Abstract
Background/Aim:
Endoscopic tunneling resection is a relatively novel endoscopic technology for removing gastric submucosal tumors. Our study aimed to compare the differences between tunneling and nontunneling resection for gastric submucosal tumors.
Materials and Methods:
Resections of gastric submucosal tumors (n = 97) performed from 2010 to 2015 at our endoscopy center were reviewed, and PubMed was searched for clinical studies on gastric submucosal tumor resection by endoscopic nontunneling and tunneling techniques.
Results:
At our endoscopy center, nontunneling (Group 1) and tunneling resection (Group 2) were performed for 78 and 19 submucosal tumors, respectively; median tumor diameters were 15 and 20 mm (P = 0.086), median procedural times were 50 and 75 min (P = 0.017), successful resection rates were 94.9% (74/78) and 89.5% (17/19) (P = 0.334), and en bloc resection rates were 95.9% (71/74) and 94.1% (16/17) (P = 0.569) in the Groups 1 and 2, respectively. Postoperative fever, delayed hemorrhage and perforation, hospitalization time, and hospitalization expense were statistically similar between the 2 groups. A literature review on gastric submucosal tumor resection suggested that the en bloc resection rates of the two methods for tumors with a median diameter of 15–30 mm were also high, and there were no relapses during the follow-up period.
Conclusions:
Both endoscopic nontunneling and tunneling resection seem to be effective and safe methods for removing relatively small gastric submucosal tumors. Compared with endoscopic nontunneling, tunneling resection does not seem to have distinct advantages for gastric submucosal tumors, and has a longer mean operative time.
Key Words: Endoscopic full-thickness resection, endoscopic submucosal dissection, endoscopic submucosal tunnel dissection, gastric submucosal tumor, submucosal tunneling endoscopic resection
To date, studies[1,2] on successful endoscopic resection of gastric submucosal tumors have been reported, suggesting that endoscopic resection of gastric submucosal tumors with relatively small size seems to be feasible. Ye et al.[1] retrospectively analyzed 773 cases of submucosal tumors resected endoscopically. The median diameter of submucosal tumors was 1.7 (1.0–4.0) cm, en bloc resection rate was 97.1% without severe complications, and no recurrence occurred during a median follow-up period of 28 months. Another study[2] was from Korea, which indicated that it was feasible to selectively resect gastrointestinal stromal tumors by endoscopic methods and the recurrence rate was low during the 4-year follow-up period. With the development of endoscopic technology, endoscopic resection of gastric submucosal tumors may be gradually recognized and accepted.
At present, the main methods for resecting submucosal tumors are endoscopic submucosal dissection (ESD) and endoscopic full-thickness resection (EFTR), and they are considered endoscopic as nontunneling resections. A relatively new method for removing submucosal tumors is endoscopic tunneling resection, which was first reported by Inoue et al.[3] and initially used for the tumors of the esophagus and cardia. In contrast to endoscopic nontunneling resection (ESD and EFTR), tunneling resection is performed to resect a tumor through a tunnel created in the submucosal layer, and maintains the structural integrity of the mucosa, which facilitates postoperative recovery.[4] The main difference between endoscopic nontunneling and tunneling resection is that a tunnel is needed in the latter technique. For tunneling resection, the same method as in ESD or EFTR is used to resect the tumor after creating a submucosal tunnel. A recent study compared tunneling resection with endoscopic excavation for treating submucosal tumors of the esophagus and cardia, and concluded that tunneling resection was the better choice for submucosal tumor sizes of >10 mm.[5] However, there have been no comparative studies of nontunneling and tunneling resection for gastric submucosal tumors.
To compare the differences between the two endoscopic methods, we retrospectively analyzed the cases of gastric submucosal tumors resected at our endoscopy center, and systematically reviewed the published studies on the endoscopic resection of gastric submucosal tumors.
