] CD44v6, miRNA,[20] RPL27A, CK19,[21] etc. Fecal RNA can be extracted in theory, however, it contains large amounts of impurities, such as bacteria, mucus, food, that can lead to RNA degradation. According to literature, low temperature and short time to collect sample is associated with a large number of exfoliated cells.[18] Our experience is that stool collection is the key step before fecal RNA extraction, and low temperature (0–4°C) and shortening acquisition time (less than 3 hours) can reduce the number of exfoliated cell death and degradation of RNA. At the same time, wearing sterile gloves during the testing avoids RNA degradation and pollution; the room of extraction, separation, and amplifying RNA must be kept separate.
Feces RNA detection technology is based on PCR detection technology, mainly including RT-PCR, real-time fluorescence quantitative RT-PCR technique, and nested RT-PCR. Over the past 20 years, some researchers have successfully extracted fecal RNA. Leung et al.[22] analyzed the expression of fecal COX-2 in CRC by RT-PCR, 10 cases detected COX-2 mRNA in 20 cases of CRC, only 1 case was detected COX-2 mRNA in 30 cases of advanced adenomas, 2 cases detected COX-2 mRNA in 30 cases of normal control group. Takai et al.[13] found that the expression of feces MMP-7 mRNA in CRC increased with RT-PCR. Kanaoka et al.[14] detected the sensitivity and specificity of feces COX-2 mRNA by nested RT-PCR. Lagerholm et al.[19] used nested RT-PCR methods to analyze the expression level of c-myc gene. Yang et al.[21] examined the fecal cytokeratin CK19 and ribosomal protein RPL19 in CRC with real-time quantitative RT-PCR, and found that it were associated with distant metastasis. Atmar et al.[23] detected the fecal RNA calicivirus by real-time RT-PCR. Dkhil et al.[24] detected the expression of jejunum of miRNA in mice by quantitative RT-PCR technologies. Koga et al.[25] reported that the sensitivity and specificity of feces miRNA were 74.1% and 79%, respectively, in patients with CRC. Therefore, on the basis of these studies, we further investigated feces COX-2 mRNA expression and compared the sensitivity of RT-PCR method and FOBT to provide the theoretical basis for fecal COX-2 mRNA in early screening for CRC, as well as the mechanism of COX-2 in the development of CRC.
Our study adopted the RT-PCR method to detect feces COX-2 mRNA expression. The positive rate of feces COX-2 mRNA in normal control group, colorectal adenoma, and CRC was 18.2% (2/11), 59.3% (32/54), and 82.1% (25/28), respectively. It indicated that fecal COX-2 mRNA can be detected in CRC and colorectal adenomas, with high sensitivity (82.1%) in CRC. Our results are consistent with those of Hamaya et al.[26] There are also other authors[12] who detected fecal COX-2 mRNA in exfoliated cells with nested RT-PCR, evaluating the analysis application efficiency of feces COX-2 mRNA; the results showed that the positive rate of feces COX-2 mRNA was 80.9%. The positive rate of our study was higher than this research; the reason may be related to different research methods or that the sample size was not sufficient. The methods and results mentioned above were not completely consistent with ours but showed that fecal COX-2 expression was high in CRC, and has good value in the early diagnosis of CRC.
We also compared the sensitivity and specificity between the two methods. The sensitivity of RT-PCR and FOBT was 82.1% and 33.3%, respectively, in the CRC group, 59.3% and 10.7%, respectively, in the colorectal adenomas group, and 18.2% and 9.1%, respectively, in the normal control group. The specificity of RT-PCR and FOBT were 81.8% and 90.9%, respectively, in the CRC group. Our results showed that the sensitivity of RT-PCR method was obviously higher than FOBT, however, the specificity of FOBT was slightly higher than RT-PCR. Hence, we can detect feces COX-2 mRNA expression by RT-PCR for early CRC detection, diagnosis, and treatment, and combined with FOBT will offer better utility.
