Abstract
Background and Aim:
Candida spp. have emerged as successful pathogens both in invasive and mucosal infections. C. albicans is the sixth cause of most common nosocomial infections according to studies by the Centers for Disease Control and Prevention. A shift toward non-albicans species has been reported. There is a dearth of knowledge regarding the virulence factors of Candida, especially from this part of India. The aim was to study the prevalence of Candida, speciate, and determine antifungal sensitivity along with the detection of in vitro production of phospholipases in 100 Candida isolates.
Materials and Methods:
A total of 100 Candida isolates from various clinical specimens were studied (February 1, 2015–May 31, 2015; 4 months). Speciation was done by conventional methods and antifungal drugs fluconazole and voriconazole tested. Phospholipase activity (Pz value) was determined.
Results:
Of the 100 Candida spp., 35% were C. albicans and 65% were nonalbicans Candida (NAC). Species spectrum was of the 100 isolates as follows: 35 were C. albicans, 17 Candida tropicalis, 6 Candida glabrata, 8 Candida guilliermondi, 1 Candida kefyr, 6 Candida krusei, 14 Candida parapsilosis, 2 Candida lusitaniae, and 1 Trichosporon and 10 Candida spp. (not speciated). Phospholipase production was seen in 81 (81%) of the total isolates. The majority (63%) of phospholipase producers were NAC. Among NAC spp., the maximum phospholipase activity was seen in C. tropicalis (30%) and C. parapsilosis (24%). Of these, 60% of Candida was from patients admitted to the hospital. Sensitivity rates of C. albicans for fluconazole and voriconazole were 89.5% and 90.5%, respectively.
Conclusion:
Increasing usage of devices, total parenteral nutrition, broad-spectrum antibiotics, chemotherapies, and transplantation are factors contributing to the increase of candidal infections. Recent studies underline the increasing frequency of infections by NAC. The present study showcases the increased prevalence as well as virulence of NAC. In addition, early detection of virulence factors by Candida is useful in clinical decision-making.
Keywords: Candida, phospholipase activity, virulence
Introduction
Infections caused by Candida species are termed candidiasis or candidosis. It exhibits a varied spectrum of organ involvement ranging from colonization of mucosa to multi-systemic organ invasion, thus expressing the variety of relations that occur between the host and commensal microorganisms. Candidemia corresponds to the most important opportunistic mycosis in the world, besides being among the leading causes of nosocomial infections. C. albicans is the sixth cause of most common nosocomial infections according to studies by the Centers for Disease Control and Prevention.[1] The past two decades have seen an increase in the number of patients in Intensive Care Units (ICUs), neutropenia related to cancer, major surgery, and preterm infants.[1] Nosocomial infections constitute a serious public health problem, and they are among the major causes of morbidity and mortality in humans, leading to increased hospitalization time and consequently, generating high costs for patient treatment.[1,2]
Candida tropicalis has a high prevalence in cases of candidemia in Brazil and worldwide.
Its overall incidence raised five folds in the past 10 years and it is currently one among the top six nosocomial bloodstream isolates in American and European studies.[2] Candidemia has been reported to contribute to attributable mortality ranging from 5% to 71% and crude mortality rates as high as 81%.[2] A shift toward nonalbicans species was reported by some authors, especially in hematological, transplanted, and ICU patients. Chang et al. reported nearly 50% of Candida infections by nonalbicans Candida (NAC).[3] Although C. albicans is the most prevalent species involved in invasive fungal infections, the incidence of infections due to nonalbicans species is increasing.[4]
Materials and Methods
A total of 100 Candida isolates from various clinical specimens were taken up for the study. Duration of the study was from February 1, 2015, to May 31, 2015 (4 months) (blood, urine, sputum, pus, swab, lung aspirate, catheter tip, Endotracheal ET fluid/aspirate, internal jugular vein tip, vaginal swab, etc.) All the respiratory specimens and exudates were examined in 10% KOH. In addition, the smears were Gram stained and examined. They were inoculated on to Sabouraud's dextrose agar (SDA) as the main isolation medium. The identification of the species was conducted by assessing the germ tube formation and chlamydospore formation on corn meal agar whereby yeast cells were plated onto cornmeal agar under a glass coverslip to maintain a semianaerobic condition and grown in dark for 7 days at 25°C. Plates were examined over the following 21 days for chlamydospores.
