Abstract
Background and Aims:
Sevoflurane is the most often used inhalational agent in paediatric anaesthesia, but emergence agitation (EA) remains a major concern. Oral midazolam and parenteral dexmedetomidine are known to be effective in controlling EA. We attempted to elucidate whether oral dexmedetomidine is better than midazolam in controlling EA.
Methods:
Prospective double-blinded study involving ninety patients aged 1–10 years, undergoing elective surgeries of <2 h of expected duration under sevoflurane general anaesthesia, randomised to receive either midazolam (Group A) or dexmedetomidine (Group B) as oral premedication was carried out to record level of sedation before induction, haemodynamic parameters and recovery time. Incidence and severity of EA, post-operative pain and requirement of rescue analgesic were assessed at 0, 5, 15, 30 and 60 min postoperatively.
Results:
Data were analysed applying Student's t-test and Chi-square test using SPSS software. Mask acceptance was better in Group B (97.8% vs. 73.4%, P < 0.001). Mean arterial pressure was lower in Group B (P < 0.001) though clinically not significant. More rescue analgesic was required in Group A (5.6% vs. 0%). There was no significant difference in adverse effects. Although there was a higher incidence of EA in Group A (Aono's score 3 and 4; 40% vs. 4.4%), none of them required intervention (paediatric anaesthesia emergence delirium score >10; 0 vs. 0).
Conclusion:
Premedication with oral dexmedetomidine provides smooth induction and recovery, reduces the EA and provides better analgesia and sedation as compared to oral midazolam.
Keywords: Dexmedetomidine, emergence agitation, midazolam, oral premedication, sevoflurane
INTRODUCTION
With the introduction of shorter onset inhalational agents such as sevoflurane, incidence of emergence agitation (EA) has become a significant hindrance in quality post-operative care and early discharge from post-operative care unit.[1]
EA is defined as a disturbance in a child's awareness of and attention to his/her environment with disorientation and perceptual alterations including hypersensitivity to stimuli and hyperactive motor behaviour in the immediate post-anaesthesia period.[2]
Of several interventions tried for controlling EA, premedication has some advantage, as it is a routine practice in paediatric anaesthesia, especially to favour parental separation with additional benefit of reducing the EA.[3] Although multiple studies have indicated that midazolam, a most commonly used and a near ideal premedicant, is also effective in reducing the incidence of EA, unfortunately it is not effective in all cases.[3,4,5] Meanwhile, intraoperative use of dexmedetomidine has shown promising results in reducing the EA.[6,7] Dexmedetomidine has also been tried as an effective premedicant with favourable outcomes.[8,9,10] Despite different mechanism of action and pharmacological profiles, both midazolam and dexmedetomidine have been used to reduce the incidence of EA after sevoflurane anaesthesia. Thus, we have compared effectiveness of these two drugs when used orally as premedication, in prevention of EA after sevoflurane anaesthesia in paediatric patients. The primary outcomes were quality of sedation and incidence and severity of EA, whereas secondary outcomes were haemodynamic stability, incidence of adverse effects of drugs and rescue analgesics requirements.
