Abstract
Background and Aims:
Various anaesthetic drugs, in addition to airway block, are used for producing favourable intubation conditions during awake fibre-optic intubation (AFOI), but most of them cause respiratory depression and hypoxaemia. The aim of this study was to evaluate the efficacy of intravenous (IV) dexmedetomidine (DEX) on sedation, patient comfort and cardiovascular responses during AFOI in patients with cervical spondylotic myelopathy (CSM).
Methods:
This randomised, placebo-controlled, double-blinded, prospective study was conducted on 56 adult patients with cervical spondylotic myelopathy (CSM) undergoing elective cervical fixation, who were randomly allocated into two groups - Group D and Group C. Group D patients received DEX infusion at a rate of 1 μg/kg for the first 10 min followed by 0.5 μg/kg/h and Group C received 0.9% normal saline infusion in the same manner. Airway blocks with lignocaine were given to all patients before undergoing AFOI. Patient's alertness, sedation and cardiorespiratory changes during the procedure were assessed by the Observer Assessment Awareness and Sedation (OAA/S) scale. On the 1st post-operative day, patient's' comfort during AFOI was assessed by visual analogue scale (VAS).
Results:
Patients of Group D had an acceptable level of sedation (OAA/S score: 20 to 17 with greater comfort and satisfaction (VAS: 40–60), compared to control group (VAS: 50–90, P < 0.001.). Moreover, haemodynamic parameters were less significantly altered in the DEX group during AFOI.
Conclusions:
IV DEX infusion during AFOI improves patient's tolerances with an acceptable level of sedation without significant haemodynamic instability and respiratory depression.
Keywords: Airway block, awake fibre-optic intubation, intravenous dexmedetomidine
INTRODUCTION
Cervical spondylotic myelopathy (CSM) occurs due to degenerative changes in the cervical spine leading to progressive spinal cord compression and dysfunction.[1] Cervical fixation along with spinal cord decompression is the most commonly performed surgical procedure for medically intractable patients.[2] As there is concern about the possibility of cervical spinal cord injury when extending the head and flexing the neck for direct laryngoscopic intubation, awake flexible fibre-optic intubation (AFOI) is often the preferred method for airway management in patients with CSM.[3] The success of AFOI is highly dependent on adequate preparation and sedation techniques. Adequate sedation with topical anaesthesia of the airway may minimise undue discomfort, anxiety and sympathetic surge during AFOI, but respiratory depression and hypoxaemia may occur with excessive sedation.[4]
Although various pharmacological agents such as fentanyl, midazolam, ketamine, propofol and remifentanil have been used for conscious sedation during AFOI, most of them have respiratory depressant effect in higher doses.[4,5] Hence, there is a need for the ideal sedative agent for AFOI that will not cause respiratory depression, and will allow patients to maintain spontaneous respiration and protect their own airway with full cooperation during the passage of fibre-optic scope. Dexmedetomidine (DEX) is a highly selective centrally acting α2-adrenoceptor agonist widely used in intensive care units for its sedative, hypnotic, anxiolytic, sympatholytic, antisecretory and analgesic properties. As it does not cause respiratory depression, the role of intravenous (IV) DEX for sedation during AFOI was assessed in this study.
The main objectives of this study were to assess the level of patient's satisfaction, comfort and sedation along with haemodynamic changes such as heart rate (HR) and mean arterial pressure (MAP) with pre-operative DEX infusion, compared to the placebo group during AFOI.
METHODS
This prospective, randomised, double-blind, controlled trial was conducted in our neurosurgical unit from March 2012 to February 2013. After obtaining the Institutional Ethics Committee clearance and written informed consent from each patient, 56 adult patients with CSM of American Society of Anesthesiologists (ASA) Physical Status I and II, aged 18–50 years, undergoing elective cervical fixation, were included in this study. Patients with higher degree atrioventricular block, obstructive sleep apnoea, morbid obesity and treated with angiotensin converting enzyme inhibitor, α-antagonist, and long-term use of benzodiazepines or tricyclic antidepressants were excluded from this study.