MATERIALS AND METHODS
Retrospective analysis of submucosal tumor cases at our endoscopy center
Case selection
We retrospectively reviewed the case histories of submucosal tumor patients who underwent endoscopic nontunneling or tunneling resection from 2010 to 2015 at our digestive endoscopy center, Nanfang Hospital, Southern Medical University, Guangzhou, China. The submucosal tumors in these patients mainly consisted of gastric stromal tumor, leiomyoma, or lipoma. Prior to resection, these patients underwent endoscopic ultrasonography examinations to evaluate the indications for endoscopic treatment. All patients gave signed informed consent for endoscopic treatment as well as for the study. The study protocol was approved by the institute's ethics committee on human research. All the submucosal tumors were resected by endoscopy physicians who had plenty of experience on ESD, peroral endoscopic myotomy (POEM), and tunneling resection.
Endoscopic method and pathological assessment
Our protocols for resecting tumors were in accordance with those reported in the literature for endoscopic nontunneling (ESD[6,7,8] and EFTR[9,10]) and tunneling resection.[11] Tumors were subjected to pathological examination after resection, and were independently reviewed by two pathologists. En bloc resection was defined as complete resection of a submucosal tumor in a single piece.
Collection of basic information on the cases at our endoscopy center
Detailed information on the cases included age, gender, tumor size, location, origin of tumor in the submucosa or muscularis propria, resection method, operative time, complications during resection, successful and complete resection, postoperative fever, severe abdominal pain (requiring analgesic drugs in the clinic), and postoperative complications.
Statistical analysis
All data were analyzed using the Statistical Package for the Social Sciences version 19.0 software. Quantitative data with a normal distribution were expressed as mean ± standard deviation (SD) and analyzed by t-test; quantitative data with a non-normal distribution were expressed as median (interquartile range) and analyzed using the Mann–Whitney U test. Classifiable variables were tested by Chi-square analysis.
When the expected frequency in more than 20% frames of R×C contingency table was <5 or the expected frequency in any frame was <1, Fisher's exact probability test was used to analyze the data. P < 0.05 in a two-sided hypothesis test was considered to be a significant difference.
Literature review of submucosal tumor resection by endoscopic nontunneling (endoscopic submucosal dissection and endoscopic full-thickness resection) and tunneling resection
The PubMed database was searched for clinical studies regarding endoscopic nontunneling and tunneling resection of tumors. The search terms were (gastric OR stomach) and (submucosal neoplasm OR submucosal lesion OR submucosal tumor) and (stromal tumor OR stromal neoplasm). The retrieval fields were titles and abstracts.
All selected studies conformed to the following criteria: Resection of submucosal tumors were performed by endoscopic nontunneling (ESD and EFTR) or tunneling resection, including endoscopic submucosal tunnel dissection (ESTD) and submucosal tunneling endoscopic resection (STER); all the information required for our study could be extracted from the original research. Excluded from the present study were case reports, reviews, and meta-analyses, or unavailable full paper text. We summarized and analyzed the following data: Resected tumor size, location, and origin of tumor from the submucosa or muscularis propria, pathological type, operative time, complications, rate of complete resection, and follow-up information.
