Abstract
Background/Aims:
5-Fluorouracil (5-FU) is widely used in the treatment of patients with colorectal cancer (CRC). However, the efficacy of 5-FU as a single agent is limited, with multiple undesired side effects. Therefore, the aim of the current study was to assess the efficacy of CUDC-907 (a dual inhibitor of histone deacetylase and phosphatidylinositide 3-kinase) in combination with 5-FU against CRC cells.
Materials and Methods:
Cell viability was determined using AlamarBlue and colony formation assays. Acridine orange/ethidium bromide staining and flow cytometry were used to measure apoptotic and necrotic events, as well as cell cycle progression. Immunoblotting was used to assess acetylation of histone H3 and phosphorylation of AKT.
Results:
Our data revealed enhanced toxicity of CUDC-907 against HCT116, RKO, COLO-205, and HT-29 CRC cells when combined with 5-FU. Similarly, the colony formation capability of HCT116 cells was suppressed by the combination treatment. Cells treated with CUDC-907 and 5-FU underwent apoptosis and necrosis, and exhibited increased polyploidy. Furthermore, CRC cells treated with CUDC-907 exhibited a higher degree of histone H3 lysine 9 acetylation (H3K9ac) and reduced AKT phosphorylation (Ser473).
Conclusion:
Our data revealed, for the first time, the enhanced inhibitory effect of CUDC-907 against CRC cells when combined with 5-FU, supporting the application of this combination as a potential therapeutic strategy in CRC treatment.
Key Words: Colorectal cancer, histone deacetylase inhibitors, phosphatidylinositide 3-kinase
Colorectal cancer (CRC) is ranked the third leading cause of cancer-related deaths in the United States, and the fourth globally.[1,2,3] In Saudi Arabia, CRC is the most common cancer type in men and the second most common in women.[4] For decades, the fluoropyrimidine class of anticancer agents, such as 5-fluorouracil (5-FU), has been the preferred therapy for CRC. Chemotherapeutic approaches for CRC have improved significantly in recent years, with combination therapies of 5-FU with newer agents such as oxaliplatin, irinotecan, cetuximab, and bevacizumab showing response rates of up to 50%.[5]
Epigenetic alterations such as DNA methylation and histone modification play a crucial role in the initiation and progression of cancer, including CRC.[6] Therefore, inhibition of key epigenetic modifiers has been utilized in the clinical management of various human malignancies. Histone deacetylase inhibitors (HDACis), such as vorinostat and romidepsin, have been approved for the treatment of cutaneous and peripheral T-cell lymphomas, respectively.[7] In vitro experiments and animal model studies have shown that DNA methyltransferase and HDAC inhibitors exhibit anti-tumor activities in CRC. Thus far, seven classes of HDACis have been developed, with four of them, i.e., short-chain fatty acids, cyclic peptides, hydroxamic acids, and benzamides, currently being investigated in the clinic.
CUDC-907 is a small-molecule, dual inhibitor of HDAC, and phosphatidylinositide 3-kinase (PI3K) that is currently in Phase 1 clinical testing for the treatment of patients with lymphoma and multiple myeloma. It has been shown to inhibit class I and class II HDAC enzymes as well as suppress the PI3K-AKT-mTOR pathway in solid tumors, in addition to inducing apoptosis and inhibiting cancer cell proliferation in xenograft tumors.[8] The aim of the current study is to investigate the effect of CUDC-907 as a single agent and in combination with 5-FU against CRC at the cellular and molecular levels.
MATERIALS AND METHODS
Cell culture and viability
HCT116 human colorectal cell line was obtained from ATCC (Manassas, VA, USA) and was subsequently authenticated by Genetica DNA Laboratories, Inc. (Burlington, NC, USA). The RKO cell line was obtained from ATCC, while the HT-29 and COLO-205 cell lines were obtained from CLS Cell Lines Service (CLS Cell Lines Service GmbH, Eppelheim, Germany). Cells were cultured in Dulbecco's modified Eagle's medium supplemented with 4500 mg/L D-glucose, 4 mM L-glutamine, 110 mg/L sodium pyruvate, 10% fetal bovine serum, 1× penicillin–streptomycin (Pen–Strep), and non-essential amino acids (all purchased from Gibco-Invitrogen, Waltham, MA, USA).[9] For cell viability assays, 1 × 104 cells were seeded in 96-well flat bottom plates and incubated for 24 h. Thereafter, cells were treated with the indicated dose of 5-FU (Sigma, St. Louis, MO, USA), CUDC-907 (Selleckchem Inc., Houston, TX, USA), or combination of both CUDC-907 and 5-FU at the same concentration. AlamarBlue assay (BUF012B; AbD Serotec, Kidlington, UK) was used to measure cell viability, as we previously described.[10] Briefly, cells under different treatment conditions were incubated with 10 μL (10% of total volume) of AlamarBlue substrate in the dark at 37°C for 60 min. Subsequently, plate readings were taken using the fluorescent mode (ex 530 nm/em 590 nm) with a BioTek Synergy II microplate reader (BioTek Inc., Winooski, VT, USA).