METHODS
With the Institutional Ethical Committee permission (Order No: INST.EC/E.C/60/2013-14, dated 19th September 2013), a prospective double-blinded study was conducted at a tertiary teaching hospital from January 2014 to August 2015, enrolling ninety patients of 1–10 years' age, belonging to the American Society of Anesthesiologists Physical Status (ASA-PS) I and II, undergoing elective surgeries of <2 h of expected duration under sevoflurane general anaesthesia. Patients were thoroughly evaluated during their pre-anaesthetic visit. Patients with known allergies to the drugs used in the study, those on chronic analgesic or sedative medication, children with known central nervous system disorders including developmental delay or mental retardation and patients with the presence of anticipated difficult airway were excluded from the study. Written informed consent was obtained from parents/guardian of eligible children. Patients were fasted for 6 and 2 h before surgery for solids and clear fluids, respectively. After shifting the patient to the pre-operative room, they were given premedication orally either midazolam or dexmedetomidine, approximately 45 min before surgery where, Group A patients received midazolam 0.5 mg/kg, dosage rounded to nearest digit and Group B patients received dexmedetomidine 4 μg/kg, dosage rounded to nearest multiples of 10, mixed with 5 ml of honey based on block randomisation protocol, in which participants were divided into 15 blocks of 6 each. Fifteen envelopes were prepared by the investigator, each containing six chits, three each having A or B on it. The preoperative ward nurse assigned participants to the groups by picking a chit each time from the respective envelope, and premedication was given as per the group allocation decided by the chit drawn. The pre-operative nurse administering premedication did not reveal either block to which patient belonged or the group allocation till the procedure, and data collection was over in respective case. The patient/parent and anaesthesiologist managing the procedure were unaware of the group allocation or premedication given. After 45 min, patient was taken to the operation theatre. An electrocardiogram, pulse oximeter and non-invasive blood pressure monitor were attached. General anaesthesia was induced with 8% sevoflurane in 60% nitrous oxide and oxygen. After achieving adequate depth of anaesthesia, an intravenous (IV) line was secured with appropriate gauge cannula and injection fentanyl 2 μg/kg IV was given. Injection vecuronium bromide 0.1 mg/kg IV or injection atracurium 0.5 mg/kg IV was given for neuromuscular blockade. After ventilation with sevoflurane 5% in oxygen for 3 min, the airway was secured with an appropriate size endotracheal tube/laryngeal mask airway (LMA). Anaesthesia was maintained with 66% nitrous oxide in oxygen, titrated concentration of sevoflurane and positive pressure ventilation. Neuromuscular blockade was maintained with additional dose of neuromuscular blocking drugs if train-of-four (TOF) count was >2. Diclofenac suppository 1 mg/kg rounded to nearest multiples of 12.5 mg was placed per rectal before the onset of procedure. At the end of procedure, after discontinuing sevoflurane and when the TOF count was >3, neuromuscular blockade was reversed with appropriate doses of neostigmine and glycopyrrolate. Once the child was awake, breathing spontaneously and TOF ratio was 1, the trachea was extubated or LMA removed. After shifting the child to the post-anaesthesia care unit (PACU), a parent was allowed to stay with the child till the discharge. The following parameters were monitored and recorded: level of sedation before induction (mask acceptance scale [1 = excellent, 2 = good, 3 = fair and 4 = poor]); haemodynamic parameters including mean arterial pressure (MAP), heart rate (HR), SpO2 every 5 min during the procedure and at 0, 5, 15, 30, 60 min post-operatively; time to recovery (defined as time interval between discontinuation of sevoflurane and extubation); quality of recovery (Modified Aldrete Score) in the post-operative ward on arrival; post-operative pain (objective pain discomfort score [OPDS] [blood pressure, crying, moving, agitation, verbal evaluation (language), each criterion was categorised into 0, 1 or 2 with maximum possible score of 10, where score exceeding 6 required rescue analgesic, (paracetamol infusion at 15 mg/kg was given)]) at 0, 5, 15, 30 and 60 min; incidence of EA (Aono's four-point scale, where 1 = calm, 2 = not calm but easily calmed, 3 = not easily calmed, moderately agitated or restless and 4 = excited or disoriented; for scores 1 and 2, EA was absent and for scores 3 and 4, EA was present) and severity of agitation (paediatric anaesthesia emergence delirium [PAED] score containing five behavioural classes: makes eye contact with caregiver, actions are purposeful, aware of his or her surroundings, restless and inconsolable, where each class was scored from 0 to 4, with maximum possible score of 20 and a total score of more than ten was indication for treatment for the severity of agitation) both scores assessed at 0, 5, 15, 30 and 60 min postoperatively.[10,11,12]
Sample size (n) was calculated based on findings of Mountain et al. using the formula for comparison of two means; where mean PAED scores for dexmedetomidine and midazolam groups were 7 and 6 (7.42 and 5.62, respectively, rounded off to full digit), level of significance as 5% and precision of 2. A minimum of 82 patients were required totally in both the groups, and hence we enrolled ninety.[10] Data were compiled using Microsoft Excel 2007 (Microsoft Corporation, Redmond, Washington, 2007) and were analysed using SPSS version 20 software (IBM Corporation, New York, USA, 2014). Student's t-test was used for comparing independent samples such as age, weight, duration of surgery, haemodynamic parameters and incidence of EA. Sex distribution, ASA-PS distribution, mask acceptance score, side effects and need for rescue drugs were compared by Chi-square test. Aono's four-point score, PAED score and OPDS scores were tabulated using median and interquartile range. P <0.05 was considered statistically significant and P < 0.001 was considered highly significant.