For the purpose of sample size calculation, the Observer Assessment Awareness and Sedation (OAA/S) scale was used as the primary outcome measure. The standard deviation (SD) for this parameter, when used as the marker for the success of premedication, was expected to be approximately 4 on the basis of a published study.[6] It was calculated that 28 patients were required for the group to detect the difference of 3 in anxiety scoring with 80% power and 5% probability in Type I error, using SD of 4. The total number of patients in the study population was 56. Randomisation was done on the basis of a computer-generated random number list that was in the custody of a senior anaesthesiologist who was not otherwise involved in the day-to-day care and monitoring of the study participants. Once the details of a study participant were recorded, the designated study nurse had the responsibility of contacting the senior anaesthesiologist for obtaining the randomisation code and preparation of the study drug. The independent nurse who prepared the study drug refrained from further study participation. This randomisation schedule facilitated patient disposition into two equal groups; Group C (control group [n = 28]) and Group D (DEX group [n = 28]).
At pre-operative visit, the nature of OAA/S scale and visual analogue scale (VAS) were explained to each patient, and proper counselling for airway block was done. All patients fasted for at least 6 h, and tablet diazepam 10 mg and tablet ranitidine 150 mg were given orally, the night before surgery. In the operating, room after attaching standard ASA monitors, patient's' neck was immobilised with a semi-rigid cervical collar (Philadelphia cervical collar). Intravenous (IV) cannulation was done with 18-gauge IV cannula in a peripheral vein and IV glycopyrrolate 0.2 mg was administered. Group D patients had received DEX infusion through an infusion pump (200 μg/2 ml DEX in 48 ml of 0.9% saline solution in a 50-ml syringe - concentration of 4 μg/ml) at a rate of 1 μg/kg for the first 10 min followed by 0.5 μg/kg/h. On the other hand, Group C patients received 0.9% normal saline infusion in the same manner to ensure blinding. After 15 min of administering the study drug and saline infusion, the nasal mucosa was packed with xylometazoline 0.05% and cotton-tipped applicator soaked in 2% lignocaine with adrenaline was applied to block the sphenopalatine ganglion and anterior ethmoidal nerve after performing a nasal patency test. The glossopharyngeal and superior laryngeal nerves were blocked bilaterally with 2 ml of 2% lignocaine and the recurrent laryngeal nerve was blocked by a transtracheal approach with 2 ml of 4% lignocaine. Then, an experienced anaesthesiologist performed nasotracheal fibre-optic intubation loaded with a lubricated flexo-metallic armoured endotracheal (ET) tube of appropriate size. After visualisation of the vocal cords, the bronchoscope was further advanced up to the level of the carina. Then, the armoured ET tube was introduced over the bronchoscope and fixed 3 cm above the carina. Confirmation of the tube position was done by chest auscultation bilaterally and by end-tidal CO2 concentration. Patients' alertness and sedation during the procedure were assessed by the OAA/S scale by an anaesthesiologist who was blind to the study drug. HR, MAP, percentage saturation of oxygen (SpO2) and respiratory rate (RR) were recorded at baseline, during study drug infusion at 3 min intervals until AFOI had been completed and then at 1 min interval up to 5 min after intubation. Hypotension (reduction of MAP >20% from baseline) was treated with IV fluid and/or phenylephrine 50 μg IV bolus, repeated if necessary after 5 min. Bradycardia (HR <60 beats/min) was treated with atropine 0.6 mg IV. Oxygen desaturation (SpO2 <95% for >10 s) was treated with oxygen supplementation either through a nasal cannula or the suction port of the bronchoscope. If the patient became uncomfortable during AFOI, additional IV fentanyl 25 μg was given. Then, induction of anaesthesia was done by IV propofol 2 mg/kg along with IV fentanyl 2 μg/kg and IV rocuronium bromide 0.6 mg/kg as the muscle relaxant. Maintenance of anaesthesia was with an oxygen: nitrous oxide mixture (3:5) along with isoflurane. Muscle relaxation was maintained with IV rocuronium 0.1 mg/kg as and when necessary. When the surgical procedure was completed, the residual neuromuscular block was reversed with IV neostigmine 0.05 mg/kg and glycopyrrolate 0.02 mg/kg. On the 1st post-operative day, patients' comfort during AFOI was assessed on a 100 mm VAS by an investigator blinded to the procedure. Primary outcomes of the study were patient's satisfaction and sedation during AFOI assessed by OAA/S scale based on speech, facial expression and eye movement (Score: 20-18 = response readily to name/alert, 17-15 = lethargic response/light sedation, 14-11 = response only after name spoken loudly/heavy sedation and under 10 = unable to respond) recorded from baseline before the study drug infusion up to ET intubation. Secondary outcomes were haemodynamic parameters such as HR and MAP which were measured from the baseline before the study drug infusion up to 5 min after intubation.