Immunoblotting
HCT116 cells were treated with 10 nM CUDC-907, and 48 h later, cells were lysed using RIPA buffer (Norgen-Biotek Corp., Thorold, Canada) supplemented with 1× Halt protease inhibitor cocktail (Pierce Inc., Rockford, IL, USA). Twenty micrograms of total protein were isolated and blotted using the Bio-Rad V3 Western work flow system, as previously described.[10,11] Immunoblotting was performed against acetyl-histone H3 (Lys9, C5B11) rabbit mAb (1:1000 dilution, #9649; Cell Signaling Technology, Danvers, MA, USA) and phospho-Akt (Ser473, D9E) XP rabbit mAb (1:2000 dilution, #4060; Cell Signaling Technology). The membrane was subsequently incubated with anti-rabbit IgG-horseradish peroxidase (HRP) conjugated antibody (1:3000 dilution, #7074p2; Cell Signaling Technology), and probed with mouse anti-human β-actin antibody (1:1000 dilution; GenWay Biotech, Inc., San Diego, CA, USA) followed by secondary anti-mouse IgG-HRP conjugated antibody (1:2500 dilution; GE Healthcare, Buckinghamshire, UK). For phospho-Akt (Ser473) detection, cells were starved for 4 h and incubated with insulin (20 mg/mL) for 30 min in order to activate the PI3K pathway in the absence or presence of CUDC-907 before cell pellet collection. Imaging was performed using the ChemiDoc™MP imager (Bio-Rad Laboratories, Hercules, CA, USA).
Flow cytometry (Cell cycle)
HCT116 cells were treated with dimethyl sulfoxide (DMSO) as control, 5-FU, CUDC-907, or combination of 5-FU and CUDC-907. On day 5, cell pellets were collected and washed with phosphate-buffered saline (PBS), and then were resuspended in 1 ml of FACS buffer (PBS/0.5% BSA), and then 3 ml of ice-cold 70% ethanol was added to fix the cells for 1 h on ice. Cell pellets were subsequently centrifuged, and re-suspended in 500 μL of PBS supplemented with 40 µg/mL RNAse A (Sigma) and 50 µg/mL propidium iodine (PI), before being analyzed using a Navious flow cytometer (Beckman Coulter, Miami, FL, USA). Staining was detected in the green fluorescence channel (FL1) and the data were analyzed by Kaluza software (Beckman Coulter).
Colony forming assay
Cell survival was measured by colony formation assay. HCT116 cells were seeded at different densities (15, 30, 60, 120, 240, and 500 cells) in 24-well tissue culture plates and were treated with CUDC-907 (10 nM), 5-FU (1.5 µM), or a combination of both. Cells were allowed to grow, and on day 6, cells were fixed with 70% ethanol and stained with Diff-Quik Stain Set (Siemens Healthcare Diagnostics, Inc., Newark, DE, USA), as previously described.[10] Images of formed colonies in each well were taken using a Carl Zeiss-Axio Observer 1 microscope equipped with an Axiocam MRc5 digital camera (Carl Zeiss, Oberkochen, Germany) at 20× magnification.
Apoptosis and viability assay
Apoptosis of cells was determined by fluorescence microscopy after exposure to DMSO (control), 5-FU (1.5 µM), CUDC-907 (10 nM), or CUDC-907 (10 nM) +5-FU (1.5 µM) using acridine orange and ethidium bromide (AO/EB) staining.[10] HCT116 cells were stained with dual fluorescent staining solution (1 µL) containing 100 µg/mL AO and 100 µg/mL EB (AO/EB; Sigma). Cells were washed twice with PBS and were gently mixed with AO/EB (1:100 dilution) dye solution for 1 min; afterwards, the cells were observed and photographed under a Nikon Eclipse Ti fluorescence microscope (Nikon, Tokyo, Japan). Acridine orange/ethidium bromide staining uses a combination of two dyes to visualize cells with aberrant chromatin organization. The differential uptake of AO/EB allows the identification of viable and non-viable cells. In particular, AO is used to visualize the number of cells, which have undergone apoptosis.