Antifungal susceptibility test was done using the National Committee for Clinical Laboratory Standards 2011 method for antifungal disc diffusion susceptibility for yeasts with approved guideline M44-A 12. We used the following antifungal discs: fluconazole (25 mcg) and voriconazole (1 mcg) (Hi-Media, Mumbai).
Phospholipase estimation
Phospholipase activity assays were performed according to Samaranayake et al.[5] The extracellular phospholipase activity was determined by the egg yolk agar method. The egg yolk medium used consisted of 13.0 g SDA, 11.7 g NaCl, 0.111 g CaCl2, and 10% sterile egg yolk. The egg yolk was centrifuged at 500 g for 10 min at room temperature, and 20 ml of the supernatant was added to the sterilized medium. Extracellular phospholipase activity was detected by inoculating 10 µl aliquots of the yeast suspension (approximately 108 yeast cells/ml) into the wells punched onto the surface of the egg yolk medium. The diameter of the precipitation zone around the well was measured after incubation at 37°C for 48 h. Phospholipase activity (Pz value) was determined. When Pz = 1, no phospholipase activity was detected in the strain. Thus, low Pz means high production of the enzyme. Five classes were described for phospholipase activity including; Pz = 1 means that the test strain is negative for phospholipase, while a value of Pz < 0.90–0.99 = weak phospholipase activity (+), 0.80–0.89 = poor phospholipase activity (++); 0.70–0.79 = moderate phospholipase activity (+++), and < 0.70 = large phospholipase activity (++++). C. albicans ATCC10231 was used as a positive control.
Results
Of the 100 Candida spp. isolated from various clinical specimens, 35% were C. albicans and 65% were NAC. Urine samples (51%) were the most common clinical specimen followed by sputum [Table 1].
Table 1.
Candida spp. Isolated from Various Clinical Samples along with Phospholipase Activity (Percentage)
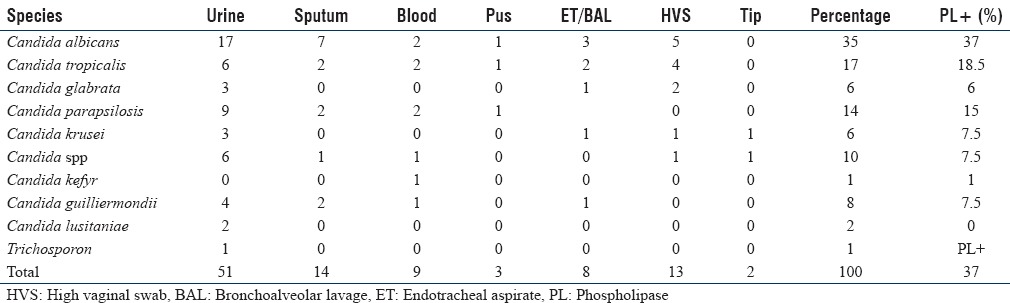
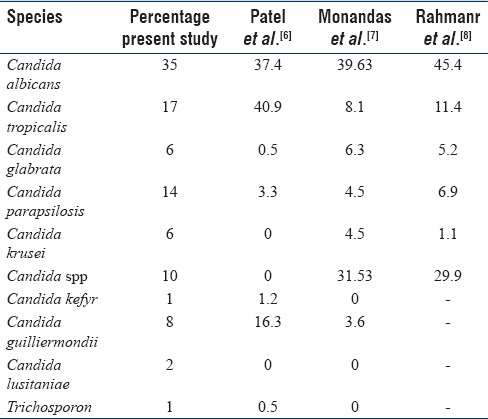
The species spectrum was as follows: of the 100 isolates, 35 were C. albicans, 17 C. tropicalis, 6 Candida glabrata, 8 Candida guilliermondi, 1 Candida kefyr, 2 Candida krusei, 14 Candida parapsilosis, 2 Candida lusitaniae, and 1 Trichosporon and 10 Candida spp. Candida spp. isolated from various clinical samples and its comparison with other studies is shown in Table 2.
Table 2.