RESULTS
All children (n = 90) enrolled for the study completed the protocol [Figure 1]. The groups were comparable in terms of demography, type and duration of surgery [Tables 1 and 2]. Mask acceptance was better in Group B i.e., 97.8% versus 73.4% [P < 0.001, Table 3]. MAP was lower in Group B though clinically not significant [P < 0.001, Figure 2]. More rescue analgesia was required in Group A i.e., 5.6% versus 0%. No significant difference in adverse effects. Although there was a higher incidence of EA in Group A [Aono's four-point score 3 and 4, 40% vs. 4.4%, Figure 3], none of them had severe EA requiring intervention [PAED > 10, 0 vs. 0, Table 4].
Figure 1.

CONSORT flow chart
Table 1.
Demography of the groups
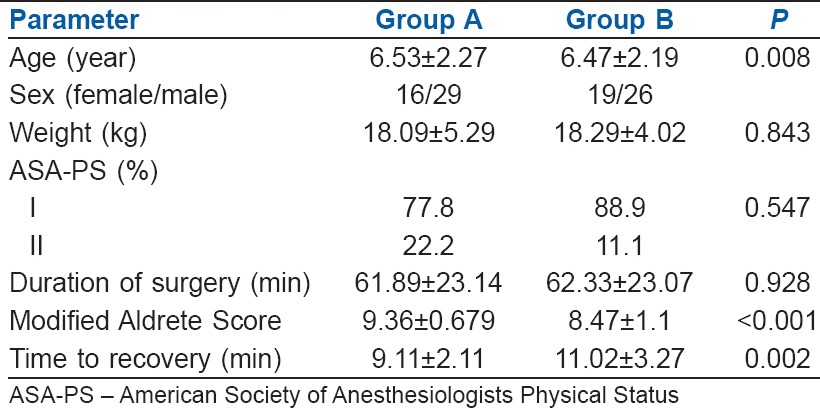
Table 2.
Distribution of surgeries amongst the two groups
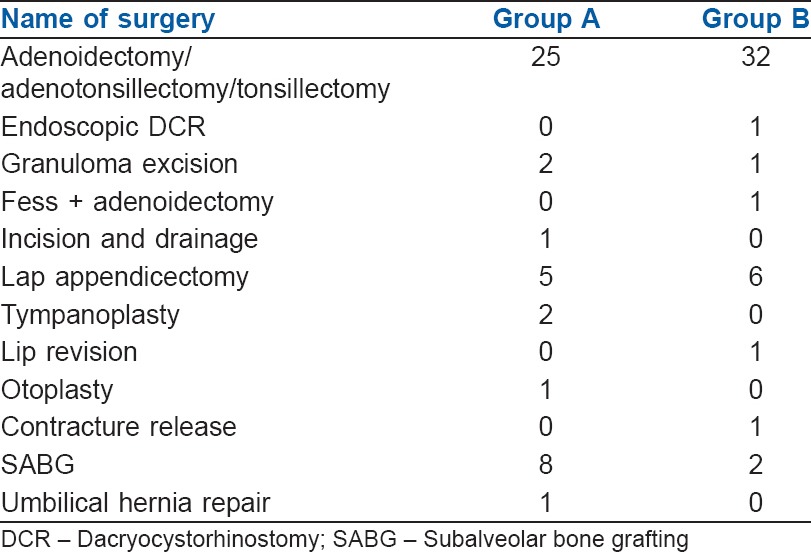
Table 3.