Statistical analysis
Statistical analyses and graphing were performed using Microsoft Excel 2010 and GraphPad Prism 6.0 software (GraphPad Software Inc., San Diego, CA, USA). Unpaired two-tailed t-test was used to calculate P- values. P-values < 0.05 were considered significant.
RESULTS
Enhanced efficacy of 5-FU when combined with CUDC-907 in HCT116 cells
First, we assessed the effect of CUDC-907 as a single agent (2.5, 5.0, and 10.0 nM) and in combination with 5-FU (1.5 µM) on HCT116 cell line. Low concentrations of CUDC-907 were used in this experiment to determine the lowest dose of CUDC-907 that could potentially synergize with 5-FU against CRC cells. The data presented in Figure 1 demonstrated no significant inhibition of cell viability when CUDC-907 or 5-FU was used as single agents at the indicated concentrations. Interestingly, when the two agents were combined, we observed significant toxicity against the HCT116 cells, with the maximum toxicity observed when 5-FU was combined with 10.0 nM CUDC-907 [Figure 1]. Similar results were obtained when using the RKO, COLO-205, and HT-29 CRC models [Supplementary Figure 1]. We subsequently employed the colony formation assay (CFU) to assess the effect of CUDC-907, 5-FU, and the combination of both on the ability of HCT116 cells to form colonies in vitro. The data presented in Figure 2 demonstrated significant inhibition of colony formation, especially in the combination group, which suggested possible targeting of CRC colony forming units or tumor initiating cells (TICs) by the combination treatment.
Figure 1.
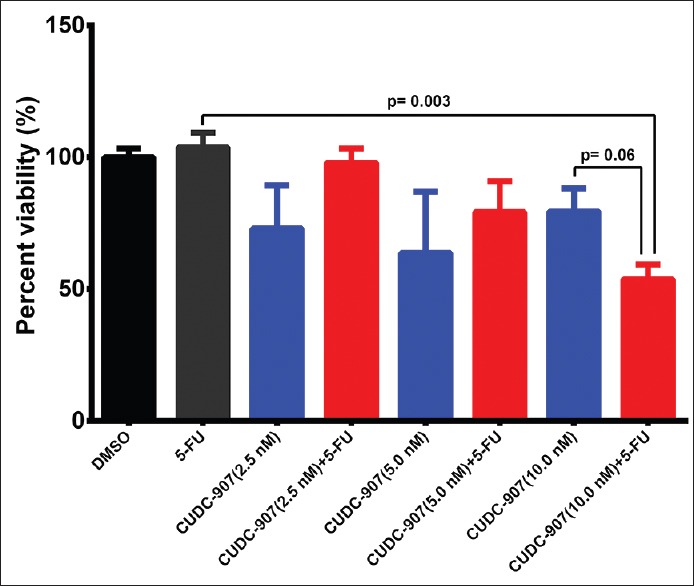
CUDC-907 in combination with 5-FU inhibits CRC cell proliferation. HCT116 cells were treated with different doses (2.5, 5, and 10 nM) of CUDC-907 in the absence and presence of 1.5 μM 5-FU. Cell viability was determined on day 5 posttreatment using alamarBlue assay. Data are presented as mean ± S.E., n = 8
Figure 2.