Prevalence of Candida spp. in Comparison with other Studies
Phenotypic screening of extracellular phospholipase enzyme
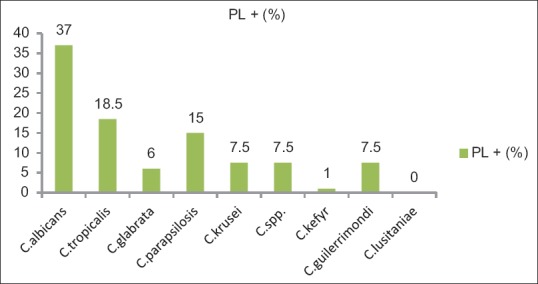
Phospholipase production was seen in 81 (81%) of the total isolates. In the present study, majority (63%) of phospholipase producers were NAC while only 37% (30) of C. albicans were positive for phospholipases [Figure 1]. Among NAC spp., the maximum phospholipase activity was seen in C. tropicalis (30%) and C. parapsilosis (24%). Sensitivity rates of C. albicans for fluconazole and voriconazole were 89.5% and 90.5%, respectively [Table 3].
Figure 1.
Phospholipase activity of the various Candida spp. (in percentage)
Table 3.
Antifungal Susceptibility of Albicans and Nonalbicans Candida

Discussion
Both albicans and NAC cause invasive candidiasis, and the antifungal resistance of invasive Candida aggravates the situation. Increasing number of treatment failures, associated mortality, and shift to more resistant isolates advocate the need for species identification in Candida. Phenotypic methods for speciation are effective and economic but they can be time consuming and expensive albeit molecular techniques such as polymerase chain reaction and restriction fragment length polymorphism are promising but come with the sanction of economic burden, thereby restricting utility to research and reference laboratories.
The present study reports 35% of C. albicans and 65% of NAC of all clinical specimens, which is in concordance with the results of Sachin et al. whereby C. albicans was 44.4% and NAC spp. was 55.5%. Among NAC spp., C. tropicalis was the major isolate followed by C. glabrata, C. parapsilosis, C. krusei, and C. kefyr [Table 1].
The present study showed the distribution of Candida species in different clinical samples and the predominance of NAC, as was also shown by Mujica et al.[9] The most common isolate from all samples was C. tropicalis. NAC (41.37%) was the predominant species recovered from all samples except sputum. Mohandas et al.[7] and the ARTEMIS DISK global antifungal surveillance project undertaken between 1997 and 2007 observed that 90% of infections were caused by four Candida species, i.e., C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis[10] [Table 2].
Numerous virulence factors of Candida have been reported that enable a mucosal colonizer to transform into a disseminating pathogen causing mortality. Adhesins help the yeast in adhering to the host cell surfaces whereas hydrolytic enzymes such as proteinases and phospholipases along with hyphal forms promote the penetration through cells and hence internalization. Phospholipases are a heterogeneous group of enzymes produced by Candida species that share the ability to hydrolyze one or more esterlinkage in glycerophospholipids, thereby leading to cell lysis of host cells. Ma et al. experimented with corneal stroma and reported that PLB comes into action immediately after fungal adherence by decomposing the phospholipid of cell membranes and leading to cell lysis.[11] In Candida, phospholipase activity is concentrated at the growing tip of the pseudohyphae. Ibrahim et al. found that chemically induced mutants of C. albicans deficient in extracellular phospholipase activity caused ≥30% less damage to endothelial cells in comparison to the parent strain.[12] Production of extracellular phospholipase was predictive of mortality.
These mechanisms put together initiate the host cell damage and thereby hasten the dissemination of fungal infections. C. albicans, Cryptococcus neoformans, and Aspergillus also exhibit phospholipase as a potential virulence factor. The secretion of these hydrolytic enzymes is a process that requires attention, especially in ICU patients with candidemia associated with the use of catheters. Phospholipase production was seen in 81 (81%) of the total isolates. In the present study, majority (63%) of the phospholipase producers were NAC while only 37% (30) of C. albicans were positive for phospholipases [Figure 1]. Among NAC spp., the maximum phospholipase activity was seen in C. tropicalis (30%) and C. parapsilosis (24%). A study from Egypt observed 100% phospholipase production from Candida spp. including albicans and nonalbicans which is certainly higher than seen presently. A study from Bengaluru reported 23 (46.93%) C. albicans isolates and 26 (42%) non-C. albicans isolates producing phospholipase activity.[7] Tellapragada et al. reported that 93% of phospholipase producers were C. albicans while only 7% of NAC were positive for phospholipases which is in contrast to the present findings.[13]
Sensitivity rates of fluconazole and voriconazole are shown in Table 3 and these rates are similar to those reported by Pahwa et al., the present rates were 88% and 92%, respectively. Tellapragada et al. found fluconazole and voriconazole sensitivity rates to be 95% and 97.5%, respectively.
