Abstract
Background/Aims:
In patients with colon cancer who undergo resection for potential cure, 40–60% have advanced locoregional disease (stage III). Those who are suitable for adjuvant treatment had a definite disease-free-survival benefit. The aim of the present study was to demonstrate whether the presence of desmoplasia influenced the mortality rate of stage III colorectal cancer (CRC) within 5 years from the surgery and adjuvant therapy.
Patients and Methods:
Sixty-five patients with stage III CRC underwent resection and adjuvant therapy. Qualitative categorization of desmoplasia was obtained using Ueno's stromal CRC classification. Desmoplasia was related to mortality using Spearman correlation and stratified with other histological variables (inflammation, grading) that concurred to the major determinant of malignancy (venous invasion and lymph nodes) using the Chi-square test.
Result:
The 5-year survival rate was 65% and the relapse rate was 37%. The mortality rate in patients with immature desmoplasia was 86%, 27% in intermediate desmoplasia, and 0% in mature desmoplasia (Spearman correlation coefficient: −0.572, P = 0.05).
Conclusion:
Immature desmoplasia appears to be associated with disease recurrence and mortality in stage III CRC patients.
Key Words: Colorectal cancer, desmoplasia, disease recurrence, leucovorin, mortality
Approximately 1 million patients receive a diagnosis of colorectal cancer (CRC) annually, and half a million deaths occur annually worldwide from this neoplasm.[1] In 2004, approximately 106,000 people living in the United States were diagnosed with CRC.[2,3] Of these patients, just less than one-third had node-positive disease (stage III), and approximately one-third had node-negative (stage II) disease. Each year, approximately 230,000 patients with CRC are eligible for adjuvant chemotherapy.[1,4,5] The benefits of fluorouracil (FU)-based adjuvant chemotherapy is reducing the risk of relapse and prolonging survival. After a complete surgical resection, stage III patients face a 50–60% chance of developing recurrent disease[6,7]. However, randomized trials conducted in the 1980s demonstrated that FU-based therapy could decrease the chance of death by approximately 30% (relative risk reduction), which is greater than the 10% absolute improvement in 5-year survival.[8] As a result of these trials, in 1990, the National Institutes of Health (NIH) convened a consensus conference panel that recommended the administration of FU-based adjuvant therapy for all medically fit patients with completely resected stage III CRC.[9] It has been argued that fibrotic stroma could represent an attempt by the host to ward-off tumor cells, thereby exerting antagonistic biological forces. Alternatively, this process may benefit the tumor by neovascularization and impeding access to host lymphocytes, macrophages, and other immune regulator cells.[10,11] This study addresses this question; is it possible to identify the characteristics of desmoplastic response that are involved in lymphatic and venous invasion as risk factors for poor prognosis in curative resected stage III CRC?
PATIENTS AND METHODS
Sixty-five consecutive patients, having recovered from surgery and with histological confirmation of stage III CRC, from Policlinico Umberto I Hospital, were eligible for recruitment. Some patients underwent screening colonoscopy without symptoms whereas others presented with nonspecific signs and symptoms such as asthenia, weight loss, appetite loss, iron deficiency anemia, and increased active phase indices. Others had specific symptoms such as recent changes in bowel habit, rectal bleeding, abdominal pain, tenesmus, and anemia. All patients who were diagnosed with CRC were admitted to the surgical unit to undergo computed tomography (CT) and anesthesiological, respiratory, and cardiovascular examinations before surgery. Subsequently, patients were assigned to six cycles of rapid intravenous infusion of leucovorin, at a dose of 10 mg per square meter, followed immediately by an intravenous bolus of FU, at a dose of 370 mg per square meter, on days 1 through 5 every 28 days, along with infusion of oxaliplatinum at a dose of 85 mg per square meter for 12 cycles at intervals of 2 weeks (6 months of treatment).
Exclusion criteria
Patients with rectal cancer were excluded from the study because they were assigned to neoadjuvant radiotherapy treatment before surgery, according to the recent guidelines, as this would have altered the histological status of the neoplastic tissue when staging.[12,13,14,15] Patients with evidence of metastatic disease on CT or presence of tumor cells in ascites were ineligible for participation. Other exclusion criteria considered were patient's refusal of the advised treatment, patients with extremely bad clinical conditions, heart or respiratory failure, or old age (>90 years, except after family discussion and written informed consent). Patients were also excluded on the basis of prior cytotoxic chemotherapy, organ allograft, state of pregnancy, or lactation.