We found that the level of feces COX-2 mRNA had no obvious correlation with gender, age, location, and histology in colorectal adenomas group (P > 0.05). No significant correlation was noted between the level of fecal COX-2 mRNA and clinical pathological parameters in CRC (P > 0.05), including differentiation, lymphatic and distant metastasis, Dukes stage, etc., which is consistent with Kanaoka's research, who found that COX-2 mRNA was not correlated with tumor size, tumor location, and Dukes stage.[14] However, some studies have shown that the expression feces COX-2 in colorectal adenomas were associated with the clinical pathological parameters. Hamaya et al.[26] examined the factors that affect feces COX-2 mRNA expression, they found that the expression of feces COX-2 mRNA was influenced by many factors, including the number of total exfoliated tumor cells, shedding of inflammatory cells, the size of tumor surface, and the amount of COX-2 mRNA in the organization, but not with the part of the tumor. Thus far, the relationships between the expression of fecal COX-2 mRNA and clinical pathological parameters in colorectal adenomas and CRC is still controversial, the reason may be related to the number of the sample, testing methods and test reagents, the evaluation criteria, etc., thus necessitating large number of samples for further research. In conclusion, these studies (including ours) suggest that the expression of feces COX-2 mRNA in CRC and colorectal adenomas is increased, and our study found that the level of fecal COX-2 mRNA in CRC were obviously higher than that of colorectal adenomas. Maybe, it is an important early event in the process of colorectal adenomas to adenocarcinoma evolution.
In future, preparing a storage reagent which can keep stool samples at room temperature is needed, which will not only favor the collection and preservation of feces samples but also increase the purity and reduce the degradation of stool RNA, increasing the practicability of stool RNA detection. Further, stool RNA detection can be combined with gene chip technology to improve the sensitivity and specificity. In addition, continue to screening diagnosis indicators with high sensitivity and specificity, and searching a convenient and rapid reagent which can detect a variety of gene for clinical use.
Financial support and sponsorship
Nil.
Conflicts of interest
All of the authors declare that they have no conflicts of interest regarding this paper.
REFERENCES
- 1.Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. doi: 10.3322/caac.21220. [DOI] [PubMed] [Google Scholar]
- 2.Zheng ZX, Zheng RS, Zhang SW, Chen WQ. Colorectal cancer incidence and mortality in China, 2010. Asian Pac J Cancer Prev. 2014;15:8455–460. doi: 10.7314/apjcp.2014.15.19.8455. [DOI] [PubMed] [Google Scholar]
- 3.Walsh JM, Terdiman JP. Colorectal cancer screening. JAMA. 2003;289:1288–96. doi: 10.1001/jama.289.10.1288. [DOI] [PubMed] [Google Scholar]
- 4.Settmacher U, Dittmar Y, Knösel T, Schöne U, Heise M, Jandt K, et al. Predictors of long-term survival in patients with colorectal liver metastases: A single center study and review of the literature. Int J Colorectal Dis. 2011;26:967–81. doi: 10.1007/s00384-011-1195-7. [DOI] [PubMed] [Google Scholar]
- 5.Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: A review of cur-rent American cancer society guidelines and issues in cancer screening. CA Cancer J Clin. 2010;60:99–119. doi: 10.3322/caac.20063. [DOI] [PubMed] [Google Scholar]
- 6.Kahi CJ, Imperiale TF, Juliar BE, Rex DK. Effect of screening colonoscopy on colorectal cancer incidence and mortality. Clin Gastroenterol Hepatol. 2009;7:770–5. doi: 10.1016/j.cgh.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Tam TK, Ng KK, Lau CM, Lai TC, Lai WY, Tsang LC. Fecal occult blood screening: Knowledge, attitudes, and practice in four Hong Kong primary care clinics. Hong Kong Med J. 2011;17:350–7. [PubMed] [Google Scholar]
- 8.Castells A. The usefulness of fecal tests in colorectal cancer screening. Gastroenterol Hepatol. 2014;37(Suppl 3):71–6. doi: 10.1016/S0210-5705(14)70085-8. [DOI] [PubMed] [Google Scholar]
- 9.Hamza S, Cottet V, Touillon N, Dancourt V, Bonithon-Kopp C, Lepage C, et al. Long-term effect of faecal occult blood screening on incidence and mortality from colorectal cancer. Dig Liver Dis. 2014;46:1121–5. doi: 10.1016/j.dld.2014.08.041. [DOI] [PubMed] [Google Scholar]