Demographic data were expressed as mean ± SD (age, weight, height) or proportion (sex and ASA physical status). All the raw data were entered into a Microsoft Excel spread sheet and analysed using Statistica version 6 (StatSoft Inc., 2001, Tulsa, Oklahoma, USA), SPSS Statistics version 17 (SPSS Inc., 2008, Chicago, Illinois. USA) and Graph Prism version 5 (graph Pad Software Inc., 2005, San Diego, California, USA) software. Numerical parameters were compared between the two groups by Student's independent t-test if normally distributed or by Mann–Whitney U-test, if otherwise. Normality was tested using Kolmogorov–Smirnov goodness-of-fit test. Pearson's Chi-square test and Fisher's exact test were employed for intergroup comparison of categorical variables. All the analyses were two tailed, and confidence level was 95%. P < 0.001 was considered statistically significant.
RESULTS
In the final analysis, 56 patients were randomised for assessment, and Figure 1 illustrates the flow of patients. All the demographic data on patients' characteristics such as age, height, body weight, body mass index, sex and ASA status were comparable between the two groups.[Table 1] Figure 1: Consort transparent reporting of trials - Flow of patients in the trial. (AV, atrioventricular; DEX, dexmedetomidine)
Figure 1.
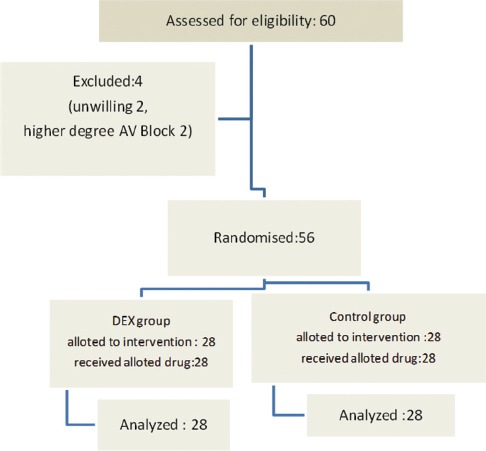
Consort transparent reporting of trials -- Flow of patients in the trial. (AV, atrioventricular; DEX, dexmedetomidine)
Table 1.
Demographic characteristics of the patients in two groups
All the patients of both groups were alert and awake at the beginning of the study i.e., comparable in terms of OAA/S scale. Each patient underwent successful fibre-optic intubation, but satisfaction score was more favourable in DEX group. Out of 28 patients in Group D, 75% of the patients were lightly sedated and 25% of the patients were alert. Despite sedation, all patients in DEX group responded and cooperated well during AFOI with stable haemodynamics. In control group, the verbal response to the speech was more prominent, but comfort level and satisfaction were less with the haemodynamic surge.
In control group, total OAA/S score remained same (OAA/S score: 20) throughout the study period, but in Group D, the score was insignificantly decreased during fibre-optic bronchoscopy (FOB) (20 to 17, P = 0.328). The comparison of intergroup changes of OAA/S score was statistically significant (P < 0.001) from 9 min after the study drug infusion to ET tube introduction [Figure 2]. Patient's comfort level during the procedure was measured postoperatively after 24 h by VAS. Higher VAS score was found in the control group (50–90) than Group D (40–60), which was statistically significant at P < 0.001 [Figure 3].