Comparison of mask acceptance score
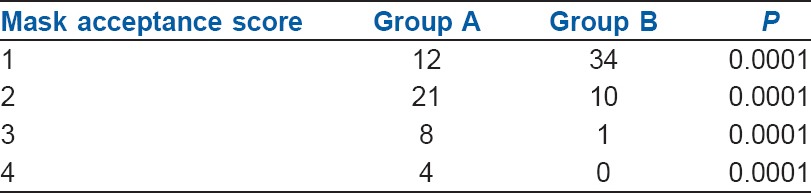
Figure 2.
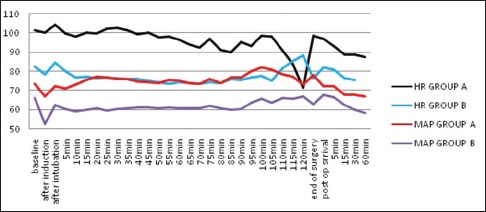
Comparison of heart rate and mean arterial pressure between the two groups. MAP – Mean arterial pressure; HR – Heart rate
Figure 3.
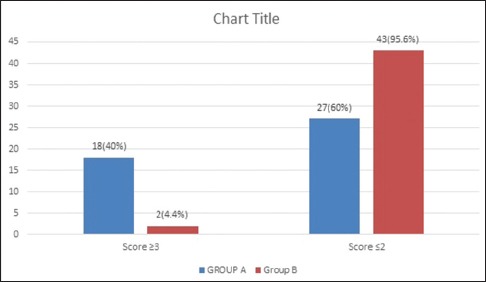
Incidence of emergence agitation as per Aono's four-point scale
Table 4.
Objective pain discomfort score, Aono's and paediatric anaesthesia emergence delirium scores
DISCUSSION
Dexmedetomidine was more effective in suppressing EA compared to midazolam in a dose of 4 μg/kg as evident by significantly less number of children in Group B had Aono's four point of 3 or 4 [P = 0.001, Table 4]. This trend remained so up to 30 min in the post-operative period, thereafter values were similar between the groups with most of the children having a score of 1 or 2 (calm or easily consolable). However, Aono's scale being a qualitative measure does not indicate when to treat EA. Therefore, PAED scale was used to measure the severity of EA and to initiate treatment. PAED scores were significantly lower in Group B [median of 1–2 vs. 3–5, P < 0.001, Table 4]. While 19 patients in Group A had PAED score more than 6, just two patient of Group B had score more than 6 and 16 patients had score 0. α2 agonists are known to decrease EA in the post-operative ward.[10,11] Dexmedetomidine decreased incidence of EA from 40% to 4.4%, this goes well with the finding of Shukry et al.[6] Although these findings support the reports of significant reduction in EA with α2 agonists, the incidence in the current study is least compared to available data. However, due to differing working definition of EA and dosages of dexmedetomidine used, reported absolute incidence may not be comparable between various studies.[10,11,12,13,14,15,16,17,18] The only available meta-analysis concluded that both the agents are of equal efficiency in preventing EA. Authors were of the opinion that doses of midazolam used in the studies analysed were well accepted (oral 0.5 mg/kg; intranasal 0.2 mg/kg), and the same is widely used in clinical practice, while that of dexmedetomidine was empirical (oral 2.5 μg/kg; intranasal 1 μg/kg). Probably, oral dose of dexmedetomidine was too small compared to IV loading dose due to first pass metabolism. The mean bioavailability of dexmedetomidine is 15.6% by oral route, while it is about 81.8% through transmucosal. Considering four times more bioavailability by transmucosal route as compared to oral, an oral dose of 4 μg/kg may be considered equivalent to transmucosal (transbuccal or transnasal) dose of 1 μg/kg. However, loss of drug due to movement of saliva/mucus and swallowing, causes the absorption to be much lesser.[19] Therefore, the results of several existing studies and meta-analysis by Peng et al. may not be comparable with the current study.[20]
In the current study, Group B had three times better mask acceptance and six times less need for forcing the face mask induction, which indicates dexmedetomidine ensured better cooperation, adequate sedation and smooth induction conditions [Table 3]. We observed better mask acceptance with dexmedetomidine premedication as compared to other studies, probably due to smaller dosage used (1, 2 μg/kg) as well as route of administration (intranasal) in those studies.[10,15] Peng et al. found better mask acceptance with dexmedetomidine from a meta-analysis involving 425 patients but cautioned that the results are confounded by heterogeneity.[20]