- 10.van Veldhuizen H, Bonfrer JM, Kuipers EJ. Faecal occult blood test for colorectal cancer screening: High quality for a good price. Ned Tijdschr Geneeskd. 2013;157:A6330. [PubMed] [Google Scholar]
- 11.Stracci F, Zorzi M, Grazzini G. Colorectal cancer screening: Tests, strategies, and perspectives. Front Public Health. 2014;2:210. doi: 10.3389/fpubh.2014.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu H, Yang L, Yan F, Li Y, Sun X, Zhou Z. Detection of fecal peroxisome proliferator-activated receptor delta and cyclooxygenase 2 mRNA in screening of colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2014;17:26–30. [PubMed] [Google Scholar]
- 13.Takai T, Kanaoka S, Yoshida K, Hamaya Y, Ikuma M, Miura N, et al. Fecal cyclooxygenase 2 plus matrix metalloproteinase 7 mRNA assays as a marker for colorectal cancer screening. Cancer Epidemiol Biomarkers Prev. 2009;18:1888–93. doi: 10.1158/1055-9965.EPI-08-0937. [DOI] [PubMed] [Google Scholar]
- 14.Kanaoka S, Yoshida K, Miura N, Sugimura H, Kajimura M. Potential usefulness of detecting cyclooxygenase 2 messenger RNA in feces for colorectal cancer screening. Gastroenterology. 2004;127:422–7. doi: 10.1053/j.gastro.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Roelofs HM, Te Morsche RH, van Heumen BW, Nagengast FM, Peters WH. Over-expression of COX-2 mRNA in colorectal cancer. BMC Gastroenterol. 2014;14:1. doi: 10.1186/1471-230X-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lobo Prabhu KC, Vu L, Chan SK, Phang T, Gown A, Jones SJ, et al. Predictive utility of cyclooxygenase-2 expression by colon and rectal cancer. Am J Surg. 2014;207:712–6. doi: 10.1016/j.amjsurg.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 17.Eisenberg B, Decosse JJ, Harford F, Michalek J. Carcinoma of the colon and retum: The natural history reviewed in 1704 patients. Cancer. 1982;49:1131–4. doi: 10.1002/1097-0142(19820315)49:6<1131::aid-cncr2820490611>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Loktionov A. Cell exfoliation in the human colon: Myth, reality and implications for colorectal cancer screening. Int J Cancer. 2007;120:2281–9. doi: 10.1002/ijc.22647. [DOI] [PubMed] [Google Scholar]
- 19.Lagerholm S, Lagerholm S, Dutta S, Nair P. Non-invasive detection of c-myc p64, c-myc p67 and c-erbb-2 in colorectal cancer. Scand J Gastroenterol. 2005;40:1343–50. doi: 10.1080/00365520510023549. [DOI] [PubMed] [Google Scholar]
- 20.Phua LC, Chue XP, Koh PK, Cheah PY, Chan EC, Ho HK. Global fecal microRNA profiling in the identification of biomarkers for colorectal cancer screening among Asians. Oncol Rep. 2014;32:97–104. doi: 10.3892/or.2014.3193. [DOI] [PubMed] [Google Scholar]
- 21.Yang SH, Huang CJ, Lee CL, Liu CC, Chien CC, Chen SH. Fecal RNA detection of cytokeratin 19 and ribosomal protein L19 for colorectal cancer. Hepatogastroenterology. 2010;57:710–5. [PubMed] [Google Scholar]
- 22.Leung WK, To KF, Man EP, Chan MW, Hui AJ, Ng SS, et al. Detection of hypermethylated DNA or cyclooxygenase-2 messenger RNA in feces samples of patients with colorectal cancer or polyps. Am J Gastroenterol. 2007;102:1070–6. doi: 10.1111/j.1572-0241.2007.01108.x. [DOI] [PubMed] [Google Scholar]
- 23.Atmar RL, Neill FH, Le Guyader FS. Detection of human caliciviruses in fecal samples by RT-PCR. Methods Mol Biol. 2011;665:39–50. doi: 10.1007/978-1-60761-817-1_3. [DOI] [PubMed] [Google Scholar]
- 24.Dkhil M, Abdel-Baki AA, Delić D, Wunderlich F, Sies H, Al-Quraishy S. Eimeria papillata: Upregulation of specific miRNA-species in the mouse jejunum. Exp Parasitol. 2011;127:581–6. doi: 10.1016/j.exppara.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Koga Y, Yasunaga M, Takahashi A, Kuroda J, Moriya Y, Akasu T, et al. MicroRNA expression profiling of exfoliated colonocytes isolated from feces for colorectal cancer screening. Cancer Prev Res. 2010;3:1435–42. doi: 10.1158/1940-6207.CAPR-10-0036. [DOI] [PubMed] [Google Scholar]
- 26.Hamaya Y, Yoshida K, Takai T, Ikuma M, Hishida A, Kanaoka S. Factors that contribute to fecal cyclooxygenase-2 mRNA expression in subjects with colorectal cancer. BJ Cancer. 2010;102:916–21. doi: 10.1038/sj.bjc.6605564. [DOI] [PMC free article] [PubMed] [Google Scholar]