Histology and staging
The surgical specimens obtained were first conserved in formalin, and then cut, fixed and stained with hematoxylin–eosin. The specimens were divided into right and left colon in order to separate the colonic vascularization; they were also classified according to parietal infiltration status, histological phenotype, growth pattern, lymph node invasion, stage, and grading.[16] Dysplastic and atypical cells were detected following the architectural (gland and villous conformation) and cytological (mucus secretion, stratifying, polarity, hyperchromatosis, and nuclear polymorphism, mitosis) deviations.[17] Other variables were considered such as, vascular and perineural invasion, growth type (spreading or invasive), degree of lymph node infiltration along the invasive edge, presence of lymph node aggregates along the invasive edge (lymph node reaction, “Crohn's disease-like”), and number of tumour infiltrating lymphocytes (TIL). The Union for International Cancer Control-Tumor-Node-Metastasis (UICC-TNM) classifying system was utilised for disease staging (sixth edition, 2002).[16]
The presence of desmoplasia, inflammatory infiltration, and extension of necrotic area were graded semi-quantitatively into low (if the variables were poorly represented), moderate, and high (if the variables were moderately or abnormally represented in the tumor mass). In particular, the presence of desmoplasia and its qualitative evaluation was obtained with the hematoxylin–eosin staining method and in some cases with Van Gieson staining method, which has the advantage of separating the different types of collagen fibres present in the stroma tissue, according to the latest guidelines.[17] The histological examination of the regional lymphadenectomy comprised twelve or more lymph nodes, in agreement with the latest edition of the TNM classifying system.[16] According to this system, at least 7 to 14 lymph nodes are needed after a radical resection of the colon and/or rectum in order to determine if metastasis is present.[18,19,20] Nevertheless, the number of tumor residuals present along the radial margin was evaluated, and with radiological examinations, the presence of metastasis or micrometastasis in different organs was evaluated.
Statistical analysis
The statistical analysis was performed using the following variables: degree of tumor mass invasiveness, grading, desmoplasia, inflammatory infiltration status, compared with the presence or absence, during the postsurgical staging, of lymph node or distant metastasis, and of venous or lymphatic vessel embolization. Data were particularly relevant in patients with disease extending to the serosa or subserosa, classified as metastasis positive (M+) or metastasis negative (M−), and/or lymph-node positive (N+) or lymph-node negative (N−).
The prevalence rates were compared using Fisher's exact test. A value of P < 0.05 was considered statistically significant, with α error of 5%. All calculations were done using the Statistical Package for the Social Sciences (SPSS, IBM, Chicago, Illinois).
RESULTS
A total of 65 patients, 42 males and 23 females, with a median age of 67 ± 17.6 years, underwent hemicolectomy for CRC. Twenty-five of the patients underwent right hemicolectomy and 40 left hemicolectomy. Out of the 65 patients examined, 3 (4.6%) were symptom-free and had undergone colonoscopy for screening purpose and 2 (3%) had already been operated on an emergency basis for subacute obstruction. Sixty patients (92%) presented with anemia, 55 (85%) with rectal bleeding, and 63 (97%) referred for recent changes in bowel habit. Other general symptoms found were weight loss in 56 patients (86%) and asthenia in 32 patients (49%). A mean 13 ± 2 lymph nodes were obtained. Nineteen patients (29%) presented satellite adenomas with low grade dysplasia.
Fifty out of the 65 tumor masses revealed a tubular histotype (77%), 9 (14%) presented a mucinous histotype (more than 50% of mucinous pattern) and 7 (9%) a tubulovillous histotype. All patients were classified in stage III according to the UICC-TNM staging system.[20] At 5 years, crude survival rate was 65% and the relapse rate was 37%. Immature desmoplasia was present in 14 patients (21.5%), intermediate desmoplasia was present in 40 (61.5%) patients, and mature desmoplasia in 11 patients (17%), Mortality rate in patients with immature desmoplasia was 86%, 27% in intermediate desmoplasia, and 0% in mature desmoplasia (Spearman correlation coefficient: −0.572, P = 0.05) [Figure 1].
Figure 1.