Dexmedetomidine is known to lower both HR and blood pressure.[5,6,11] Similar trend was witnessed in the current study. HR remained consistently lower in Group B where four patients required atropine to counter bradycardia. This trend continued in post-operative period for first 60 min. However, mean HR in Group B was more stable compared to that of Group A. Although MAP also remained lower in Group B, it did not require any corrective measures, and the difference was clinically insignificant [Figure 2] akin to Peng et al.[20] The finding support the observation that dexmedetomidine provides better stability of MAP.[10,15,20] Oxygen saturation was never affected intraoperatively as well as postoperatively in either of the groups, reinforcing the safety of both drug usages in paediatric population.[20]
Effective treatment of post-operative pain is known to reduce the incidence of EA. This includes multimodal pain management with non-steroidal anti-inflammatory drugs, ketorolac, α2-agonist such as clonidine, dexmedetomidine, regional anaesthesia including caudal blocks and narcotics. However, controlling pain was not able to abolish EA completely. In the current group of study participants, there was no need for intervention to control EA as it was of very low intensity. There was significantly lower OPDS in Group B [median 0, 1 vs. 3, 4 and P < 0.001, Table 4] and none of these patients required rescue analgesic as against five patients in Group A. This reinforces the belief that dexmedetomidine has analgesic property and analgesic-sparing effect in the PACU.
Dexmedetomidine causes anxiolysis, sedation and profound analgesia which also contribute to the depth of anaesthesia.[9,21] We noticed slight delay in recovery (time to recovery and recovery score) amongst children receiving dexmedetomidine [P = 0.002, Table 1]. However, Peng et al. found no such difference in comparison to midazolam in either recovery time or discharge from PACU.[20] Available data are inadequate to infer, due to heterogeneity in the definition, dexmedetomidine dosage and anaesthetic protocol. However, lately, dexmedetomidine is being preferred even in children undergoing cardiac catheterisation procedures as it provides good sedation along with cardiovascular and respiratory stability.[22,23,24] There was no significant difference in adverse effects between two groups except for bradycardia with dexmedetomidine which is pharmacologically well described.
Duration of surgery has importance as the half-lives of premedicants used is comparatively short. Midazolam has elimination half-life of 1–4 h and dexmedetomidine has elimination half-life of between 2.0 and 2.5 h.[21] The effect of premedicant has outlasted the surgical time as the mean duration of surgery in both the groups was approximately 1 h, while duration of action of the drugs is much longer (approximately 2–5 h). Most of the studies in the past carried out in the context of EA were based on patients undergoing single procedure.[12] In the current study; patients included underwent different procedures with different intensity of post-operative pain. Majority of the procedures in both the groups were adenotonsillectomy followed by lap appendicectomy. Distribution of types of procedures between the groups was not different [Table 2]. Hence, it may be concluded that duration, type and heterogeneity of surgical procedures did not affect the outcome of the study. In addition, participant allocation and blinding were strictly adhered to for minimising the distributive and observer bias.