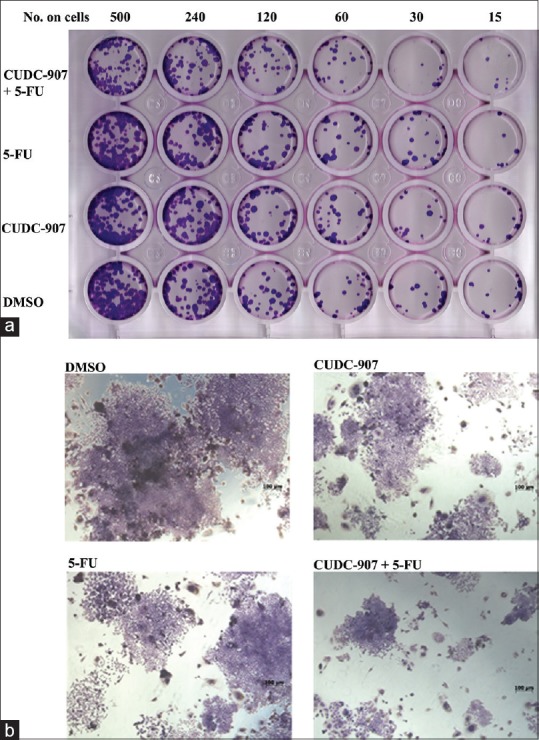
Inhibition of CRC colony formation by CUDC-907 when combined with 5-FU. (a) Relative cell survival as determined by colony forming assay at different cell counts of HCT116 (15, 30, 60, 120, 240, and 500) cells/well treated with 10 nM CUDC-907 alone, 1.5 μM 5-FU alone, or with combination of CUDC-907 and 5-FU. (b) Image representing colony formation of HCT116 cells treated with 10 nM CUDC-907 alone, 1.5 μM 5-FU alone, or with combination of CUDC-907 and 5-FU
CUDC-907 sensitizes CRC cells to 5-FU-induced cell death
The AlamarBlue cell viability and clonogenic data presented in Figures 1 and 2 revealed that the combination of CUDC-907 (10 nM) +5-FU (1.5 µM) markedly inhibited the proliferation and colony formation ability of HCT116 CRC cells. We subsequently used the AO/EB assay to characterize the mode of cell death (apoptosis or necrosis) in HCT116 cells that were exposed to CUDC-907, 5-FU, or the combination of CUDC-907 + 5-FU compared to control HCT116 cells. Our data revealed that the combination of CUDC-907 + 5-FU was more toxic to the cells than either of the drugs alone [Figure 3]. The combination group had more cells undergoing apoptosis (bright green with condensed chromatin, white arrow head) and necrosis (red, yellow arrow head).
Figure 3.
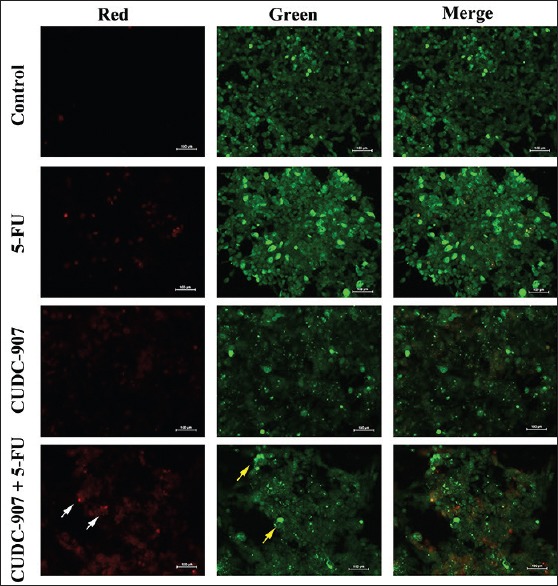
Combination of CUDC-907 and 5-FU induces cell death in CRC cells. Representative fluorescence images of HCT116 cells treated with DMSO (control), 5-FU, CUDC-907, or combination of both. Cells were stained with AO/EB to detect apoptotic (cells with green condensed chromatin, white arrow head) and necrotic cells (red, yellow arrow head)
Effect of combination treatment of CUDC-907 and 5-FU on cell cycle
To elucidate the mechanism of CUDC-907 + 5-FU-induced cell growth inhibition, we utilized flow cytometry to assess changes in cell cycle progression under different treatment conditions. Treatment with 5-FU resulted in partial G2/M cell cycle arrest at a concentration of 1.5 µM, whereas CUDC-907 treatment enhanced cell polyploidization (cells >2n of DNA content, 20.8% vs 9.7% in the control group) [Figure 4a and b], as a result of cells undergoing endoreplication (also known as endoreduplication). Interestingly, combination of the two compounds led to a further increase in apoptosis (7.0% vs 3.0% in the control group) and polyploidy (20.6% vs 9.7% in the control group).
Figure 4.