Figure 2.
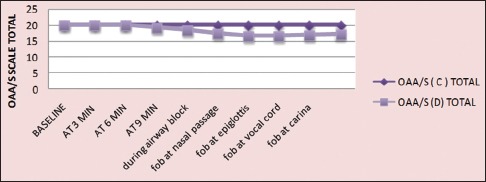
Procedural alertness and sedation assessed by OAA/S scale (OAA/S: Observer assessment of alertness /sedation scale, C-control; D-dexmedetomidine)
Figure 3.
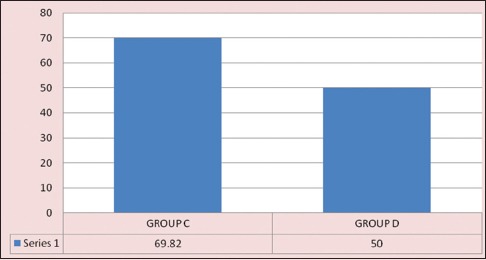
Comparison of procedural comfort by postoperative VAS (VAS = Visual analogue scale C-control;D-dexmedetomidine)
In the control group, there was a significant increase in HR (92.67 + 11.47/min) during FOB from baseline value (74 + 14.54/min) (P < 0.001), whereas in Group D, HR was significantly decreased (64.25 ± 8.92/min) during FOB from baseline (72 + 12.54/min) (P < 0.001). The comparison of intergroup changes of HR during AFOB was also statistically significant (P < 0.001) [Figure 4]. In the control group, there was a significant rise of MAP during and after FOB (94.8–109.1 mmHg - P < 0.001), but changes in MAP in Group D were not statistically significant (94.8–101.1 mmHg - P = 0.129). The comparison of intergroup changes of MAP during AFOB was also statistically significant (P < 0.001) [Figure 5].
Figure 4.
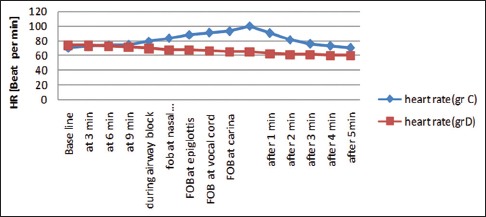
Comparison of procedural heart rate (HR= heart rate, C-control; D-dexmedetomidine)
Figure 5.
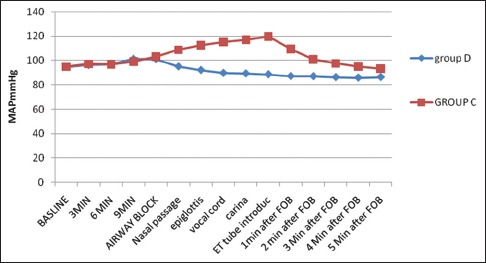
Comparison of procedural mean arterial pressure (MAP= mean arterial pressure, C-control; D-dexmedetomidine)
All the patients of both groups maintained arterial SpO2 within the satisfactory level (98%–99%) during the study period and the changes were statistically insignificant (P = 0.321). There was a gradual increase in RR in the control group from baseline up to ET intubation which was statistically significant (14–23/min, P < 0.001), but in DEX group, the changes in RR were statistically insignificant (14–16/min, P = 0.328). Moreover, the comparison of intergroup changes of RR was statistically significant (P < 0.001) from FOB at the nasal passage to intubation. IV fentanyl 25 μg was required in 60% of the patients of control group during AFOI, but no patient in DEX group required IV fentanyl.
There was no incidence of hypoxia, respiratory depression (RR <8/min), significant bradycardia (HR <60/min) and hypotension (SBP <30% from baseline) in either group of patients.