RESULTS
Retrospective analysis of submucosal tumor cases at our endoscopy center
Characteristics of gastric submucosal tumors
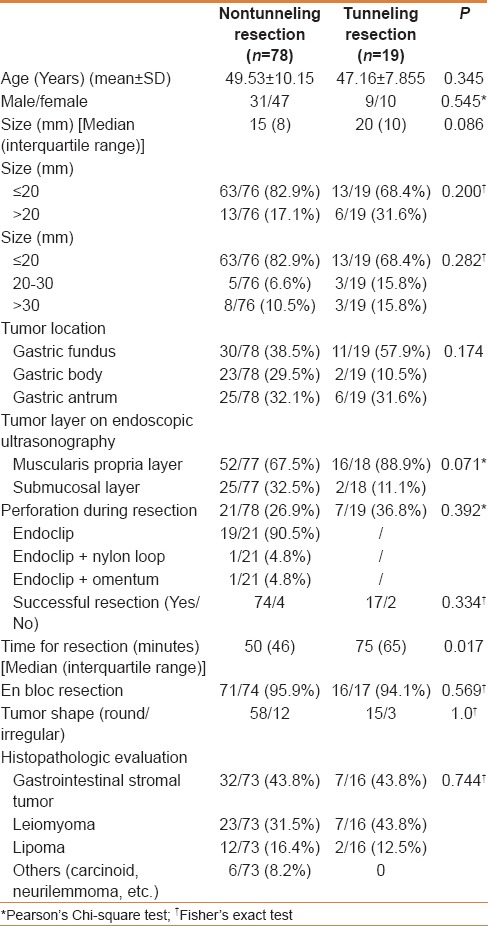
Data of 97 submucosal tumors were collected in our retrospective study. Of these, 78 were resected by endoscopic nontunneling (Group 1) and 19 were resected by tunneling (Group 2). As shown in Table 1, the median tumor sizes for the 2 groups were 15 mm and 20 mm (P = 0.086), respectively. The 2 groups were statistically similar in the distribution of pathological types of submucosal tumors (P = 0.744); the proportions of stromal tumor, leiomyoma, and lipoma were 43.8%, 31.5%, and 16.4% in Group 1; and 43.8%, 43.8%, and 12.5% in Group 2, respectively. The tumor locations of the two groups were not significantly different (P = 0.174); in Groups 1 and 2, there were 30 (38.5%) and 11 (57.9%), respectively, in the fundus; 23 (29.5%) and 2 (10.5%), respectively, in the gastric body; and 25 (32.1%) and 6 (31.6%), respectively, in the gastric antrum. In addition, the two groups were similar in the proportions of tumors originating from the submucosa and muscularis propria (P = 0.071).
Table 1.
Resection-related parameters and histopathologic evaluation of gastric submucosal tumors removed by endoscopic nontunneling and tunneling resection at our endoscopy center
Feasibility evaluation of endoscopic resection of submucosal tumors
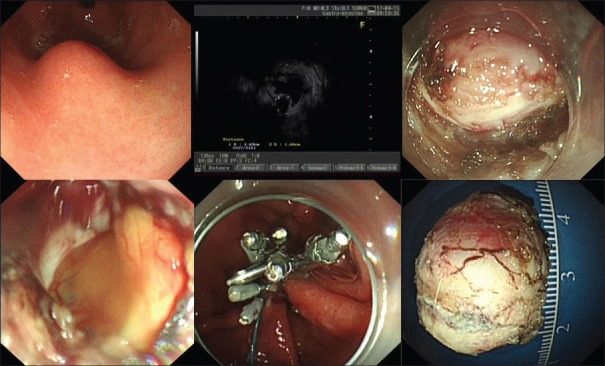
The median operative times for the nontunneling and tunneling resection were 50 (46) min and 75 (65) min (P = 0.017), respectively. The rates of successful resection of tumors in the nontunneling resection and tunneling groups were 94.9% (74/78) and 89.5% (17/19; P = 0.334), and the rates of complete resection were 95.9% (71/74) and 94.1% (16/17; P = 0.569), respectively. The perforation rates in the two groups were 26.9% (21/78) and 36.8% (7/19; P = 0.392) respectively, and all the perforations were successfully closed in both groups. For example, a submucosal tumor was successfully resected by nontunneling resection, and the other by tunneling resection, as shown in Figures 1 and 2, respectively. Perforations that occurred during nontunneling and tunneling resection were successfully closed with endoclips combined with endoloops or by endoclips alone.
Figure 1.
A submucosal tumor was successfully resected by endoscopic nontunneling resection
Figure 2.
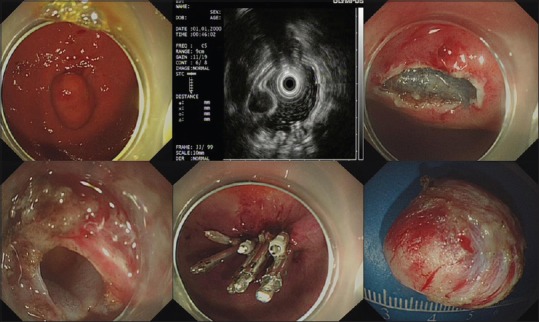
A submucosal tumor was successfully resected by endoscopic tunneling resection
Postoperative conditions
As shown in Table 2, some cases of fever was reported in patients with resected submucosal tumors developed on the first day after resection. The proportions of patients with fever in the nontunneling and tunneling groups were similar (P = 0.10): 29.4% (20/68) and 50% (9/18), respectively. Most of these patients had low-grade fever: 12 (70.6%) in the nontunneling group and 6 (85.7%) in the tunneling group. No patient in either group experienced postoperative perforation. In the nontunneling and tunneling groups, 11 and 4 patients had obvious abdominal pain after resection (P = 0.731), respectively.