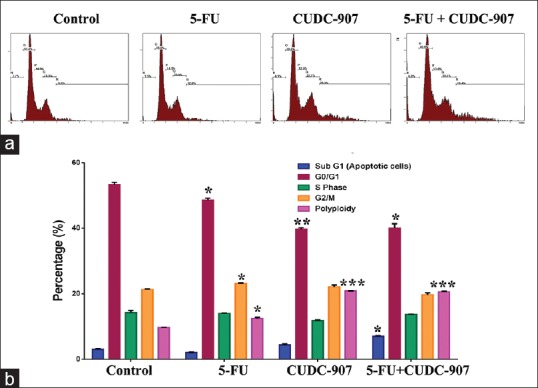
Effect of 5-FU and CUDC-907 on cell cycle in CRC cells. (a) Representative flow cytometry blots depicting changes in cell cycle of HCT116 cells exposed to DMSO (control), 5-FU, CUDC-907, or combination of both. (b) Quantification of the percentage of cells in each stage of the cell cycle. *P < 0.05, **P < 0.005, ***P < 0.0005
Suppression of HDAC and PI3K signaling by CUDC-907 in HCT116 cells
To identify whether CUDC-907 promoted HCT116 cell death via inhibition of HDAC activity and PI3K signaling, we utilized western blotting to determine the level of histone H3 lysine 9 acetylation (H3K9ac) and inhibition of the PI3K pathway post exposure to CUDC-907. The data presented in Figure 5 demonstrated a marked increase in H3K9ac epigenetic mark. However, CUDC-907 treatment suppressed AKT phosphorylation (Ser473) in response to insulin stimulation, which clearly suggested that CUDC-907 targets HDAC and the PI3K pathway in HCT116 cells.
Figure 5.
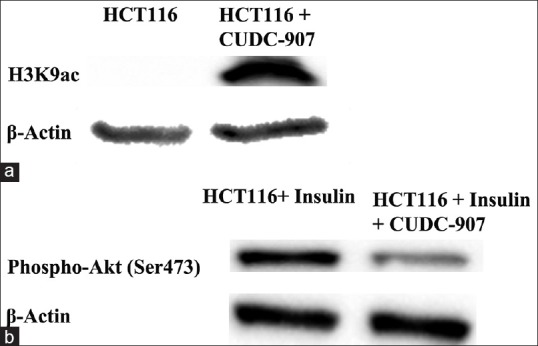
Suppression of HDAC and PI3K signaling by CUDC-907 in CRC cells. Immunoblotting of H3K9ac in CUDC-907-treated HCT116 cells (a), and Akt (Ser473) in CUDC-907-treated HCT116 cells post insulin activation of the PI3K pathway (b). β-Actin was used as loading control
DISCUSSION
Colorectal cancer is the fourth leading cause of cancer-related deaths worldwide.[12] Metastasis, recurrence, and drug resistance are among the most common causes of morbidity and mortality associated with CRC.[13] 5-FU is one of the most commonly used chemotherapeutic agents in the clinical management of metastatic CRC, either as a single agent or in combination with other therapeutic agents.[14] A number of pre-clinical studies have suggested the potential application of HDAC inhibitors in CRC therapy.[15] However, the utilization of CUDC-907, a dual inhibitor of HDAC and PI3K, as single agent or in combination with 5-FU against CRC has not been explored thus far. In the current study, we report significant cytotoxicity and suppression of CRC colony formation by CUDC-907. Interestingly, the combination of CUDC-907 at a very low dose (10 nM) and 5-FU was more efficacious than either of the drugs alone, which supports the potential application of this combined treatment for CRC in an attempt to minimize the side effect and toxicity often observed with high-dose 5-FU therapy.[16] Our data suggested the increased apoptosis, necrosis, and defect in cell division observed in the combination group, as potential mechanisms leading to cell death in CRC. Concordant with our findings, Xu et al.[17] reported induction of polyploidy as a potential mechanism of anti-tumor effect of HDAC inhibitors. CUDC-907 treatment led to substantial increase in H3K9ac, suggesting possible unfolding of the chromatin structure, resulting in active transcription of pro-apoptotic genes in response to 5-FU treatment. In addition, reduced phosphorylation of AKT (Ser473) was observed in cells exposed to CUDC-907; therefore, inhibition of PI3K signaling may be another mechanism by which CUDC-907 + 5-FU induced cell death in CRC.