The current study has several limitations. Inclusion of control group would have made the inferences robust as incidence of EA requiring treatment was very low in the study group. We have not recorded the pre-operative sedation. Our surgical population was heterogeneous and the intensity of pain varied with the surgical procedure, this might have influenced EA. We took surgical procedures with expected duration of <2 h, so the results may not be suitable to be extrapolated to surgical procedures of longer duration. Finally, equipotent dosages of midazolam and dexmedetomidine have not been fixed.
Although dexmedetomidine was used orally for premedication or procedural sedation amongst children, it was not used much for testing its efficacy in controlling EA following sevoflurane anaesthesia [Table 4]. However, IV and intranasal routes have been tested and reported to be effective in controlling EA in comparison to midazolam. The current study establishes clearly the superiority of oral dexmedetomidine over midazolam as a premedicant as well as to control EA. However, the incidence of EA requiring treatment was nil in the present study, forcing us to believe that both drugs are of equal efficiency in controlling the overall severity of EA. However, study involving larger sample population to assess the incidence of EA requiring treatment and to calculate numbers needed to treat in each group is needed to answer the question. Further, the effect on duration of stay in the post-operative ward and time to discharge from the hospital are to be studied, which were not part of the observations.
CONCLUSION
Oral dexmedetomidine is superior to oral midazolam when used as premedication to reduce the incidence and severity of the EA. Dexmedetomidine provided better induction environment and reduced need for rescue analgesia postoperatively.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wang M, Zhang JH, Applegate RL., 2nd Adverse effect of inhalational anesthetics on the developing brain. Med Gas Res. 2014;4:2. doi: 10.1186/2045-9912-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwak KH. Emergence agitation/delirium: We still don't know. Korean J Anesthesiol. 2010;59:73–4. doi: 10.4097/kjae.2010.59.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nasr VG, Hannallah RS. Emergence agitation in children – A view. Middle East J Anaesthesiol. 2011;21:175–82. [PubMed] [Google Scholar]
- 4.Lapin SL, Auden SM, Goldsmith LJ, Reynolds AM. Effects of sevoflurane anaesthesia on recovery in children: A comparison with halothane. Paediatr Anaesth. 1999;9:299–304. doi: 10.1046/j.1460-9592.1999.00351.x. [DOI] [PubMed] [Google Scholar]
- 5.Kain ZN, Mayes LC, Caldwell-Andrews AA, Karas DE, McClain BC. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics. 2006;118:651–8. doi: 10.1542/peds.2005-2920. [DOI] [PubMed] [Google Scholar]
- 6.Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth. 2005;15:1098–104. doi: 10.1111/j.1460-9592.2005.01660.x. [DOI] [PubMed] [Google Scholar]
- 7.Isik B, Arslan M, Tunga AD, Kurtipek O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr Anaesth. 2006;16:748–53. doi: 10.1111/j.1460-9592.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- 8.Kulka PJ, Bressem M, Tryba M. Clonidine prevents sevoflurane-induced agitation in children. Anesth Analg. 2001;93:335–8. [PubMed] [Google Scholar]
- 9.Ibacache ME, Muñoz HR, Brandes V, Morales AL. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg. 2004;98:60–3. doi: 10.1213/01.ANE.0000094947.20838.8E. [DOI] [PubMed] [Google Scholar]
- 10.Mountain BW, Smithson L, Cramolini M, Wyatt TH, Newman M. Dexmedetomidine as a pediatric anesthetic premedication to reduce anxiety and to deter emergence delirium. AANA J. 2011;79:219–24. [PubMed] [Google Scholar]