This study has a few limitations: correlation of specific Candida spp. along with risk factors could have defined morbidity species wise. However, this study improved our knowledge upon the epidemiology, virulence factor phospholipase, and antifungal resistance of invasive Candida spp. isolated in the present setting.
Conclusion
In summary, the present study showed a predominance of NAC over C. albicans in different clinical samples. In addition, NAC were higher producers of phospholipase. Various virulence factors are being targeted in the wake of making mucosal vaccines. Phospholipase can be regarded as a candidate for vaccine development since its production gets orchestrated in the pathogenesis. The study of these virulence factors would help in understanding the pathogenic role of NAC spp. With the broadening numbers of hospitalized patients both with and without risk factors, NAC will continue to emerge as an important cause of infection. Continuous surveillance of serious infections caused by both albicans and non-albicans spp. is necessary for monitoring changes in the epidemiology.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sheevani, Sharma P, Aggarwal A. Nosocomial Candida infection in a rural tertiary care hospital. J Clin Diagn Res. 2013;7:405–6. doi: 10.7860/JCDR/2013/4574.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: A propensity analysis. Clin Infect Dis. 2005;41:1232–9. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 3.Chang TP, Ho MW, Yang YL, Lo PC, Lin PS, Wang AH, et al. Distribution and drug susceptibilities of Candida species causing candidemia from a medical center in central Taiwan. J Infect Chemother. 2013;19:1065–71. doi: 10.1007/s10156-013-0623-8. [DOI] [PubMed] [Google Scholar]
- 4.Sardi JC, Scorzoni L, Bernardi T, Fusco-Almeida AM, Mendes Giannini MJ. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J Med Microbiol. 2013;62(Pt 1):10–24. doi: 10.1099/jmm.0.045054-0. [DOI] [PubMed] [Google Scholar]
- 5.Samaranayake YH, Dassanayake RS, Jayatilake JA, Cheung BP, Yau JY, Yeung KW, et al. Phospholipase B enzyme expression is not associated with other virulence attributes in Candida albicans isolates from patients with human immunodeficiency virus infection. J Med Microbiol. 2005;54(Pt 6):583–93. doi: 10.1099/jmm.0.45762-0. [DOI] [PubMed] [Google Scholar]
- 6.Patel L, Pethani J, Bhatia P, Rathod S, Shah P. Prevalence of Candida infection and its antifungal susceptibility pattern in tertiary care hospital. Natl J Med Res. 2012;2:439–41. [Google Scholar]
- 7.Mohandas V, Ballal M. Distribution of Candida species in different clinical samples and their virulence: Biofilm formation, proteinase and phospholipase production: A study on hospitalized patients in Southern India. J Glob Infect Dis. 2011;3:4–8. doi: 10.4103/0974-777X.77288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rahmanr ZA, Zahiraddin W, Hassan SA, Noor SS, Hassan H. Epidemiology of Candida species in tertiary-teaching hospital in Malaysia. Int Med J. 2008;4:291–4. [Google Scholar]
- 9.Mujica MT, Finquelievich JL, Jewtuchowicz V, Iovannitti CA. Prevalence of Candida albicans and Candida non-albicans in clinical samples during 1999-2001. Rev Argent Microbiol. 2004;36:107–12. [PubMed] [Google Scholar]
- 10.Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, et al. Results from the ARTEMIS DISK global antifungal surveillance study, 1997 to 2007: A 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol. 2010;48:1366–77. doi: 10.1128/JCM.02117-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma L, Xie L, Dong X, Shi W. Role of extracellular phospholipase B of Candida albicans as a virulent factor in experimental keratomycosis. Curr Eye Res. 2009;34:761–8. doi: 10.1080/02713680903056391. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim AS, Mirbod F, Filler SG, Banno Y, Cole GT, Kitajima Y, et al. Evidence implicating phospholipase as a virulence factor of Candida albicans. Infect Immun. 1995;63:1993–8. doi: 10.1128/iai.63.5.1993-1998.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tellapragada C, Eshwara VK, Johar R, Shaw T, Malik N, Bhat PV, et al. Antifungal susceptibility patterns, in vitro production of virulence factors, and evaluation of diagnostic modalities for the speciation of pathogenic Candida from blood stream infections and vulvovaginal candidiasis. J Pathog 2014. 2014 doi: 10.1155/2014/142864. 142864. [DOI] [PMC free article] [PubMed] [Google Scholar]