Table 2.
Clinical information on patients with gastric submucosal tumors resected by endoscopic nontunneling and tunneling resection at our endoscopy center
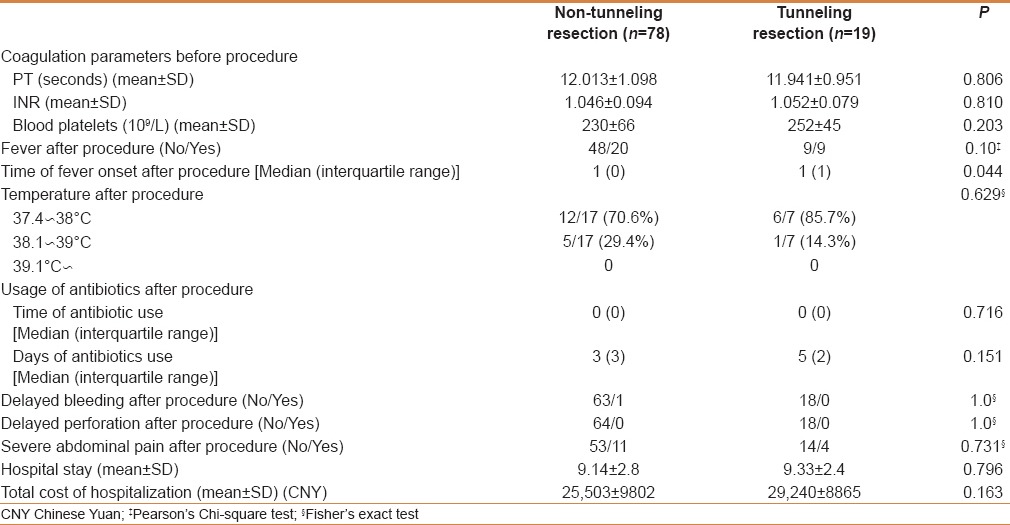
In the nontunneling and tunneling groups, the total hospitalization days were 9.14 ± 2.8 and 9.33 ± 2.4, respectively (P = 0.796), and the total hospitalization costs were 25503 ± 9802 Chinese yuan (CNY) and 29240 ± 8865 CNY (P = 0.163), respectively.
Summary and analysis of reported studies on resection of gastric submucosal tumors by endoscopic nontunneling or tunneling resection
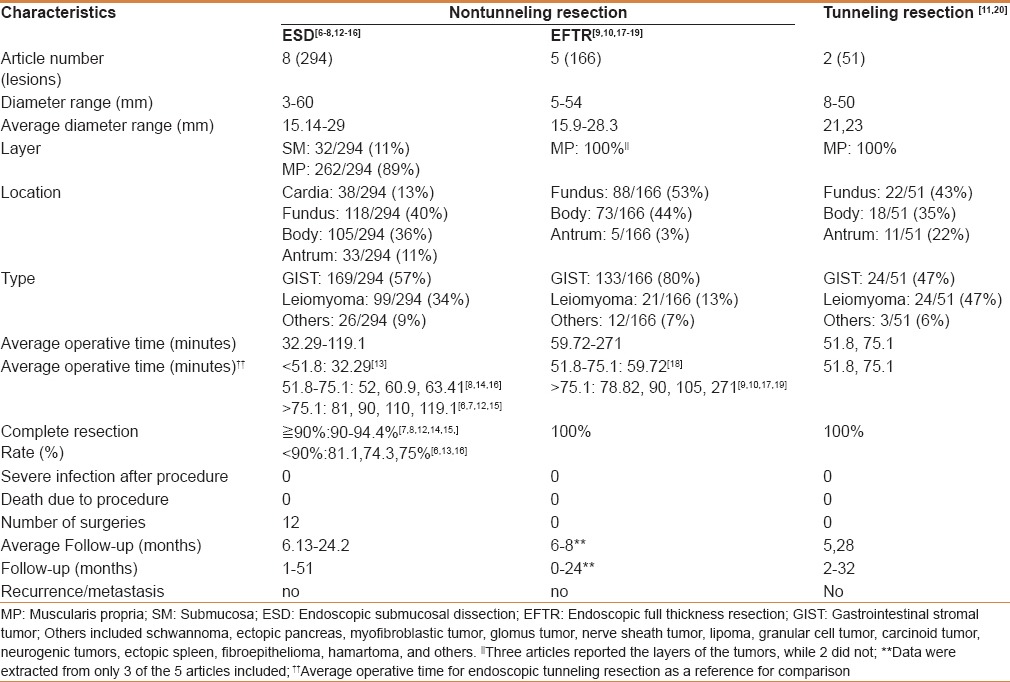
As shown in Figure 3, the search of the PubMed database yielded 8 (294 tumors), 5 (166 tumors), and 2 (51 tumors) studies on gastric submucosal tumors resected by endoscopic non-tunneling (ESD[6,7,8,12,13,14,15,16] and EFTR[9,10,17,18,19]) and tunneling resection,[11,20] respectively. Related information was extracted from each study (Supplemental data). To better understand the feasibility of each method, the information was summarized and analyzed [Table 3].
Figure 3.
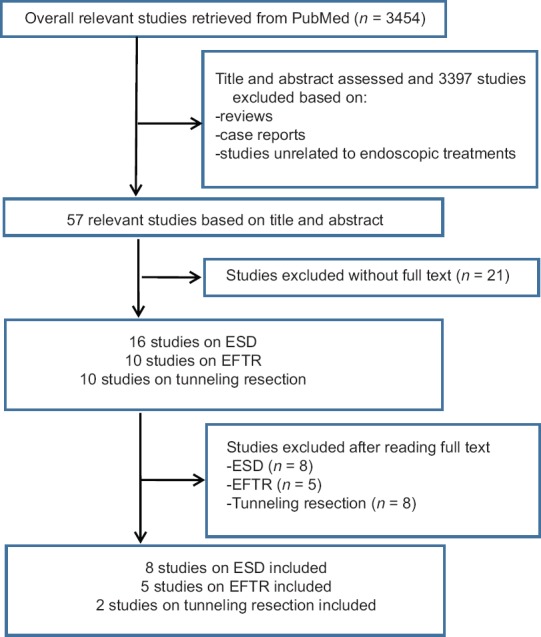
Study selection flow diagram
Table 3.
Summary of published clinical studies on gastric submucosal tumor resection by endoscopic nontunneling (ESD, EFTR) and tunneling resection