DISCUSSION
In our study, we found that patients who received IV DEX in addition to airway block during AFOI remained more comfortable than with airway block alone. During conventional direct laryngoscopy in sniffing position, there is an extension at the atlant-occipital joint and flexion of the cervical spine between C2 and C4.[7] Many anaesthesiologists prefer AFOI as it minimises the cervical spine movement and prevents further neurological damage.[8] Previously, awake flexible fibre-optic bronchoscope-aided intubation has been performed only with airway blocks by lignocaine without any sedation, but poor tolerance, patient's dissatisfaction and discomfort along with haemodynamic instability were frequently noted in these patients.[9] The advantages of using sedation during AFOI has been documented in various studies.[9,10]
Various drugs have been tried, to achieve conscious sedation during AFOI, but the incidence of hypoxaemia is high due to their respiratory depressant effect. The major advantage of DEX infusion during AFOI is a unique form of sedation where patients remain sleepy, but are easily aroused and cooperative with minimum respiratory impairment.[11,12] Neurological assessment can be done immediately after intubation as patients remain awake throughout the procedure.[13]
DEX, an α2 agonist, produces conscious sedation, hypnosis and analgesia with minimal effect on respiration. It produces sedation by pre-synaptic activation of α2-adrenoceptor in the locus ceruleus that inhibits the release of norepinephrine (NE).[14] Role of IV DEX for prevention of the conventional adrenergic surge during intubation has been well documented.[15] There is no published study to establish the effect of IV DEX, in addition to airway block, on patient's satisfaction, comfort and sedation during AFOI in cervical fixation surgery. In this study, the efficacy of DEX infusion was evaluated using OAA/S scale.
Our study indicates that the satisfaction score during AFOI is more favourable with DEX infusion at an acceptable level of sedation, in conformity with our study hypothesis. The secondary outcome haemodynamic parameters such as HR and MAP were more stable in patients with DEX premedication than in the placebo group. Post-operative VAS score for procedural comfort was also more favourable in DEX group.
One study reported that tolerance and cooperation to AFOI were better with DEX than placebo as assessed by less limb movement, and responsiveness to command was also satisfactory.[16]
These findings are also supported by other studies.[17,18] Our results are in concordance with these studies.[19,20,21,22]
The respiratory depressant effect of DEX with different IV doses is well documented in various studies. Episodes of obstructive apnoea have been reported in one study when DEX was infused at 1 and 2 μg/kg doses rapidly over 2 min.[23] Episodes of loss of airway patency with higher (10 μg/kg/h) doses of DEX infusion have been reported in another study.[24] In our study, as lower maintenance dose of DEX (0.5 μg/kg/h) was used, no such complication occurred.
Dose-dependent biphasic alteration of BP with DEX has been reported.[25] It has been documented that hypertensive episodes are more frequent at higher doses (1–2 μg/kg) and hypotension at lower doses (0.25–0.5 μg/kg) when bolus infusions of DEX were given over 2 min.[26] Initial increase in BP after the loading dose may be due to vasoconstriction caused by direct stimulation of α1 receptors on blood vessels and decrease in BP in the subsequent period due to inhibition of norepinephrine release from sympathetic terminals.[14] In our study, this biphasic BP response of DEX was decreased by increasing the duration of the loading dose (1 μg/kg over 10 min) and lowering the maintenance dose (0.5 μg/kg/h) of DEX.
It has been documented that higher doses of DEX may be used safely with minimal changes in haemodynamics when they are infused over 10 min.[27] It is also recommended that the use of DEX at 1 μg/kg bolus over 15 min, with maintenance rates of 0.2–0.7 μg/kg/h, is safe and beneficial for surgical patients.[28] On the other hand, patients in control group without DEX (Group C) have a significant rise in BP during AFOI (SBP > 30% from baseline) (P < 0.001) due to haemodynamic response to intubation.
This study has demonstrated that there was a significant decrease in HR in Group D at different time intervals during and after AFOI compared to baseline parameters (P < 0.001). The potential causes of low HR may be due to increasing vagal tone, decreased circulating levels of NE and the baroreceptor response of high vascular tone that occurs with the loading dose.[14] In our study, as all patients were pre-treated with IV glycopyrrolate, HR was not significantly decreased (<50) during the study period. In contrast, all the patients of the control group had a significant rise in HR during AFOI due to increasing sympathetic outflow (P < 0.001). These findings are closely corroborated with a similar previous study.[28]
However, there are some limitations in this study. We enlisted our study nurse to maintain allocation concealment and ensure double blinding. However, we anticipate that this arrangement would be difficult to sustain for a large number of patients unless nursing staff specially trained in this methodology can be made available. Another possible confounding factor would be variation in the level of stress enforced on the patient by varying skill of AFOI. In our case, we ensured that AFOI was done by an experienced anaesthesiologist.