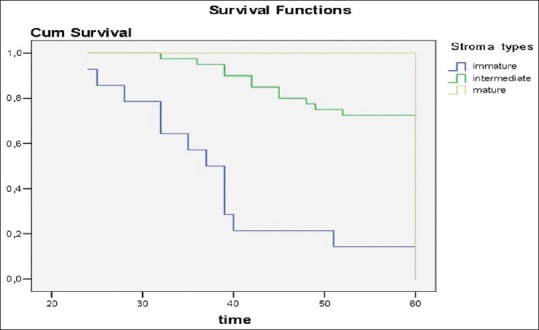
The LogRank test showed significant differences in disease recurrence and mortality comparing the mature, intermediate, and the immature stroma subtypes
Desmoplastic reaction and degree of neoplasia
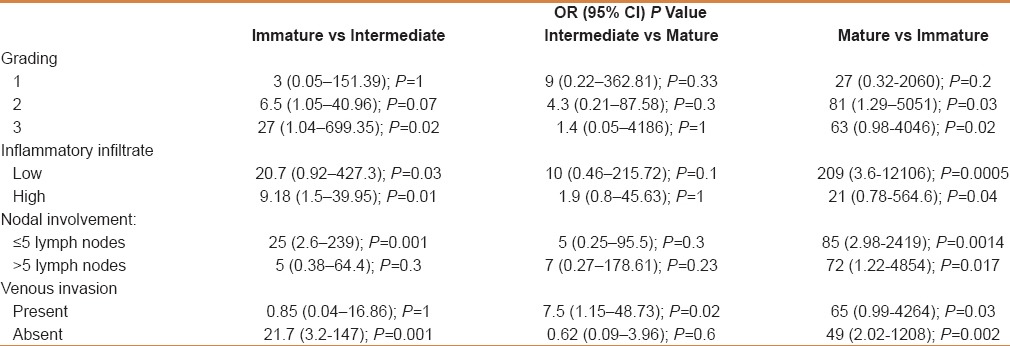
In patients with immature desmoplasia, there were 4 with first degree of differentiation, 9 with second degree of differentiation, and 1 with third degree of differentiation. In intermediate desmoplasia, there were 2 patients with first degree, 23 with second degree, and 15 with third degree of differentiation. In mature desmoplasia, the corresponding number of patients were 4, 5, and 2, respectively. No statistical differences were found in patient mortality comparing the three types of desmoplasia according to the first degree [Table 1]. For second degree, patient mortality in immature disease was significantly higher than that observed in patients with mature desmoplasia (P = 0.03) [Table 1]. For third degree, patient mortality in immature disease was significantly higher than that observed both in patients with mature and intermediate desmoplasia (P = 0.02) [Table 1].
Table 1.
Correlation between type of stroma and the principal variables of tumor invasiveness in disease recurrence and survival
Desmoplastic reaction and degree of lymphocyte infiltration
In patients with immature desmoplasia, there were 9 patients with high inflammatory infiltrate and 5 patients with low grade of infiltrate. In intermediate desmoplasia, there were 25 patients with high inflammatory infiltrate and 15 patients with a low grade of infiltrate. In mature desmoplasia, there were 9 patients with a high rate of inflammatory infiltrate and 5 with a low infiltrate. Considering cases with low grade of inflammatory infiltrate, patient mortality in immature disease was significantly higher than that observed both in patients with mature (P = 0.005) and intermediate desmoplasia (P = 0.03) [Table 1]. Similar results in patient's mortality were observed in both mature (P = 0.04) and intermediate desmoplasia (P = 0.01). In patients with high inflammatory disease, survival at 5-year follow-up was higher than in patients with immature desmoplasia [Table 1].
Desmoplastic reaction and nodal involvement
We chose the presence of five positive lymph nodes as the criterion for the categorization of the involvement of the lymphnodal system. In patients with immature desmoplasia, there were 7 with more than five lymph-nodes with neoplastic infiltration and 7 with less than five positive lymph-node. In intermediate desmoplasia, there were 8 patients with more than five lymph-nodes with neoplastic infiltration and 32 with less than five positive lymph nodes. In mature desmoplasia, there were 9 patients with less than five positive lymph nodes and 3 with more than five positive lymph nodes. Considering cases with less than 5 lymph nodes with neoplastic involvement both patients with intermediate (P = 0.001) and mature desmoplasia (P = 0.0014) have a longer disease-free period and a higher survival rate than patients with immature desmoplasia. In immature disease, both inflammatory infiltrate and patient mortality was significantly higher in comparison with that observed both in patients with mature (P = 0.005) and intermediate desmoplasia (P = 0.03), [Table 1].