Endoscopic submucosal dissection
In the selected studies,[6,7,8,12,13,14,15,16] the average diameter of gastric submucosal tumors resected by ESD was 15.14–29 mm, with 11% originating from the submucosa (32/294) and 89% from the muscularis propria (262/294). Tumors were mostly located in the fundus (118/294, 40%). Pathological types mainly included stromal tumor, leiomyoma, and lipoma, with gastric stromal tumor accounting for the highest proportion at 57% (169/294). Rates of complete resection mostly ranged from 90 to 94.4%,[7,8,12,14,15] and only 3 studies reported complete resection rates of 81.1%, 74.3%, and 75%.[6,13,16] No severe infection or death occurred in any successfully resected cases. No relapse or metastasis occurred in the follow-up period of 1–51 months.
Endoscopic full-thickness resection
The average diameter of submucosal tumors resected by EFTR was 15.9–28.3 mm. Most tumors were located in the fundus (88/166, 53%). The pathological types mainly included stromal tumor, leiomyoma, and lipoma. Gastric stromal tumor accounted for the highest proportion at 80% (133/166). The rates of complete resection were 100%. No severe infection or death occurred after the procedure. The follow-up time ranged from 0 to 24 months in 3 studies,[9,10,18] and there were no reports of relapse or metastasis.