- 11.Manaa EM, Abdelhaleem AA, Mohamed EA. Fentanyl versus dexmedetomidine effect on agitation after sevoflurane anaesthesia. Saudi J Anaesth. 2007;1:57–61. [Google Scholar]
- 12.Ali MA, Abdellatif AA. Prevention of sevoflurane related emergence agitation in children undergoing adenotonsillectomy: A comparison of dexmedetomidine and propofol. Saudi J Anaesth. 2013;7:296–300. doi: 10.4103/1658-354X.115363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng QT, Xia ZY, Luo T, Wu Y, Tang LH, Zhao B, et al. Dexmedetomidine reduces emergence agitation after tonsillectomy in children by sevoflurane anesthesia: A case-control study. Int J Pediatr Otorhinolaryngol. 2012;76:1036–41. doi: 10.1016/j.ijporl.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 14.Özcengiz D, Gunes Y, Ozmete O. Oral melatonin, dexmedetomidine, and midazolam for prevention of postoperative agitation in children. J Anesth. 2011;25:184–8. doi: 10.1007/s00540-011-1099-2. [DOI] [PubMed] [Google Scholar]
- 15.Akin A, Bayram A, Esmaoglu A, Tosun Z, Aksu R, Altuntas R, et al. Dexmedetomidine vs midazolam for premedication of pediatric patients undergoing anesthesia. Paediatr Anaesth. 2012;22:871–6. doi: 10.1111/j.1460-9592.2012.03802.x. [DOI] [PubMed] [Google Scholar]
- 16.Linares Segovia B, García Cuevas MA, Ramírez Casillas IL, Guerrero Romero JF, Botello Buenrostro I, Monroy Torres R, et al. Pre-anesthetic medication with intranasal dexmedetomidine and oral midazolam as an anxiolytic. A clinical trial. An Pediatr (Barc) 2014;81:226–31. doi: 10.1016/j.anpedi.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Sheta SA, Al-Sarheed MA, Abdelhalim AA. Intranasal dexmedetomidine vs midazolam for premedication in children undergoing complete dental rehabilitation: A double-blinded randomized controlled trial. Paediatr Anaesth. 2014;24:181–9. doi: 10.1111/pan.12287. [DOI] [PubMed] [Google Scholar]
- 18.Pant D, Sethi N, Sood J. Comparison of sublingual midazolam and dexmedetomidine for premedication in children. Minerva Anestesiol. 2014;80:167–75. [PubMed] [Google Scholar]
- 19.Anttila M, Penttilä J, Helminen A, Vuorilehto L, Scheinin H. Bioavailability of dexmedetomidine after extravascular doses in healthy subjects. Br J Clin Pharmacol. 2003;56:691–3. doi: 10.1046/j.1365-2125.2003.01944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng K, Wu SR, Ji FH, Li J. Premedication with dexmedetomidine in pediatric patients: A systematic review and meta-analysis. Clinics (Sao Paulo) 2014;69:777–86. doi: 10.6061/clinics/2014(11)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoelting RK, Hiller SC. Benzodiazepines. In: Brown B, Murphy F, editors. Pharmacology and Physiology in Anaesthetic Practice. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. pp. 134–49. [Google Scholar]
- 22.Koruk S, Mizrak A, Kaya Ugur B, Ilhan O, Baspinar O, Oner U. Propofol/dexmedetomidine and propofol/ketamine combinations for anesthesia in pediatric patients undergoing transcatheter atrial septal defect closure: A prospective randomized study. Clin Ther. 2010;32:701–9. doi: 10.1016/j.clinthera.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Tosun Z, Akin A, Guler G, Esmaoglu A, Boyaci A. Dexmedetomidine-ketamine and propofol-ketamine combinations for anesthesia in spontaneously breathing pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2006;20:515–9. doi: 10.1053/j.jvca.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Young ET. Dexmedetomidine sedation in a pediatric cardiac patient scheduled for MRI. Can J Anaesth. 2005;52:730–2. doi: 10.1007/BF03016562. [DOI] [PubMed] [Google Scholar]