Desmoplastic reaction and venous invasion
In patients with immature desmoplasia, there were 6 patients with venous involvement and 8 patients with no venous involvement. In intermediate desmoplasia, there were 7 patients with venous involvement and 33 with no venous involvement. In mature desmoplasia, there were 9 patients negative for venous involvement and 2 with venous involvement. Considering cases with venous involvement, patients with mature desmoplasia showed a longer disease free interval and higher survival at 5-year follow-up in comparison to both patients with intermediate (P = 0.02) and immature desmoplasia (P = 0.03). Considering cases with absence of venous involvement, survival rates of patients with mature (P = 0.002), and intermediate (P = 0.001) desmoplasia were significantly higher in comparison to the survival rate of patients with immature desmoplasia [Table 1].
DISCUSSION
In recent years, broad involvements of lymph nodes and neoplastic embolization have been considered prognostic factors in patients operated for CRC. In fact, in 1999 and later in 2003, The College of American Pathologist[18] stated that a number of 12–15 histologically negative lymph nodes and the absence of blood and lymphatic involvement were predictive signs of a radical surgical treatment. This evidence led us to investigate the protective or facilitating role played by different histological variables in locoregional or distant invasion of CRC. Various histological variables were the object of study on the operative specimen for disease staging, such as desmoplasia stroma tissue proliferation, degree of intra and peritumoral inflammatory infiltration, degree of morphological anaplasia, and necrotic area extension. Although its precise role has not been clearly defined, the desmoplastic tissue response has always generated long discussions because it is thought to represent the most important biological factor of a malignant neoplasm.[20,21,22,23] Presence of a connective tissue reaction around the invasive edge of neoplasia can block cancer cell migration because its collagen fibres can trap the tumour cells;[21,22,23,24,25,26] however, on the other hand, if it were constituted by mixoid stroma, it could represent a barrier against inflammatory infiltration and promote neo-vascularization, consequently sustaining and nourishing the tumor mass. In doing so, it would permit de-differentiation of cancer cells, which later form venous and lymphatic vessels emboli.[23,24,27] Following the histological stroma classification of Schurlchs et al.,[24] we can conclude that differentiated neoplasia produces mature stroma tissue, while moving toward an undifferentiated neoplastic pattern, the stroma tissue also becomes disorganized and mixoid. This report supports the hypothesis that a mature desmoplastic response, characterized by a considerable amount of collagen fibers against the relatively low presence of matrix, limits stage III CRC tumor aggressiveness and contradicts the concept that tumors eliciting the greatest desmoplastic reaction behave more aggressively than those eliciting a less pronounced response. This fact suggests that there are multiple processes in the desmoplastic reaction which have opposing effects on cancer behavior. It is conceivable that such different processes may operate independently during neoplastic growth and could simultaneously occur at different locations in the same tumor. In order to understand such complex interactions between neoplastic cells and the surrounding matrix, it is necessary to examine not only the amount of fibrosis, which has been the pathological index for stromal reaction, but also its qualitative nature. Fibrous connective tissue includes matrix components (for example, fibronectin, interstitial collagens, elastin, and glycosaminoglycans) and the cells responsible for matrix synthesis (for example, fibroblasts, myofibroblasts, and histiocytes).[28] With the histological classification of fibrotic cancer stroma,[29] we have identified histological features of the microenvironment associated with cancer that could explain neoplastic malignant behavior. Tumor cells proliferate and invade the stroma where host immune cells congregate around tumor nests, promoting tumor angiogenesis.[30] Since the dynamic changes in the cancer-associated stroma resemble a wound-healing reaction,[30] it is termed a desmoplastic reaction. The desmoplastic reaction is thought to be supported mainly by the activation of host fibroblasts referred to as “myofibroblast.”[21,31] Myofibroblasts are differentiated host fibroblasts that express α-smooth muscle actin (α-SMA) as cytoplasmic microfilaments, as well as desmin to a limited extent, whereas quiescent host resident fibroblasts express vimentin as intermediate filament proteins.[32] Myofibroblasts produce an extracellular matrix enriched in type III and V collagen, which is considered to be responsible for the hard consistency of many carcinomas.[33] Several fundamental studies on myofibroblast have been conducted to clarify the role of cancer-associated desmoplastic reactions. It has been argued that the process of dedifferentiation and dissociation of neoplastic cells at the invasive edge is the first and essential step in tumor invasion.