Endoscopic tunneling resection
The average diameters of gastric submucosal tumors resected by tunneling were 21 mm and 23 mm in 2 studies.[11,20] All tumors originated from the muscularis propria. Most tumors were located in the fundus (22/51, 43%). The pathological types mainly included stromal tumor, leiomyoma, and lipoma. Gastric stromal tumor and leiomyoma accounted for the highest proportion of tumors at 47% (24/51) and 47% (24/51), respectively. The rates of complete resection were 100%. No severe infection or death occurred after the procedure. The follow-up time ranged from 2 to 32 months and there was no relapse or metastasis.
DISCUSSION
In our study, the differences between endoscopic nontunneling and tunneling resection for gastric submucosal tumors were systematically analyzed and compared. Our study showed that they seem to be effective and safe for resecting some tumors; compared with nontunneling, tunneling resection as a novel endoscopic technique does not seem to have distinct advantages for resecting gastric submucosal tumors.
At our endoscopy center, the median diameters of gastric submucosal tumors resected by endoscopic nontunneling and tunneling were 15 mm and 20 mm, respectively. Published studies reported average diameters of tumors resected by non-tunneling ranged from 15.1 to 29 mm,[6,7,8,9,10,12,13,14,15,16,17,18,19] and the tumors resected by tunneling were 21–23 mm.[11,20] Thus, the average sizes of tumors resected by the two methods showed no obvious difference. In addition to tumor size, tumor location is also an important factor that should be considered to select an appropriate resection method. Normally, endoscopic nontunneling resection is not limited by tumor location. Unlike the esophagus, the gastric cavity is large and curved, and the tunneling procedure may be limited by tumor location; thus, only some gastric submucosal tumors are easily resected. Li et al. suggested that gastric submucosal tumors located close to the cardia in the lesser curvature of the gastric corpus and in the greater curvature of the gastric antrum are usually optimal indications for the tunneling method.[11] Thus, endoscopic nontunneling resection seems to be more widely used than tunneling resection for gastric submucosal tumors located in different parts of the stomach.
For gastric submucosal tumors, the complete endoscopic resection rate is an important index of the efficacy of endoscopic resection. Most studies[7,8,9,10,12,14,15,17,18,19] reported a high complete resection rate for nontunneling resection ranging from 90% to 100%. For tunneling, the complete resection rate was 94.1% at our endoscopy center, and two studies[11,20] reported complete resection rates of 100% for tumors from the muscularis propria. Consequently, both methods seem to be effective for the complete resection of gastric submucosal tumors. In addition, perforation is a major concern for tumor resection. At our endoscopy center, the perforation rates for nontunneling and tunneling resection during surgery were 26.9% and 36.8%, respectively. All perforations were closed successfully by endoscopic clips or nylon loops or by blocking the tunneling opening. In general, perforation during tunneling resection can be easily closed by blocking the tunnel opening; however, perforation during nontunneling resection can also be effectively closed with endoclips.[6,7,8,9,10,12,13,14,15,16,17,18,19] Further, to evaluate the efficacy of tumor resection, long-term follow-up is needed. In published studies, the proportions of gastric stromal tumors resected by endoscopic nontunneling[6,7,8,9,10,12,13,14,15,16,17,18,19] and tunneling resection[11,20] were 57–80% and 47%, respectively, and there were no cases of relapse or metastasis after average follow-up periods of 0–51 and 2–32 months, respectively.