The serum stress hormone (cortisol and noradrenaline) level could not be estimated in our study.
The interpretation of the study is that only airway block is inadequate for patient satisfaction and comfort during AFOI, and combination of DEX infusion (1 μg/kg for the first 10 min and followed by 0.5 μg/kg/h) with airway block is preferable. It provides better intubation condition, improves patient tolerance and enhances patient comfort without respiratory depression and haemodynamic instability.
The minimal side effects observed in our study are not sufficient to justify conclusions on individual drug safety, and further study in a large patient population will be required to establish the safety profiles of IV DEX infusion along with airway block with lignocaine.
CONCLUSIONS
For successful awake fibreoptic intubation in patients with cervical spine myelopathy, only airway block is not sufficient. Additional dexmedetomidine infusion along with airway block effectively increases patient's satisfaction and comfort with acceptable level of sedation. It improves patient tolerance without respiratory depression and haemodynamic instability.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Montgomery DM, Brower RS. Cervical spondylotic myelopathy. Clinical syndrome and natural history. Orthop Clin North Am. 1992;23:487–93. [PubMed] [Google Scholar]
- 2.Lebl DR, Hughes A, Cammisa FP, Jr, O'Leary PF. Cervical spondylotic myelopathy: Pathophysiology, clinical presentation, and treatment. HSS J. 2011;7:170–8. doi: 10.1007/s11420-011-9208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenblatt WH. Airway management. In: Barash PG, Cullen BF, Stoelting RK, editors. Clinical Anesthesia. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 595–638. [Google Scholar]
- 4.Bailey PL, Pace NL, Ashburn MA, Moll JW, East KA, Stanley TH. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology. 1990;73:826–30. doi: 10.1097/00000542-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lallo A, Billard V, Bourgain JL. A comparison of propofol and remifentanil target-controlled infusions to facilitate fiberoptic nasotracheal intubation. Anesth Analg. 2009;108:852–7. doi: 10.1213/ane.0b013e318184eb31. [DOI] [PubMed] [Google Scholar]
- 6.Bergese SD, Khabiri B, Roberts WD, Howie MB, McSweeney TD, Gerhardt MA. Dexmedetomidine for conscious sedation in difficult awake fiberoptic intubation cases. J Clin Anesth. 2007;19:141–4. doi: 10.1016/j.jclinane.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 7.American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Practice guidelines for management of the difficult airway: An updated report by the American Society of Anesthesiologists Task Force on Management of the Difficult Airway. Anesthesiology. 2003;98:1269–77. doi: 10.1097/00000542-200305000-00032. [DOI] [PubMed] [Google Scholar]
- 8.Meschino A, Devitt JH, Koch JP, Szalai JP, Schwartz ML. The safety of awake tracheal intubation in cervical spine injury. Can J Anaesth. 1992;39:114–7. doi: 10.1007/BF03008639. [DOI] [PubMed] [Google Scholar]
- 9.Wahidi MM, Jain P, Jantz M, Lee P, Mackensen GB, Barbour SY, et al. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest. 2011;140:1342–50. doi: 10.1378/chest.10-3361. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez R, De-La-Rosa-Ramirez I, Maldonado-Hernandez A, Dominguez-Cherit G. Should patients undergoing a bronchoscopy be sedated? Acta Anaesthesiol Scand. 2003;47:411–5. doi: 10.1034/j.1399-6576.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 11.Avitsian R, Lin J, Lotto M, Ebrahim Z. Dexmedetomidine and awake fiberoptic intubation for possible cervical spine myelopathy: A clinical series. J Neurosurg Anesthesiol. 2005;17:97–9. doi: 10.1097/01.ana.0000161268.01279.ba. [DOI] [PubMed] [Google Scholar]