Based on the observation that this process is always strictly confined to the tumor invasive front, inductive signals from the host microenvironment were considered to be involved in initiating and maintaining this rapid and even reversible phenotypic shift by activating or repressing the preformed genetic programme of tumor cells. We found significant correlation with the intensity of tumor spreading from venous and lymphatic vessels, which influences survival outcome in CRC patients. In particular, when intermediate and immature fibrotic stroma were confined to the tumor invasive front while the tumour centre was composed of fine and mature fibres stratified into multilayers (the mature stroma), we observed the best prognosis. Based on these findings, intermediate and immature fibrotic stroma seem to be transitory phenotypes which facilitate dedifferentiation of cancer cells, whereas mature stroma is a later and more stable phenotype, which may reduce the invasive activity of neoplastic cells whether they are located in the tumor centre and/or the invasive front. The decrease in lymphocyte infiltration in colorectal primary tumors has long been believed to correlate with poor prognosis, however, in the present study, the mechanism by which tumors inhibit lymphocyte locomotion was not clear.
Lieubeau et al. reported that myofibroblasts may prevent penetration of immune cells within tumors promoting tumor growth and progression because of their contractile properties and associated extracellular matrix.[34] The results of in vitro experiments would also support our findings showing that lymphocytes infiltrated less in immature fibrotic stroma, where myofibroblasts were extensively distributed, than in mature and intermediate fibrotic stroma. The above results might suggest that an immature fibrotic cancer stroma may assist cancer in hindering a host response, facilitating the survival of neoplastic cells.[35]
CONCLUSIONS
In conclusion, a pattern of immature desmoplastic response appears to be involved in disease recurrence and mortality in stage III CRC patients. Conversely, nodal involvement and venous embolization appear to be prevented by the presence of mature stroma.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Stewart BW, Kleihues P, editors. World cancer report. Lyon, France: IARC Press; 2003. p. 198. [Google Scholar]
- 2.SEER04-04-2016 Cancer Statistics review 1973-1999. National Cancer Institute: Bethesta MD. [Last accessed on 2016 Apr 04]. http//seer.cancer.gov.cst/1973_1999 .
- 3.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer Statistics 2003. CA Cancer J Clin. 2003;53:5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Ayanian JZ, Zaslavsky AM, Fuchs CS, Guadagnoli E, Creech CM, Cress RD, et al. Use of adjuvant chemotherapy and radiation therapy for colorectal cancer in a population-based cohort. J Clin Oncol. 2003;21:1293–1300. doi: 10.1200/JCO.2003.06.178. [DOI] [PubMed] [Google Scholar]
- 5.Benson AB, III, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22:3408–19. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 6.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: A final report. Ann Intern Med. 1995;122:321–6. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 7.O'Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15:246–50. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 8.Eisensberg B, Decossè JJ, Hartford E, Michalek J. Carcinoma of the colon and rectum: The natural history reviewed in 1704 patients. Cancer. 1982;49:1131–4. doi: 10.1002/1097-0142(19820315)49:6<1131::aid-cncr2820490611>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 9.Macdonald JS. Adjuvant therapy for colon cancer. CA Cancer J Clin. 1999;49:202–19. doi: 10.3322/canjclin.49.4.202. [DOI] [PubMed] [Google Scholar]
- 10.Grothey A, Kellermann L, Schmoll HJ. Deficits in management of patients with colorectal carcinoma in Germany: Results of multicenter documentation of therapy algorithms. Med Klin. 2002;97:270–7. doi: 10.1007/s00063-002-1153-9. [DOI] [PubMed] [Google Scholar]
- 11.Liotta LA, Rao CN, Barsky SH. Tumor invasion and the extracellular matrix. Lab Invest. 1983;49:636–49. [PubMed] [Google Scholar]
- 12.Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative Radiotherapy Combined with Total Mesorectal Excision for resectable rectal Cancer. N Engl J Med. 2001;345:638–46. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 13.Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high risk rectal carcinoma. N Engl J Med. 1991;324:709–15. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 14.Marsh PJ, James RD, Schofield PF. Adjuvant preoperative radiotherapy for locally advanced rectal carcinoma. Results of a prospective randomized trial. Dis Colon Rectum. 1994;37:1205–14. doi: 10.1007/BF02257783. [DOI] [PubMed] [Google Scholar]