In our study, the median operative time for endoscopic tunneling resection was significantly longer than that for nontunneling resection; thus, tunneling resection did not seem to be simpler and easier than nontunneling resection. The construction of a submucosal tunnel could contribute to the long operative time. In addition, other parameters such as postoperative fever, delayed hemorrhage and perforation, hospitalization time, and hospitalization expense were similar for both methods. In published studies of gastric submucosal tumors resected by the two methods,[6,7,8,9,10,11,12,13,14,15,16,17,18,19,20] no severe infections occurred after resection. In our study, the rates of postoperative fever in the patients with endoscopic surgery were relatively high, however, most of the patients had only a low-grade fever. The studies[21,22] reported that the rates of fever ranged from 12% to 41.3%, however, most of postoperative fevers were low-grade and transient. Old patients and patients with large size tumors were considered to be risk factors of postoperative fever.[23]
There were several limitations to this study. First, this was a retrospective study, which is itself a limitation. Second, we tried to compare nontunneling with tunneling resection of gastric submucosal tumors. The sample size of the tunneling resection group in our study was relatively small, which may add to the difficulty of determining which method was better. Third, Joensuu et al.[24] suggested that most patients who underwent stromal tumor resection had a relapse within the first 5 years of follow-up. However, most of the studies in our analysis had a median follow-up of 2 years. Thus, longer follow-up time may be needed.
CONCLUSION
In conclusion, both endoscopic nontunneling and tunneling resection seem to be effective and safe methods for removing gastric submucosal tumors. We found no significant differences between the two methods, except for the longer operative time in tunneling resection; hence, it appears that endoscopic tunneling resection does not have distinct advantages for gastric submucosal tumors. The number of cases of tunneling resection in this comparative study was relatively small, and a prospective study with a larger sample size may be needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ye LP, Zhang Y, Luo DH, Mao XL, Zheng HH, Zhou XB, et al. Safety of Endoscopic Resection for Upper Gastrointestinal Subepithelial Tumors Originating from the Muscularis Propria Layer: An Analysis of 733 Tumors. Am J Gastroenterol. 2016;111:788–96. doi: 10.1038/ajg.2015.426. [DOI] [PubMed] [Google Scholar]
- 2.Joo MK, Park JJ, Kim H, Koh JS, Lee BJ, Chun HJ, et al. Endoscopic versus surgical resection of GI stromal tumors in the upper GI tract. Gastrointest Endosc. 2016;83:318–26. doi: 10.1016/j.gie.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 3.Inoue H, Ikeda H, Hosoya T, Onimaru M, Yoshida A, Eleftheriadis N, et al. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy. 2012;44:225–30. doi: 10.1055/s-0031-1291659. [DOI] [PubMed] [Google Scholar]
- 4.Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, et al. Submucosal tunneling endoscopic resection: A new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos) Gastrointest Endosc. 2012;75:195–9. doi: 10.1016/j.gie.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Lu J, Jiao T, Zheng M, Lu X. Endoscopic resection of submucosal tumors in muscularis propria: The choice between direct excavation and tunneling resection. Surg Endosc. 2014;28:3401–7. doi: 10.1007/s00464-014-3610-y. [DOI] [PubMed] [Google Scholar]
- 6.Bialek A, Wiechowska-Kozlowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpinska K, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video) Gastrointest Endosc. 2012;75:276–86. doi: 10.1016/j.gie.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Chao GQ, Li M, Ni GB, Lv B. Endoscopic submucosal dissection for treatment of gastric submucosal tumors originating from the muscularis propria layer. Dig Dis Sci. 2013;58:1710–6. doi: 10.1007/s10620-013-2559-3. [DOI] [PubMed] [Google Scholar]