- 12.Neumann MM, Davio MB, Macknet MR, Applegate RL., 2nd Dexmedetomidine for awake fiberoptic intubation in a parturient with spinal muscular atrophy type III for cesarean delivery. Int J Obstet Anesth. 2009;18:403–7. doi: 10.1016/j.ijoa.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Stamenkovic DM, Hassid M. Dexmedetomidine for fiberoptic intubation of a patient with severe mental retardation and atlantoaxial instability. Acta Anaesthesiol Scand. 2006;50:1314–5. doi: 10.1111/j.1399-6576.2006.01157.x. [DOI] [PubMed] [Google Scholar]
- 14.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 15.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 16.Tsai CJ, Chu KS, Chen TI, Lu DV, Wang HM, Lu IC. A comparison of the effectiveness of dexmedetomidine versus propofol target-controlled infusion for sedation during fibreoptic nasotracheal intubation. Anaesthesia. 2010;65:254–9. doi: 10.1111/j.1365-2044.2009.06226.x. [DOI] [PubMed] [Google Scholar]
- 17.Chu KS, Wang FY, Hsu HT, Lu IC, Wang HM, Tsai CJ. The effectiveness of dexmedetomidine infusion for sedating oral cancer patients undergoing awake fibreoptic nasal intubation. Eur J Anaesthesiol. 2010;27:36–40. doi: 10.1097/EJA.0b013e32832e0d2b. [DOI] [PubMed] [Google Scholar]
- 18.Bergese SD, Patrick Bender S, McSweeney TD, Fernandez S, Dzwonczyk R, Sage K. A comparative study of dexmedetomidine with midazolam and midazolam alone for sedation during elective awake fiberoptic intubation. J Clin Anesth. 2010;22:35–40. doi: 10.1016/j.jclinane.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Ryu JH, Lee SW, Lee JH, Lee EH, Do SH, Kim CS. Randomized double-blind study of remifentanil and dexmedetomidine for flexible bronchoscopy. Br J Anaesth. 2012;108:503–11. doi: 10.1093/bja/aer400. [DOI] [PubMed] [Google Scholar]
- 20.Hu R, Liu JX, Jiang H. Dexmedetomidine versus remifentanil sedation during awake fiberoptic nasotracheal intubation: A double-blinded randomized controlled trial. J Anesth. 2013;27:211–7. doi: 10.1007/s00540-012-1499-y. [DOI] [PubMed] [Google Scholar]
- 21.Mondal S, Ghosh S, Bhattacharya S, Choudhury B, Mallick S, Prasad A. Comparison between dexmedetomidine and fentanyl on intubation conditions during awake fiberoptic bronchoscopy: A randomized double-blind prospective study. J Anaesthesiol Clin Pharmacol. 2015;31:212–6. doi: 10.4103/0970-9185.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chopra P, Dixit MB, Dang A, Gupta V. Dexmedetomidine provides optimum conditions during awake fiberoptic intubation in simulated cervical spine injury patients. J Anaesthesiol Clin Pharmacol. 2016;32:54–8. doi: 10.4103/0970-9185.175666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–33. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Ramsay MA, Luterman DL. Dexmedetomidine as a total intravenous anesthetic agent. Anesthesiology. 2004;101:787–90. doi: 10.1097/00000542-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Bhana N, Goa KL, McClellan KJ. Dexmedetomidine. Drugs. 2000;59:263–8. doi: 10.2165/00003495-200059020-00012. [DOI] [PubMed] [Google Scholar]
- 26.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–42. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the Intensive Care Unit: Patient and clinician perceptions. Br J Anaesth. 2001;87:684–90. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]
- 28.Jorden VS, Pousman RM, Sanford MM, Thorborg PA, Hutchens MP. Dexmedetomidine overdose in the perioperative setting. Ann Pharmacother. 2004;38:803–7. doi: 10.1345/aph.1D376. [DOI] [PubMed] [Google Scholar]