- 15.Medical Research Council Rectal Cancer Working Party. Randomized trial of surgery alone versus radiotherapy followed by surgery for mobile cancer of the rectum. Lancet. 1996;348:1610–4. [PubMed] [Google Scholar]
- 16.Sobin LH, Wittekind CH, editors. TNM classification of malignant tumours. 6th ed. New York: Wiley-Liss; 2002. International Union against Cancer. [Google Scholar]
- 17.Ueno H, Jones A, Jass JR, Talbot IC. Clinicopathological significance of the 'keloid-like' collagen and myxoid stroma in advanced rectal cancer. Histopathology. 2002;40:327–34. doi: 10.1046/j.1365-2559.2002.01376.x. [DOI] [PubMed] [Google Scholar]
- 18.Jass JR, Atkin WS, Cuzick J, Bussey HJ, Morson BC, Northover JM, et al. The grading of rectal cancer: Historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986;10:437–59. doi: 10.1111/j.1365-2559.1986.tb02497.x. [DOI] [PubMed] [Google Scholar]
- 19.Nakada I, Tasaki T, Ubukata H, Goto Y, Watanabe Y, Sato S, et al. Desmoplastic response in biopsy specimens of early carcinoma is predictive of deep submucosal invasion. Dis Colon Rectum. 1988;41:896–900. doi: 10.1007/BF02235375. [DOI] [PubMed] [Google Scholar]
- 20.Hewitt RE, Powe DG, Carter I, Turner DR. Desmoplasia and its relevance to colorectal tumour invasion. Int J Cancer. 1993;52:62–9. doi: 10.1002/ijc.2910530113. [DOI] [PubMed] [Google Scholar]
- 21.Lunevicius R, Nakamishi H, Ito S, Kozaki K, Kato T, Tatematsu M, et al. Clinicopathological significance of fibrotic capsule formation around liver metastasis from colorectal cancer. J Cancer Res Clin Oncol. 2001;127:193–9. doi: 10.1007/s004320000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caporale A, Vestri AR, Benvenuto E, Mariotti M, Cosenza UM, Scarpini M, et al. Is Desmoplasia a protective factor for survival in patients with colorectal carcinoma. Clin Gastroenterol Hepatol. 2005;3:370–5. doi: 10.1016/s1542-3565(04)00674-3. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura R, Hesebe T, Tsubno J, Ono M, Sugitoh M, Arai T, et al. The fibrotic focus in advanced colorectal carcinoma: A hitherto unrecognized historical predictor for liver metastasis. Virchows Arch. 1998;433:517–22. doi: 10.1007/s004280050283. [DOI] [PubMed] [Google Scholar]
- 24.Shurlch W, Seenarer TA, Gabbiani G. In: Histology for pathologist. 2nd ed. Sternberg S, editor. Philadelphia: Lippincott- Raven Publishers; 1997. pp. 129–65. [Google Scholar]
- 25.Dvorak HF. Tumors: Wounds that do not heal; similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–9. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 26.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia. 1971;27:549–50. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- 28.Barsky SH, Green WR, Grotendorst GR, Liotta LA. Desmoplastic breast carcinoma as a source of human myofibroblasts. Am J Pathol. 1984;115:329–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Yen TW, Aardal NP, Bronner MP, Thorning DR, Savard CE, Lee SP, et al. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery. 2002;131:129–34. doi: 10.1067/msy.2002.119192. [DOI] [PubMed] [Google Scholar]
- 30.Sappino AP, Schurch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: Expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–61. [PubMed] [Google Scholar]
- 31.Peyrol S, Raccurt M, Gerard F, Gleyzal C, Grimaud JA, Sommer P. Lysyl oxidase gene expression in the stromal reaction to in situ and invasive ductal breast carcinoma. Am J Pathol. 1997;150:497–507. [PMC free article] [PubMed] [Google Scholar]
- 32.Camps JL, Chang SM, Hsu TC, Freeman MR, Hong SJ, Zhau HE, et al. Fibroblast-mediated acceleration of human epithelial tumor growth in vivo. Proc Natl Acad Sci U S A. 1990;87:75–9. doi: 10.1073/pnas.87.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–11. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieubeau B, Heymann MF, Henry F, Barbieux I, Meflah K, Grégoire M. Immunomodulatory effects of tumor-associated fibroblasts in colorectal-tumor development. Int J Cancer. 1999;81:629–36. doi: 10.1002/(sici)1097-0215(19990517)81:4<629::aid-ijc20>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Ueno H, Shinto E, Shimazaki H, Kajiwara Y, Sueyama T, Yamamoto J, et al. Histologic categorization of desmoplastic reaction: Its relevance to the colorectal cancer microenvironment and prognosis. Ann Surg Oncol. 2015;22:1504–12. doi: 10.1245/s10434-014-4149-9. [DOI] [PubMed] [Google Scholar]