- 8.He Z, Sun C, Wang J, Zheng Z, Yu Q, Wang T, et al. Efficacy and safety of endoscopic submucosal dissection in treating gastric subepithelial tumors originating in the muscularis propria layer: A single-center study of 144 cases. Scand J Gastroenterol. 2013;48:1466–73. doi: 10.3109/00365521.2013.845796. [DOI] [PubMed] [Google Scholar]
- 9.Huang LY, Cui J, Lin SJ, Zhang B, Wu CR. Endoscopic full-thickness resection for gastric submucosal tumors arising from the muscularis propria layer. World J Gastroenterol. 2014;20:13981–6. doi: 10.3748/wjg.v20.i38.13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926–31. doi: 10.1007/s00464-011-1644-y. [DOI] [PubMed] [Google Scholar]
- 11.Li QL, Chen WF, Zhang C, Hu JW, Zhou PH, Zhang YQ, et al. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video) Surg Endosc. 2015;29:3640–6. doi: 10.1007/s00464-015-4120-2. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Wang F, Wu B, Wang Q, Wang C, Liu J. Endoscopic submucosal dissection of gastric fundus subepithelial tumors originating from the muscularis propria. Exp Ther Med. 2013;6:391–5. doi: 10.3892/etm.2013.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun SY, Kim KO, Park DS, Lee IJ, Park JW, Moon SH, et al. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: A preliminary analysis of appropriate indications. Surg Endosc. 2013;27:3271–9. doi: 10.1007/s00464-013-2904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu YY, Lien JM, Tsai MH, Chiu CT, Chen TC, Yang KC, et al. Modified endoscopic submucosal dissection with enucleation for treatment of gastric subepithelial tumors originating from the muscularis propria layer. BMC Gastroenterol. 2012;12:124. doi: 10.1186/1471-230X-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catalano F, Rodella L, Lombardo F, Silano M, Tomezzoli A, Fuini A, et al. Endoscopic submucosal dissection in the treatment of gastric submucosal tumors: Results from a retrospective cohort study. Gastric Cancer. 2013;16:563–70. doi: 10.1007/s10120-012-0225-7. [DOI] [PubMed] [Google Scholar]
- 16.Lee IL, Lin PY, Tung SY, Shen CH, Wei KL, Wu CS. Endoscopic submucosal dissection for the treatment of intraluminal gastric subepithelial tumors originating from the muscularis propria layer. Endoscopy. 2006;38:1024–8. doi: 10.1055/s-2006-944814. [DOI] [PubMed] [Google Scholar]
- 17.Yang F, Wang S, Sun S, Liu X, Ge N, Wang G, et al. Factors associated with endoscopic full-thickness resection of gastric submucosal tumors. Surg Endosc. 2015;29:3588–93. doi: 10.1007/s00464-015-4113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng Y, Yu L, Yang S, Li X, Ding J, Chen L, et al. Endolumenal endoscopic full-thickness resection of muscularis propria-originating gastric submucosal tumors. J Laparoendosc Adv Surg Tech A. 2014;24:171–6. doi: 10.1089/lap.2013.0370. [DOI] [PubMed] [Google Scholar]
- 19.Mori H, Kobara H, Fujihara S, Nishiyama N, Ayagi M, Matsunaga T, et al. Establishment of the hybrid endoscopic full-thickness resection of gastric gastrointestinal stromal tumors. Mol Clin Oncol. 2015;3:18–22. doi: 10.3892/mco.2014.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J, Zheng M, Jiao T, Wang Y, Lu X. Transcardiac tunneling technique for endoscopic submucosal dissection of gastric fundus tumors arising from the muscularis propria. Endoscopy. 2014;46:888–92. doi: 10.1055/s-0034-1377442. [DOI] [PubMed] [Google Scholar]
- 21.Kato M, Kaise M, Obata T, Yonezawa J, Toyoizumi H, Yoshimura N, et al. Bacteremia and endotoxemia after endoscopic submucosal dissection for gastric neoplasia: Pilot study. Gastric Cancer. 2012;15:15–20. doi: 10.1007/s10120-011-0050-4. [DOI] [PubMed] [Google Scholar]
- 22.Itaba S, Iboshi Y, Nakamura K, Ogino H, Sumida Y, Aso A, et al. Low-frequency of bacteremia after endoscopic submucosal dissection of the stomach. Dig Endosc. 2011;23:69–72. doi: 10.1111/j.1443-1661.2010.01066.x. [DOI] [PubMed] [Google Scholar]
- 23.Izumi K, Osada T, Sakamoto N, Kodani T, Higashihara Y, Ritsuno H, et al. Frequent occurrence of fever in patients who have undergone endoscopic submucosal dissection for colorectal tumor, but bacteremia is not a significant cause. Surg Endosc. 2014;28:2899–904. doi: 10.1007/s00464-014-3551-5. [DOI] [PubMed] [Google Scholar]
- 24.Joensuu H, Vehtari A, Riihimaki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol. 2012;13:265–74. doi: 10.1016/S1470-2045(11)70299-6. [DOI] [PubMed] [Google Scholar]