CONCLUSION
Our data established, for the first time, the enhanced efficacy of 5-FU against CRC when combined with CUDC-907, a dual HDAC and PI3K inhibitor. Based on recent data demonstrating the tolerability and safe administration of CUDC-907 in the clinic,[18] we propose the application of this combination treatment in the future management of CRC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank the College of Medicine Research Center (CMRC), Deanship for Scientific Research, King Saud University for supporting this work.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Alajez NM. Significance of BMI1 and FSCN1 expression in colorectal cancer. Saudi J Gastroenterol. 2016;22:288–93. doi: 10.4103/1319-3767.187602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosli MH, Al-Ahwal MS. Colorectal cancer in the Kingdom of Saudi Arabia: Need for screening. Asian Pac J Cancer Prev. 2012;13:3809–13. doi: 10.7314/apjcp.2012.13.8.3809. [DOI] [PubMed] [Google Scholar]
- 5.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–8. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 6.Na YS, Kim SM, Jung KA, Yang SJ, Hong YS, Ryu MH, et al. Effects of the HDAC inhibitor CG2 in combination with irinotecan, 5-fluorouracil, or oxaliplatin on HCT116 colon cancer cells and xenografts. Oncol Rep. 2010;24:1509–14. doi: 10.3892/or_00001012. [DOI] [PubMed] [Google Scholar]
- 7.Giannini G, Cabri W, Fattorusso C, Rodriquez M. Histone deacetylase inhibitors in the treatment of cancer: Overview and perspectives. Future Med Chem. 2012;4:1439–60. doi: 10.4155/fmc.12.80. [DOI] [PubMed] [Google Scholar]
- 8.Qian C, Lai CJ, Bao R, Wang DG, Wang J, Xu GX, et al. Cancer network disruption by a single molecule inhibitor targeting both histone deacetylase activity and phosphatidylinositol 3-kinase signaling. Clin Cancer Res. 2012;18:4104–13. doi: 10.1158/1078-0432.CCR-12-0055. [DOI] [PubMed] [Google Scholar]
- 9.Al-Toub M, Vishnubalaji R, Hamam R, Kassem M, Aldahmash A, Alajez NM. CDH1 and IL1-beta expression dictates FAK and MAPKK-dependent cross-talk between cancer cells and human mesenchymal stem cells. Stem Cell Res Ther. 2015;6:135. doi: 10.1186/s13287-015-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vishnubalaji R, Hamam R, Yue S, Al-Obeed O, Kassem M, Liu FF, et al. MicroRNA-320 suppresses colorectal cancer by targeting SOX4, FOXM1, and FOXQ1. Oncotarget. 2016;7:24. doi: 10.18632/oncotarget.8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali D, Hamam R, Fayez M, Kassem M, Aldahmash A, Alajez NM. Epigenetic library screen identifies Abexinostat as novel regulator of adipocytic and osteoblastic differentiation of human skeletal (mesenchymal) stem cell differentiation. Stem Cells Transl Med. 2016;5:1036–47. doi: 10.5966/sctm.2015-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2016 doi: 10.1136/gutjnl-2015-310912. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Bullock MD, Pickard K, Mitter R, Sayan AE, Primrose JN, Ivan C, et al. Stratifying risk of recurrence in stage II colorectal cancer using deregulated stromal and epithelial microRNAs. Oncotarget. 2015;6:7262–79. doi: 10.18632/oncotarget.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gustavsson B, Carlsson G, Machover D, Petrelli N, Roth A, Schmoll HJ, et al. A review of the evolution of systemic chemotherapy in the management of colorectal cancer. Clin Colorectal Cancer. 2015;14:1–10. doi: 10.1016/j.clcc.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Sikandar S, Dizon D, Shen X, Li Z, Besterman J, Lipkin SM. The class I HDAC inhibitor MGCD0103 induces cell cycle arrest and apoptosis in colon cancer initiating cells by upregulating Dickkopf-1 and non-canonical Wnt signaling. Oncotarget. 2010;1:596–605. doi: 10.18632/oncotarget.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee CS, Ryan EJ, Doherty GA. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: The role of inflammation. World J Gastroenterol. 2014;20:3751–61. doi: 10.3748/wjg.v20.i14.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu WS, Perez G, Ngo L, Gui CY, Marks PA. Induction of polyploidy by histone deacetylase inhibitor: A pathway for antitumor effects. Cancer Res. 2005;65:7832–9. doi: 10.1158/0008-5472.CAN-04-4608. [DOI] [PubMed] [Google Scholar]
- 18.Younes A, Berdeja JG, Patel MR, Flinn I, Gerecitano JF, Neelapu SS, et al. Safety, tolerability, and preliminary activity of CUDC-907, a first-in-class, oral, dual inhibitor of HDAC and PI3K, in patients with relapsed or refractory lymphoma or multiple myeloma: An open-label, dose-escalation, phase 1 trial. Lancet Oncol. 2016;17:622–31. doi: 10.1016/S1470-2045(15)00584-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


